Abstract
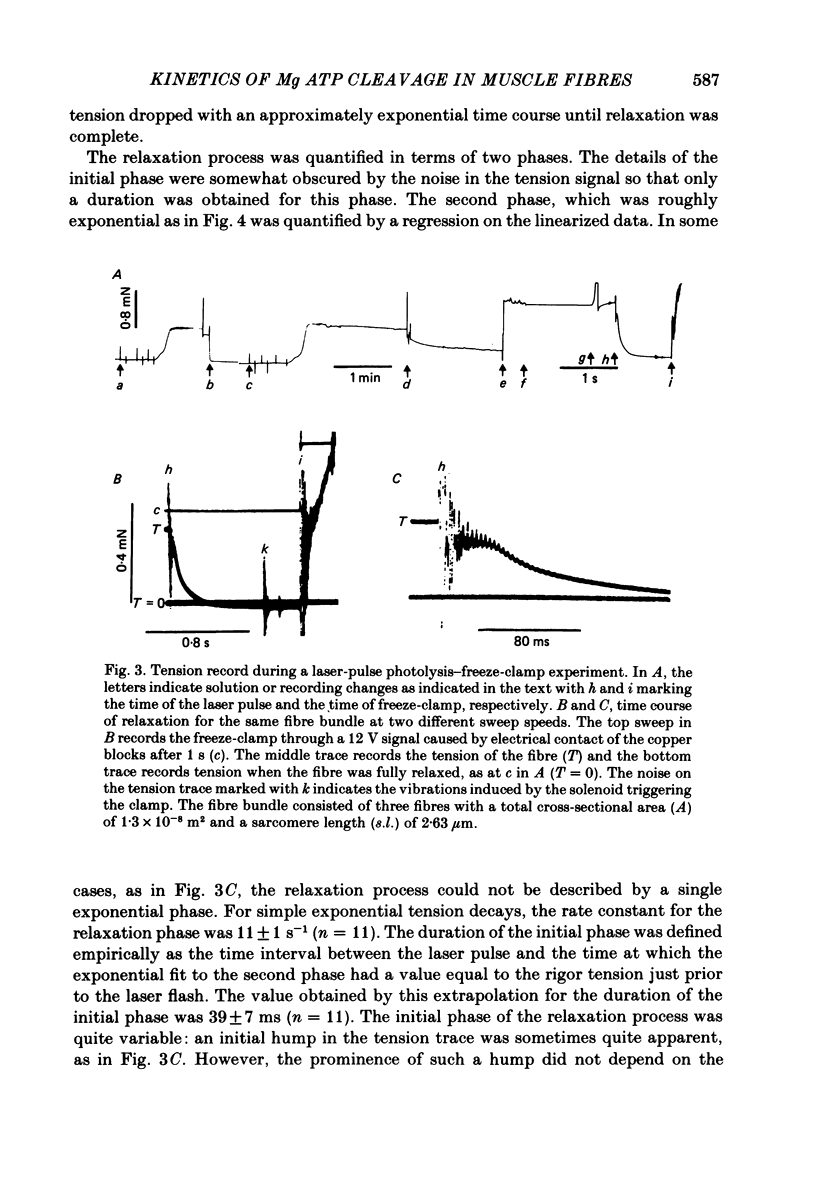
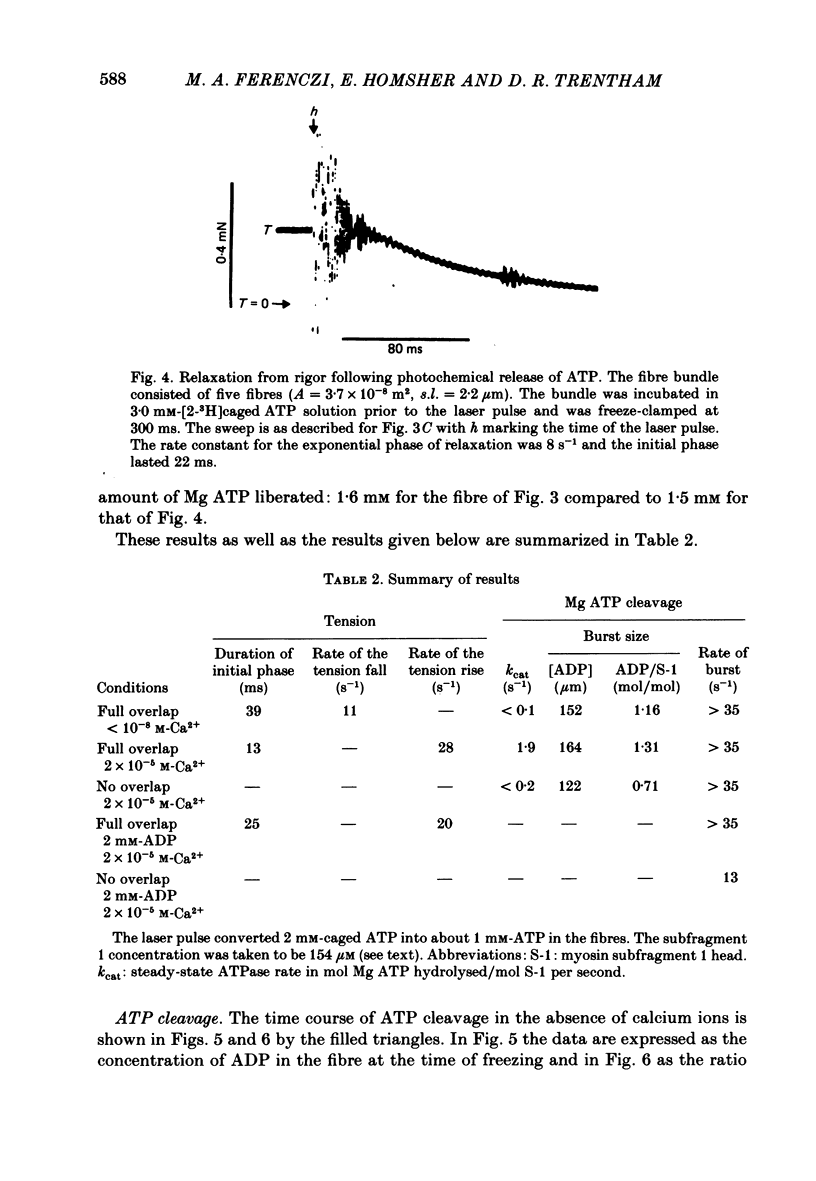
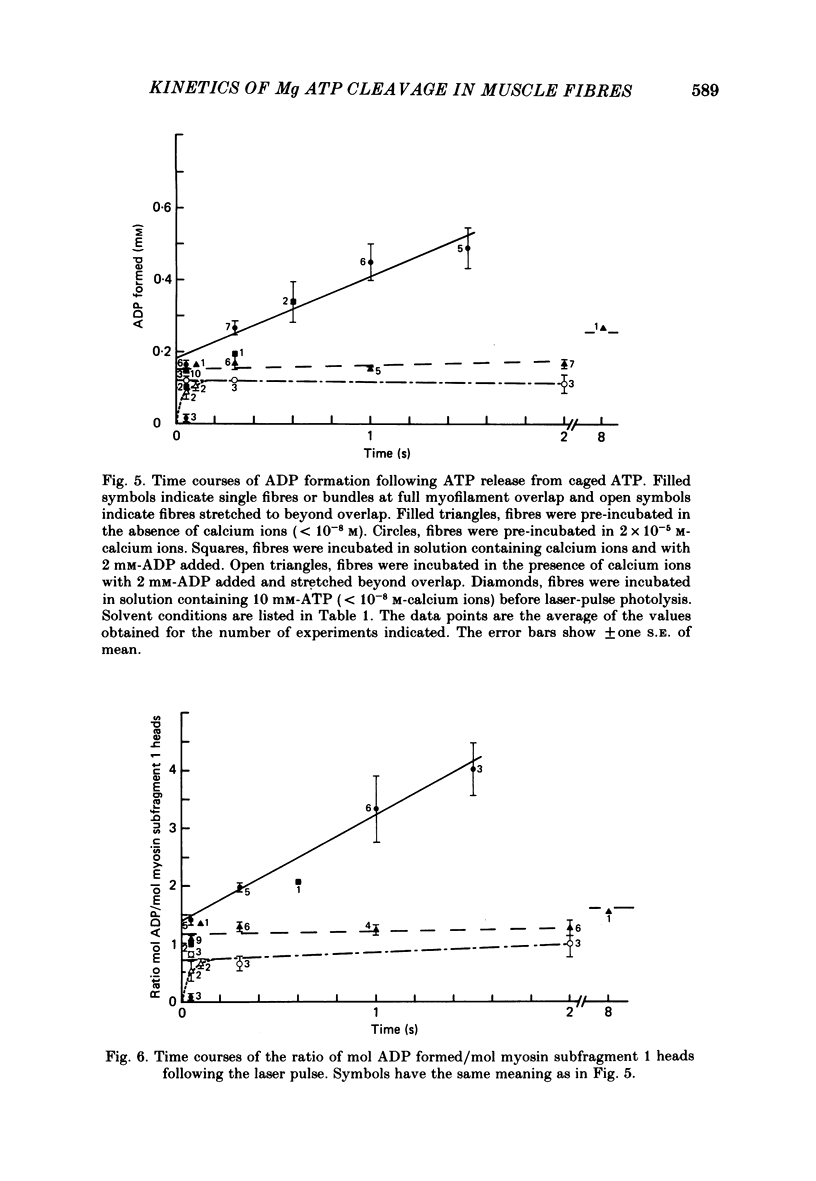
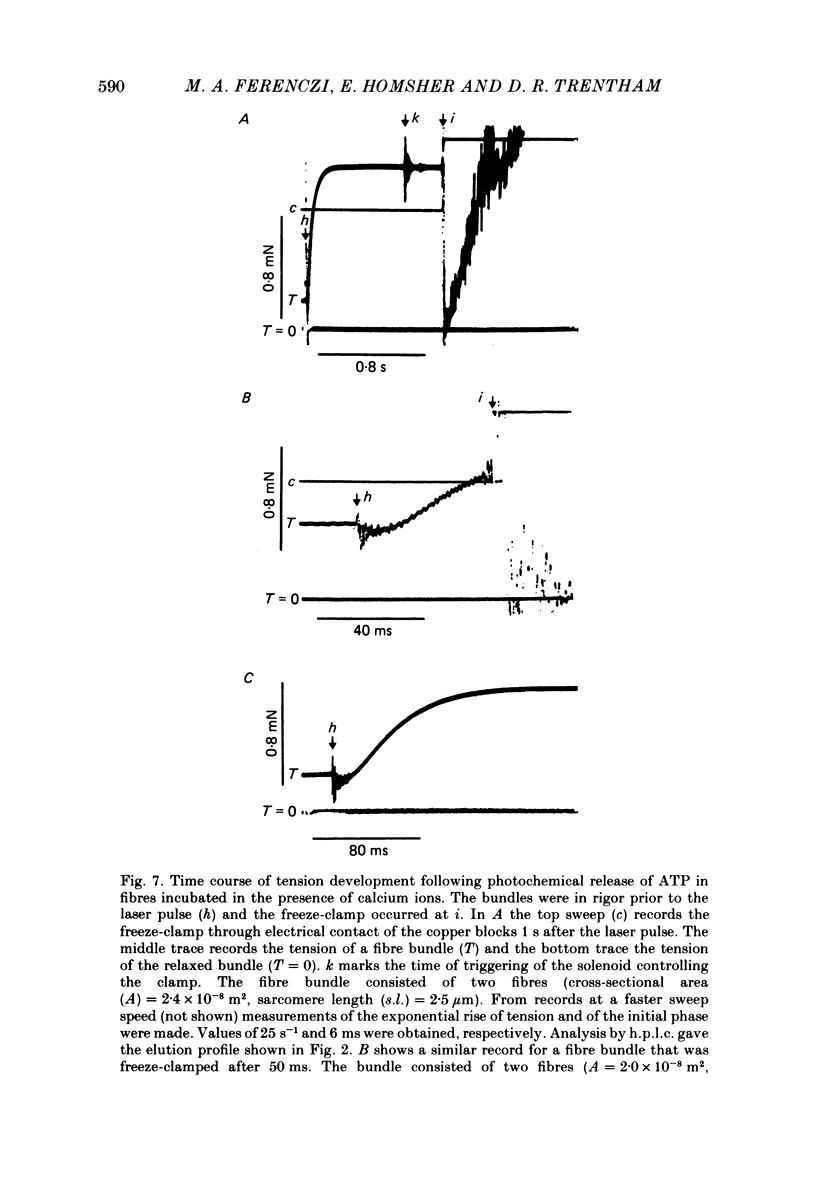
The time course of magnesium adenosine triphosphate (Mg ATP) cleavage in chemically skinned muscle fibres of the rabbit was measured by a method in which Mg ATP cleavage was initiated by photolytic release of ATP from P3-1-(2-nitro)phenylethyladenosine 5'-triphosphate (caged ATP) and terminated by rapid freezing 50 ms to 8 s later. Up to 5 mM-ATP was released following a single 50 ns laser pulse at 347 nm. Mg ATP cleavage was measured at 19 degrees C in the presence and absence of calcium ions, for fibres near rest length and stretched beyond overlap of the myofilaments. At full overlap and in the absence of calcium (less than 10(-8) M) and nucleotide, the fibres developed rigor tension. Following the laser pulse the tension decreased to that of a relaxed fibre in two distinct phases. The first phase lasted about 40 ms and was followed by a second phase during which tension decreased to zero with an approximately exponential time course with a rate constant of 11 s-1. In the presence of 2 X 10(-5) M-free calcium ions, the initial phase following the laser flash lasted approximately 13 ms, and was followed by an exponential rise of tension with a rate constant of 28 s-1. The active tension reached by the muscle fibres was 54 kN/m2. For fibres stretched beyond overlap, no change in tension was observed following the release of Mg ATP. Under all conditions the time course of Mg ATP cleavage was biphasic, and consisted of a rapid initial burst of ADP