Abstract
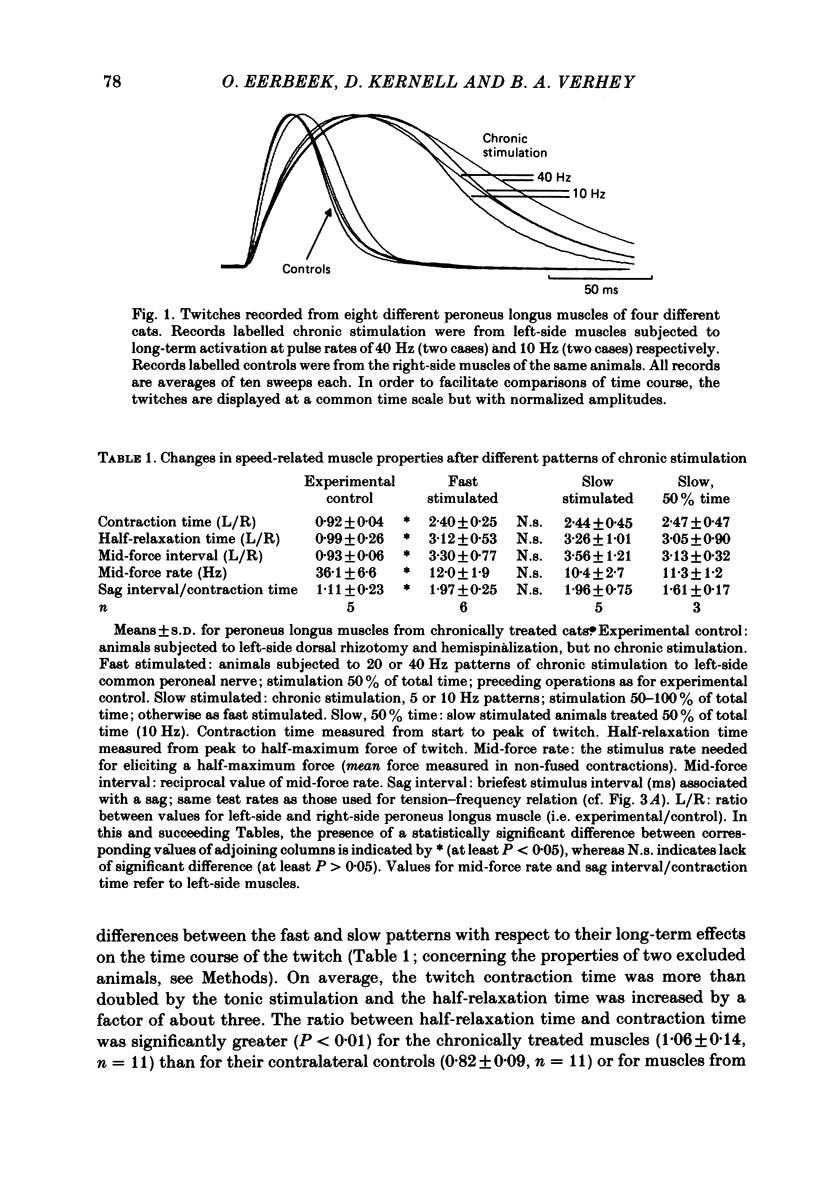
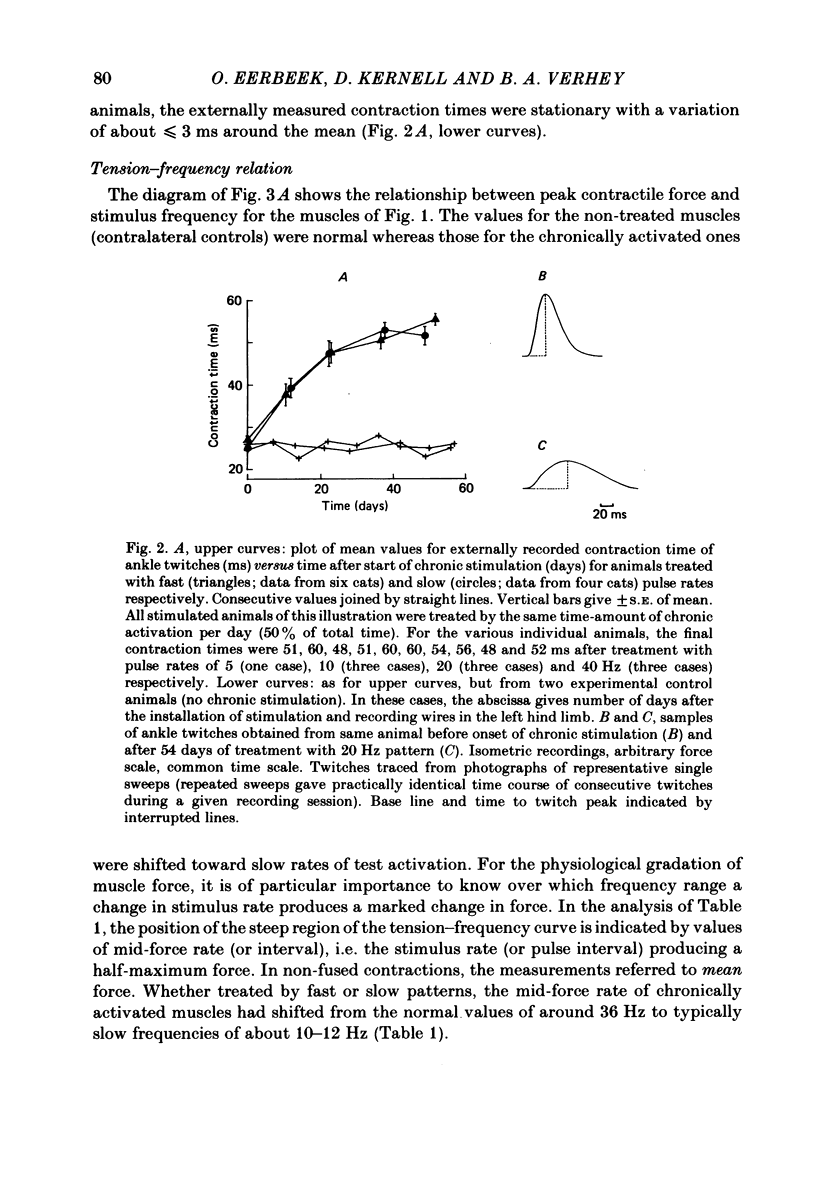
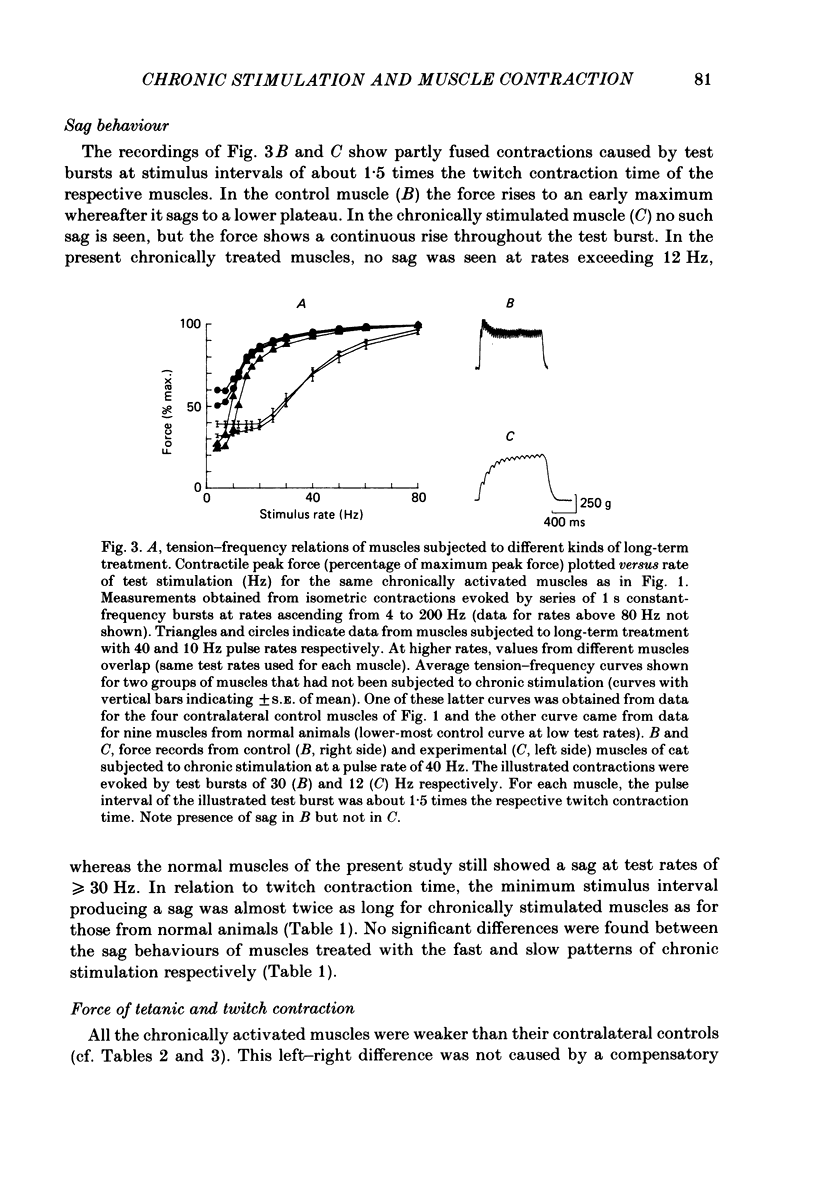
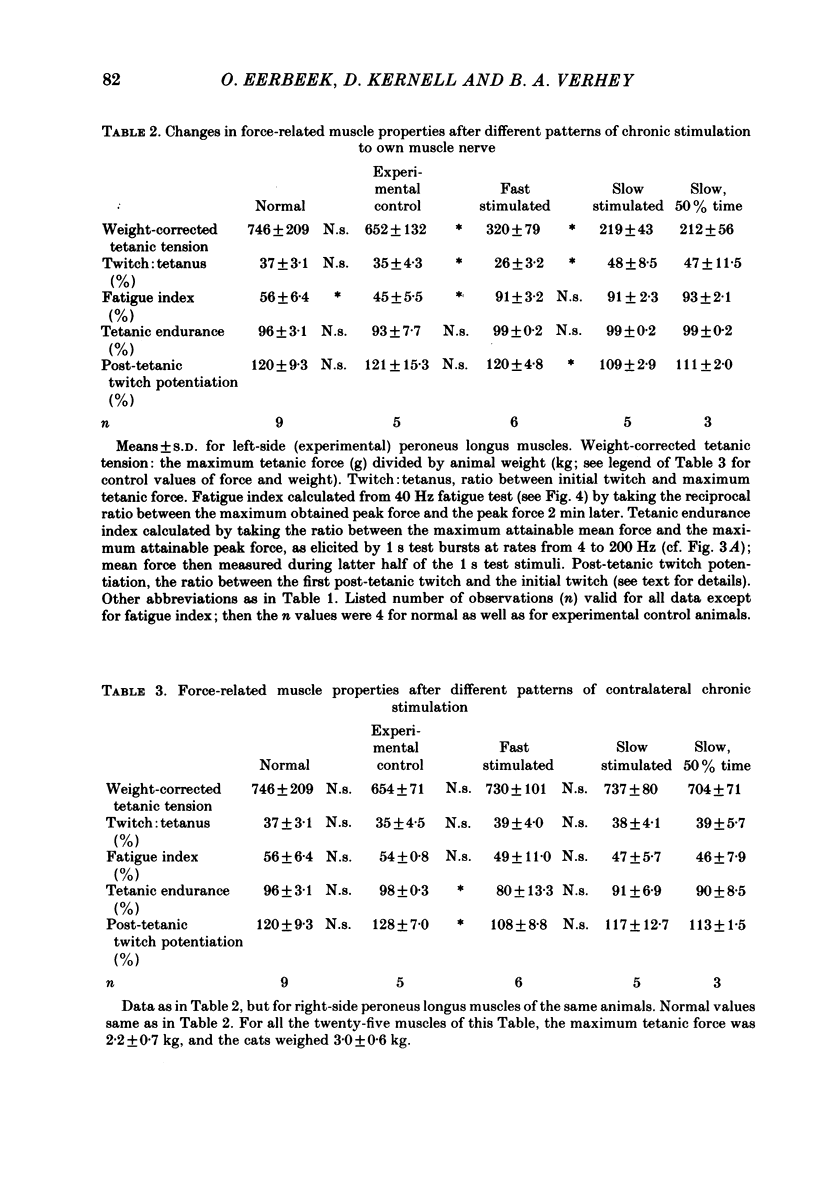
Different physiological rates of 'tonic' long-term electrical stimulation (rates 5-40 Hz; activity greater than or equal to 50% total time) were delivered to the left-side common peroneal nerve of the cat hind limb. The duration of treatment was 8 weeks, and the animals had previously been subjected to a left-side hemispinalization and dorsal rhizotomy. In the absence of stimulation, these operations had no slowing or weakening effects on peroneal muscle contraction. The minimum two-pulse interval that gave a summation of tension (neuromuscular refractory period) was longer for stimulated than for non-stimulated muscles. Twitches of chronically stimulated muscles had become prolonged by more than 100%. Corresponding changes were found in the tension-frequency relation and in the 'sag'-behaviour of the stimulated muscles. There were no differences between the 'fast' (20 or 40 Hz pulse rates) and the 'slow' (5 or 10 Hz pulse rates) patterns of tonic stimulation with respect to their effects on speed-related muscle properties. Furthermore, during the period of chronic stimulation, the prolongation of twitch contraction time occurred along the same time course