Abstract
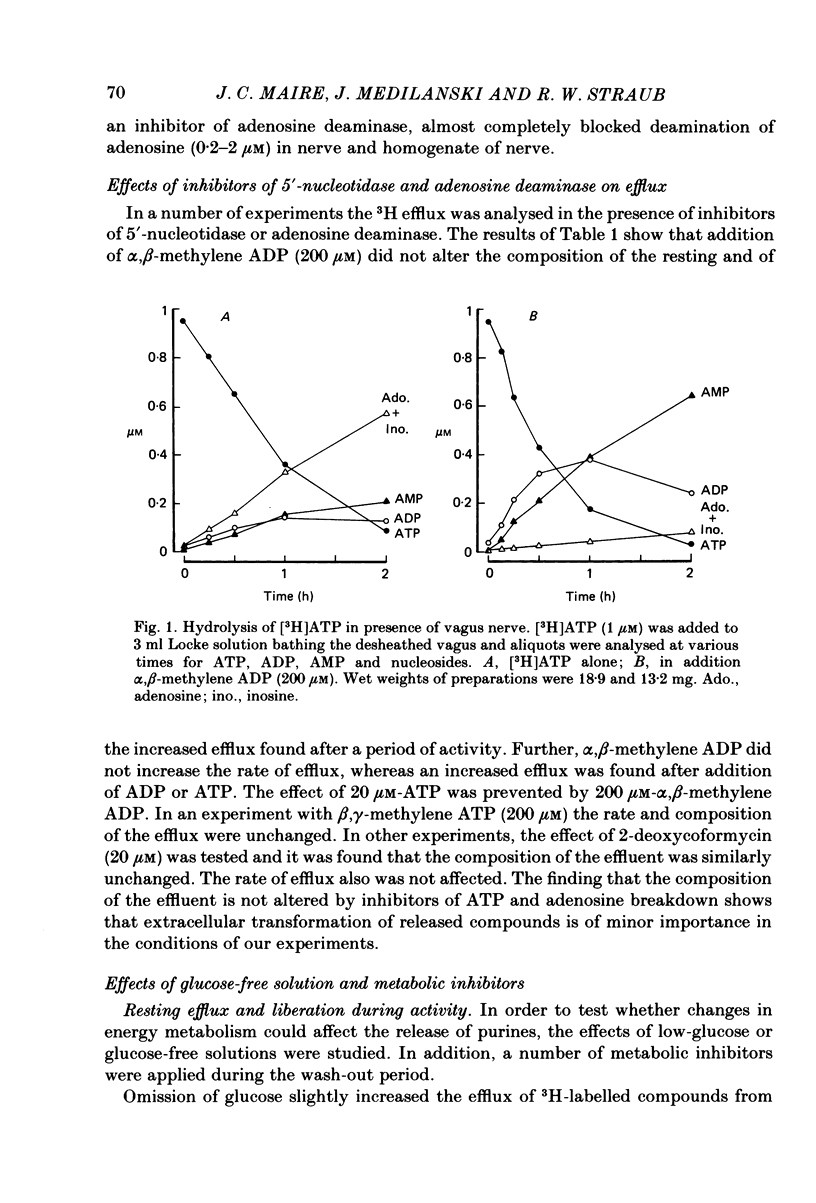
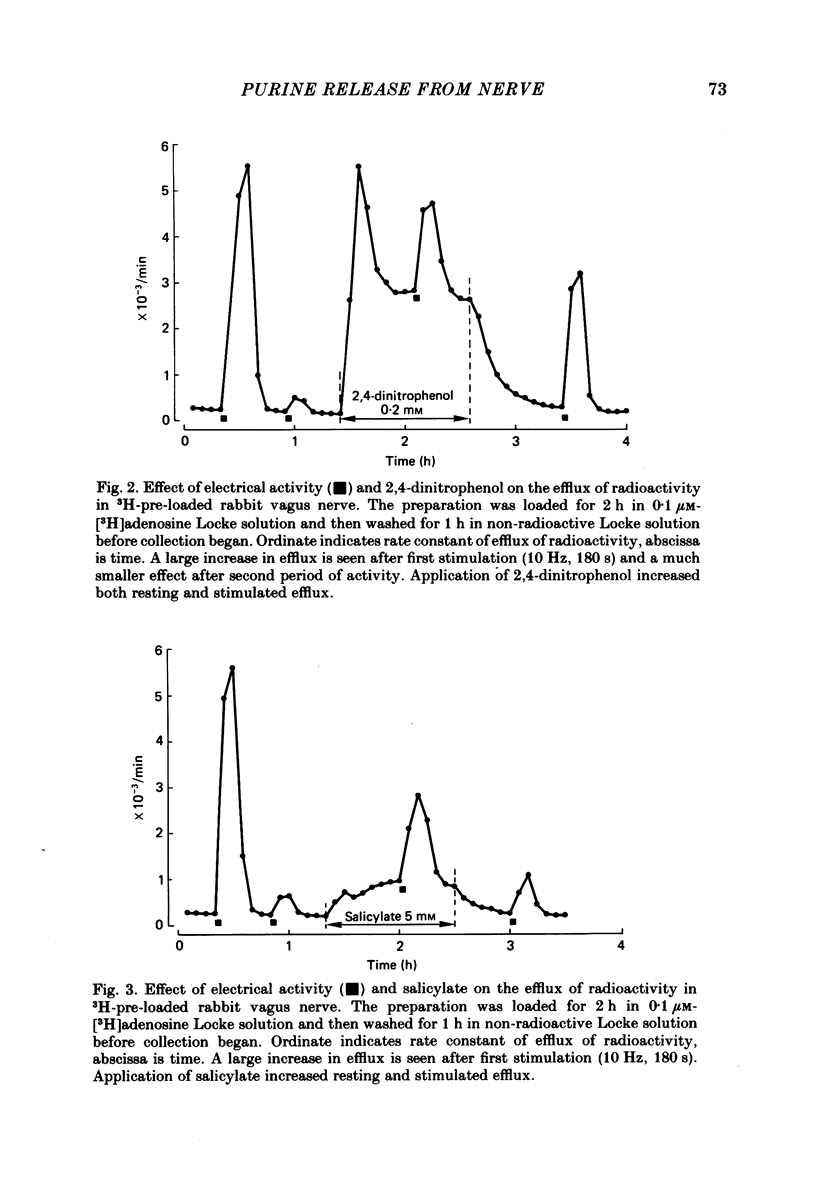
The composition of the efflux from desheathed rabbit vagus nerve, loaded with radioactivity by incubation in [3H]adenosine, was studied at rest and during electrical activity and after application of inhibitors of ecto-enzymes and modifications of intermediary metabolism. In addition, the degradation of externally applied ATP and adenosine was examined. [3H]ATP applied to the incubation medium was degraded to ADP, AMP, adenosine and inosine. The hydrolysis to nucleosides was inhibited by alpha, beta-methylene ADP; the appearance of AMP and nucleosides was slowed by beta, gamma-methylene ATP. Deamination of [3H]adenosine was blocked by 2-deoxycoformycin. The effluent from resting and stimulated preparations showed the presence of large amounts of inosine and hypoxanthine, smaller amounts of adenosine and adenine and traces of nucleotides. The composition of the effluent was not significantly altered by addition of alpha, beta-methylene ADP; beta, gamma-methylene ATP or 2-deoxycoformycin. Application of glucose-free solutions caused a large release of adenosine instead of inosine and hypoxanthine and a small increase in resting and stimulated efflux of 3H. Addition of 2-deoxyglucose produced a large increase in resting efflux and increased liberation of adenosine. Cyanide, 2,4-dinitrophenol, arsenate or salicylate increased the resting efflux of adenosine, inosine and hypoxanthine, and the effect of activity. It is concluded that electrical activity leads to release of adenosine, inosine and hypoxanthine, in various proportions depending on metabolic state, and that there is practically no liberation of nucleotides from nerve axons.