formation, complete within 50 ms, followed by a slower steady-state rate of Mg ATP cleavage. The number of molecules of Mg ATP cleaved during the burst was approximately equal to the number of myosin subfragment 1 heads for fibres at full myofilament overlap, and equal to 0.7 molecules per myosin subfragment 1 head for fibres stretched beyond overlap. At full overlap in the presence of calcium ions, the steady-state rate equalled 1.8 mol Mg ATP cleaved per mole myosin subfragment 1 head per second. In all other cases the steady-state rate of Mg ATP cleavage was at least 10-fold less. When fibres at full overlap were pre-incubated with 2 mM-ADP, the initial phase of the tension response was somewhat prolonged, but the burst of ADP formation was also complete within 50 ms.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF

















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLINKS J. R. INFLUENCE OF OSMOTIC STRENGTH ON CROSS-SECTION AND VOLUME OF ISOLATED SINGLE MUSCLE FIBRES. J Physiol. 1965 Mar;177:42–57. doi: 10.1113/jphysiol.1965.sp007574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The characterization of myosin-product complexes and of product-release steps during the magnesium ion-dependent adenosine triphosphatase reaction. Biochem J. 1974 Aug;141(2):331–349. doi: 10.1042/bj1410331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagshaw C. R., Trentham D. R. The reversibility of adenosine triphosphate cleavage by myosin. Biochem J. 1973 Jun;133(2):323–328. doi: 10.1042/bj1330323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J. 1979 Nov;28(2):241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M. J., Gadian D. G., Wilkie D. R. Contraction and recovery of living muscles studies by 31P nuclear magnetic resonance. J Physiol. 