for the fast and slow patterns of tonic treatment. All chronically stimulated muscles had become weaker than normal. In comparison to the slow patterns, the present fast patterns of long-term activation caused (1) a smaller amount of decline in maximum muscle force, (2) a smaller twitch: tetanus ratio, and (3) the retention of a normal amount of post-tetanic potentiation of twitch size (decreased by the slow patterns). When tested by a series of 40 Hz bursts, force was better maintained in chronically stimulated muscles than in normal ones. These effects on fatigue resistance were the same for the fast and slow patterns of long-term activation. In peroneus longus muscles contralateral to the side of chronic activation, an evident impairment had commonly occurred in the capability to maintain force during tetani at the high rates needed for a maximum tetanic contraction. The results are discussed in relation to problems concerning the long-term effects of motoneuronal activity patterns on the contractile properties of their muscle units.
Full text
PDF





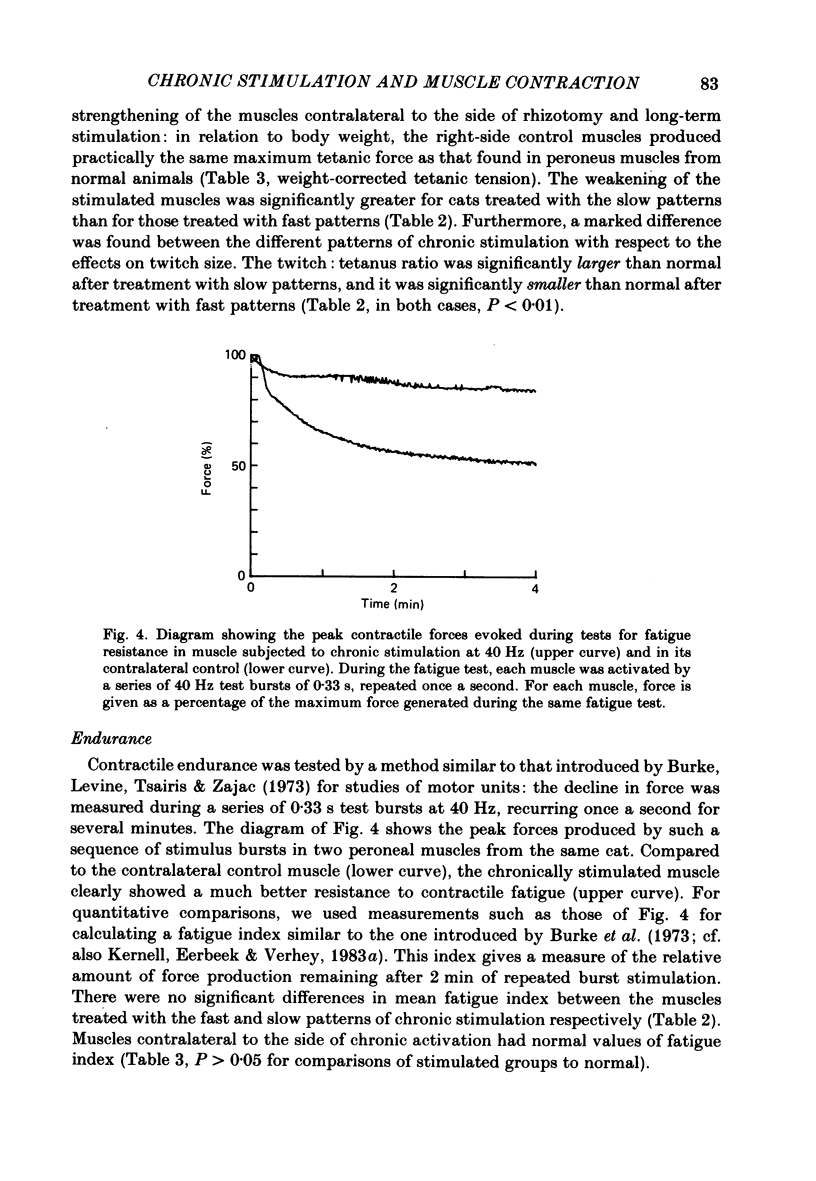
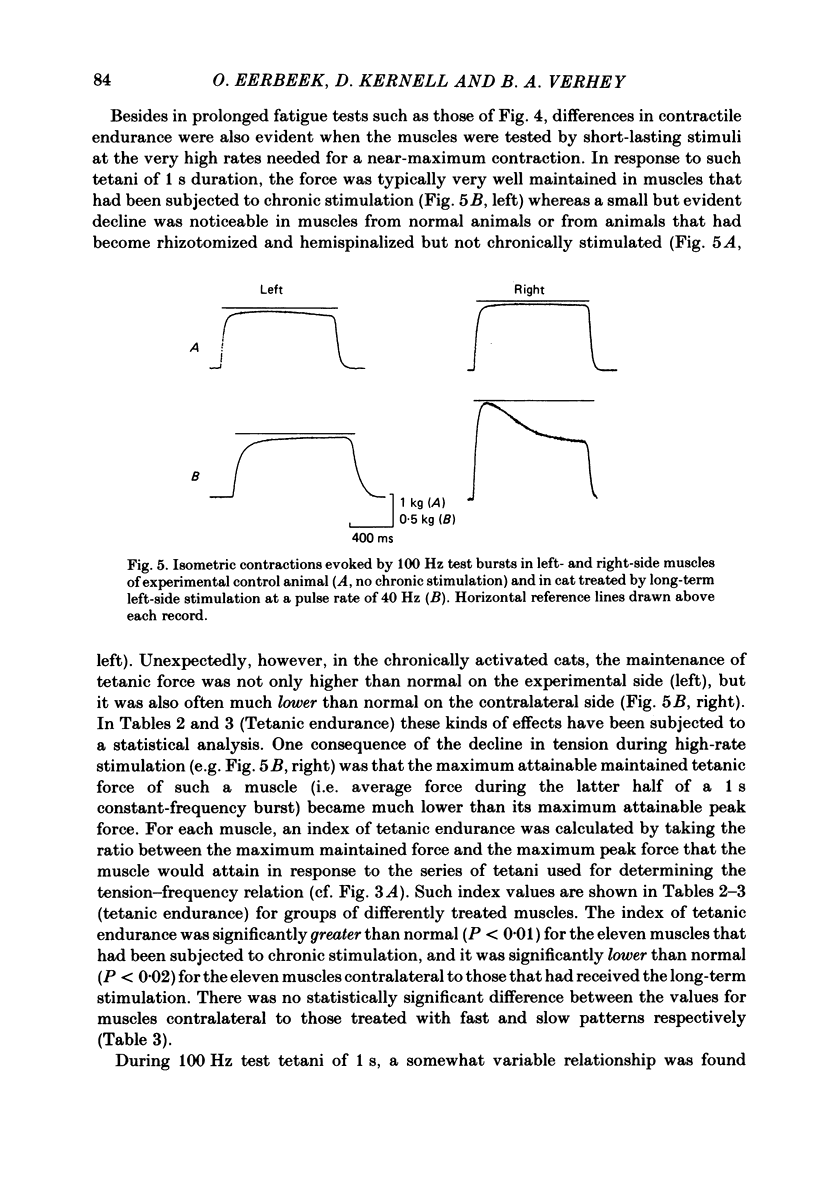