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arch J. R., Newsholme E. A. Activities and some properties of 5'-nucleotidase, adenosine kinase and adenosine deaminase in tissues from vertebrates and invertebrates in relation to the control of the concentration and the physiological role of adenosine. Biochem J. 1978 Sep 15;174(3):965–977. doi: 10.1042/bj1740965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A. S., Wu P. H., Phillis J. W. The rapid uptake and release of [3H]adenosine by rat cerebral cortical synaptosomes. J Neurochem. 1981 Feb;36(2):651–660. doi: 10.1111/j.1471-4159.1981.tb01638.x. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Review lecture. Neurotransmitters and trophic factors in the autonomic nervous system. J Physiol. 1981;313:1–35. doi: 10.1113/jphysiol.1981.sp013648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmouliovsky M., Schorderet M., Straub R. W. Effect of electrical activity on the concentration of phosphorylated metabolites and inorganic phosphate in mammalian non-myelinated nerve fibres. J Physiol. 1969 Jun;202(2):90P–92P. [PubMed] [Google Scholar]
- Coe E. L., Lee I. Y. Phosphorylation of 2-D-deoxyglucose and associated inorganic phosphate uptake in ascites tumor cells. Biochemistry. 1969 Feb;8(2):685–693. doi: 10.1021/bi00830a034. [DOI] [PubMed] [Google Scholar]
- Daval J. L., Barberis C. Release of radiolabelled adenosine derivatives from superfused synaptosome beds. Evidence for the output of adenosine. Biochem Pharmacol. 1981 Sep 15;30(18):2559–2567. doi: 10.1016/0006-2952(81)90583-9. [DOI] [PubMed] [Google Scholar]
- Ferrero J., Jirounek P., Rouiller M., Straub R. W. Efflux of inorganic phosphate from mammalian non-myelinated nerve fibres. J Physiol. 