1977 Jun;267(3):703–735. doi: 10.1113/jphysiol.1977.sp011835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood A. B., Wood D. S., Bock K. L., Sorenson M. M. Chemically skinned mammalian skeletal muscle. I. The structure of skinned rabbit psoas. Tissue Cell. 1979;11(3):553–566. doi: 10.1016/0040-8166(79)90062-4. [DOI] [PubMed] [Google Scholar]
- Eccleston J. F. Fluorescence changes associated with the binding of ribose-5-triphosphate to myosin subfragment 1. Evidence for a second triphosphate binding site. FEBS Lett. 1980 Apr 21;113(1):55–57. doi: 10.1016/0014-5793(80)80493-5. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godt R. E. Calcium-activated tension of skinned muscle fibers of the frog. Dependence on magnesium adenosine triphosphate concentration. J Gen Physiol. 1974 Jun;63(6):722–739. doi: 10.1085/jgp.63.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., McCray J. A., Trentham D. R. Relaxation of muscle fibres by photolysis of caged ATP. Nature. 1982 Dec 23;300(5894):701–705. doi: 10.1038/300701a0. [DOI] [PubMed] [Google Scholar]
- Goldman Y. E., Simmons R. M. Control of sarcomere length in skinned muscle fibres of Rana temporaria during mechanical transients. J Physiol. 1984 May;350:497–518. doi: 10.1113/jphysiol.1984.sp015215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J. H., Forbush B., 3rd, Hoffman J. F. Rapid photolytic release of adenosine 5'-triphosphate from a protected analogue: utilization by the Na:K pump of human red blood cell ghosts. Biochemistry. 