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN M. C., MATTHEWS P. B. The effect on a muscle twitch of the back-response of its motor nerve fibres. J Physiol. 1960 Feb;150:332–346. doi: 10.1113/jphysiol.1960.sp006391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., ECCLES J. C., ECCLES R. M. Interactions between motoneurones and muscles in respect of the characteristic speeds of their responses. J Physiol. 1960 Feb;150:417–439. doi: 10.1113/jphysiol.1960.sp006395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A. J., LEWIS D. M. THE RATE OF TENSION DEVELOPMENT IN ISOMETRIC TETANIC CONTRACTIONS OF MAMMALIAN FAST AND SLOW SKELETAL MUSCLE. J Physiol. 1965 Feb;176:337–354. doi: 10.1113/jphysiol.1965.sp007554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. D., Cotter M. A., Hudlická O., Vrbová G. The effects of different patterns of muscle activity on capillary density, mechanical properties and structure of slow and fast rabbit muscles. Pflugers Arch. 1976 Feb 24;361(3):241–250. doi: 10.1007/BF00587288. [DOI] [PubMed] [Google Scholar]
- Buller A. J., Proske U. Further observations on back-firing in the motor nerve fibres of a muscle during twitch contractions. J Physiol. 1978 Dec;285:59–69. doi: 10.1113/jphysiol.1978.sp012557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. I. Dynamic properties of mammalian skeletal muscles. Physiol Rev. 1972 Jan;52(1):129–197. doi: 10.1152/physrev.1972.52.1.129. [DOI] [PubMed] [Google Scholar]
- Close R. Dynamic properties of fast and slow skeletal muscles of the rat after nerve cross-union. J Physiol. 1969 Oct;204(2):331–346. doi: 10.1113/jphysiol.1969.sp008916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. The isometric responses of mammalian muscles. J Physiol. 1930 Jun 27;69(4):377–385. doi: 10.1113/jphysiol.1930.sp002657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M. T., Kushmerick M. J. Chemical energetics of slow- and fast-twitch muscles of the mouse. J Gen Physiol. 1982 Jan;79(1):147–166. doi: 10.1085/jgp.79.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh G., Gallego R., Kudo N., Kuno M. Evidence for the maintenance of motoneurone properties by muscle activity. J Physiol. 1978 Aug;281:239–252. doi: 10.1113/jphysiol.1978.sp012419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. The action potentials of the alpha motoneurones supplying fast and slow muscles. J Physiol. 1958 Jul 14;142(2):275–291. doi: 10.1113/jphysiol.1958.sp006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberger M. E., Murray M. Restitution of function and collateral sprouting in the cat spinal cord: the deafferented animal. J Comp Neurol. 1974 Nov 1;158(1):37–53. doi: 10.1002/cne.901580104. [DOI] [PubMed] [Google Scholar]
- Goldring J. M., Kuno M., Núez R., Weakly J. N. Do identical activity patterns in fast and slow motor axons exert the same influence on the twitch time of cat skeletal muscle? J Physiol. 1981 Dec;321:211–223. doi: 10.1113/jphysiol.1981.sp013980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L., Yellin H. The dynamic nature of the so-called "fiber types" of nammalian skeletal muscle. Exp Neurol. 1971 May;31(2):227–300. doi: 10.1016/0014-4886(71)90196-8. [DOI] [PubMed] [Google Scholar]
- Hudlická O., Tyler K. R., Srihari T., Heilig A., Pette D. The effect of different patterns of long-term stimulation on contractile properties and myosin light chains in rabbit fast muscles. Pflugers Arch. 1982 Apr;393(2):164–170. doi: 10.1007/BF00582940. [DOI] [PubMed] [Google Scholar]
- Kernell D., Eerbeek O., Verhey B. A. Motor unit categorization on basis of contractile properties: an experimental analysis of the composition of the cat's m. peroneus longus. Exp Brain Res. 1983;50(2-3):211–219. doi: 10.1007/BF00239185. [DOI] [PubMed] [Google Scholar]
- Kernell D., Eerbeek O., Verhey B. A. Relation between isometric force and stimulus rate in cat's hindlimb motor units of different twitch contraction time. Exp Brain Res. 1983;50(2-3):220–227. doi: 10.1007/BF00239186. [DOI] [PubMed] [Google Scholar]
- Kernell D. Functional properties of spinal motoneurons and gradation of muscle force. Adv Neurol. 1983;39:213–226. [PubMed] [Google Scholar]