1978 May 16;17(10):1929–1935. doi: 10.1021/bi00603a020. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Tregear R. T. Nucleotide binding to myosin in calcium activated muscle. Biochim Biophys Acta. 1974 Mar 26;333(3):581–584. doi: 10.1016/0005-2728(74)90143-1. [DOI] [PubMed] [Google Scholar]
- Marston S. The nucleotide complexes of myosin in glycerol-extracted muscle fibres. Biochim Biophys Acta. 1973 May 30;305(2):397–412. doi: 10.1016/0005-2728(73)90186-2. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Herbette L., Kihara T., Trentham D. R. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornet D., Bertrand R., Pantel P., Audemard E., Kassab R. Structure of the actin-myosin interface. Nature. 1981 Jul 23;292(5821):301–306. doi: 10.1038/292301a0. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Trentham D. R. Distance measurement between the active site and cysteine-177 of the alkali one light chain of subfragment 1 from rabbit skeletal muscle. Biochemistry. 1983 Nov 8;22(23):5261–5270. doi: 10.1021/bi00292a004. [DOI] [PubMed] [Google Scholar]
- Nihei T., Filipenko C. A. The effects of substrate concentration on the Mg-adenosine triphosphatase activity of myosin. Can J Biochem. 1975 Dec;53(12):1282–1287. doi: 10.1139/o75-174. [DOI] [PubMed] [Google Scholar]
- Sleep J. A., Hutton R. L. Actin mediated release of ATP from a myosin-ATP complex. Biochemistry. 1978 Dec 12;17(25):5423–5430. doi: 10.1021/bi00618a016. [DOI] [PubMed] [Google Scholar]