- Kernell D. Recruitment, rate modulation and the tonic stretch reflex. Prog Brain Res. 1976;44:257–266. doi: 10.1016/S0079-6123(08)60737-2. [DOI] [PubMed] [Google Scholar]
- Kernell D. Rhythmic properties of motoneurones innervating muscle fibres of different speed in m. gastrocnemius medialis of the cat. Brain Res. 1979 Jan 5;160(1):159–162. doi: 10.1016/0006-8993(79)90612-7. [DOI] [PubMed] [Google Scholar]
- Lomo T., Westgaard R. H., Dahl H. A. Contractile properties of muscle: control by pattern of muscle activity in the rat. Proc R Soc Lond B Biol Sci. 1974 Aug 27;187(1086):99–103. doi: 10.1098/rspb.1974.0064. [DOI] [PubMed] [Google Scholar]
- McDonagh J. C., Binder M. D., Reinking R. M., Stuart D. G. A commentary on muscle unit properties in cat hindlimb muscles. J Morphol. 1980 Nov;166(2):217–230. doi: 10.1002/jmor.1051660208. [DOI] [PubMed] [Google Scholar]
- Monster A. W., Chan H., O'Connor D. Activity patterns of human skeletal muscles: relation to muscle fiber type composition. Science. 1978 Apr 21;200(4339):314–317. doi: 10.1126/science.635587. [DOI] [PubMed] [Google Scholar]
- Peckham P. H., Mortimer J. T., Van Der Meulen J. P. Physiologic and metabolic changes in white muscle of cat following induced exercise. Brain Res. 1973 Feb 28;50(2):424–429. doi: 10.1016/0006-8993(73)90745-2. [DOI] [PubMed] [Google Scholar]