- Solaro R. J., Pang D. C., Briggs F. N. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta. 1971 Aug 6;245(1):259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- Stein L. A., Chock P. B., Eisenberg E. Mechanism of the actomyosin ATPase: effect of actin on the ATP hydrolysis step. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1346–1350. doi: 10.1073/pnas.78.3.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashi R., Putnam S. A fluorimetric method for continuously assaying ATPase: application to small specimens of glycerol-extracted muscle fibers. Anal Biochem. 1979 Jan 15;92(2):375–382. doi: 10.1016/0003-2697(79)90674-2. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Mechanism of actomyosin ATPase and the problem of muscle contraction. CRC Crit Rev Biochem. 1979;6(2):103–164. doi: 10.3109/10409237909102562. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Transient phase of adenosine triphosphate hydrolysis by myosin, heavy meromyosin, and subfragment 1. Biochemistry. 1977 Feb 22;16(4):732–739. doi: 10.1021/bi00623a027. [DOI] [PubMed] [Google Scholar]
- Travers F., Hillaire D. Cryoenzymological studies on myosin subfragment 1. Solvent, temperature and pH effects on the overall reaction. Eur J Biochem. 1979 Jul;98(1):293–299. doi: 10.1111/j.1432-1033.1979.tb13188.x. [DOI] [PubMed] [Google Scholar]
- Tregear R. T., Squire J. M. Myosin content and filament structure in smooth and striated muscle. J Mol Biol. 1973 Jun 25;77(2):279–290. doi: 10.1016/0022-2836(73)90336-7. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Yates L. D., Greaser M. L. Quantitative determination of myosin and actin in rabbit skeletal muscle. J Mol Biol. 1983 Jul 25;168(1):123–141. doi: 10.1016/s0022-2836(83)80326-x. [DOI] [PubMed] [Google Scholar]