- Pette D., Ramirez B. U., Müller W., Simon R., Exner G. U., Hildebrand R. Influence of intermittent long-term stimulation on contractile, histochemical and metabolic properties of fibre populations in fast and slow rabbit muscles. Pflugers Arch. 1975 Dec 19;361(1):1–7. doi: 10.1007/BF00587333. [DOI] [PubMed] [Google Scholar]
- Pette D., Smith M. E., Staudte H. W., Vrbová G. Effects of long-term electrical stimulation on some contractile and metabolic characteristics of fast rabbit muscles. Pflugers Arch. 1973 Feb 6;338(3):257–272. doi: 10.1007/BF00587391. [DOI] [PubMed] [Google Scholar]
- Salmons S., Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981 Mar-Apr;4(2):94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]
- Salmons S., Sréter F. A. Significance of impulse activity in the transformation of skeletal muscle type. Nature. 1976 Sep 2;263(5572):30–34. doi: 10.1038/263030a0. [DOI] [PubMed] [Google Scholar]
- Salmons S., Vrbová G. The influence of activity on some contractile characteristics of mammalian fast and slow muscles. J Physiol. 1969 May;201(3):535–549. doi: 10.1113/jphysiol.1969.sp008771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srihari T., Seedorf U., Pette D. Ipsi-and contralateral changes in rabbit soleus myosins by cross-reinnervation. Pflugers Arch. 1981 Jun;390(3):246–249. doi: 10.1007/BF00658269. [DOI] [PubMed] [Google Scholar]