1978 Sep;282:507–519. doi: 10.1113/jphysiol.1978.sp012478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flodgaard H., Torp-Pedersen C. A calcium ion-dependent adenosine triphosphate pyrophosphohydrolase in plasma membrane from rat liver. Demonstration that the adenosine triphosphate analogues adenosine 5'-[betagamma-imido]triphosphate and adenosine 5'-[betagamma-methylene]-triphosphate are substrates for the enzyme. Biochem J. 1978 Jun 1;171(3):817–820. doi: 10.1042/bj1710817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Hedqvist P. Modulation of neurotransmission by purine nucleotides and nucleosides. Biochem Pharmacol. 1980 Jun 15;29(12):1635–1643. doi: 10.1016/0006-2952(80)90117-3. [DOI] [PubMed] [Google Scholar]
- Keynes R. D., Ritchie J. M. The movements of labelled ions in mammalian non-myelinated nerve fibres. J Physiol. 1965 Jul;179(2):333–367. doi: 10.1113/jphysiol.1965.sp007666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax C. A., Bagnara A. S., Henderson J. F. Studies of the regulation of purine nucleotide catabolism. Can J Biochem. 1975 Feb;53(2):231–241. doi: 10.1139/o75-032. [DOI] [PubMed] [Google Scholar]
- Maire J. C., Medilanski J., Straub R. W. Uptake of adenosine and release of adenine derivatives in mammalian non-myelinated nerve fibres at rest and during activity. J Physiol. 1982 Feb;323:589–602. doi: 10.1113/jphysiol.1982.sp014093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C. Role of adenosine deaminase, ecto-(5'-nucleotidase) and ecto-(non-specific phosphatase) in cyanide-induced adenosine monophosphate catabolism in rat polymorphonuclear leucocytes. Biochem J. 1980 Mar 15;186(3):907–918. doi: 10.1042/bj1860907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Pull I., McIlwain H. Metabolism of ( 14 C)adenine and derivatives by cerebral tissues, superfused and electrically stimulated. Biochem J. 1972 Feb;126(4):965–973. doi: 10.1042/bj1260965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pull I., McIlwain H. Output of (14C)adenine nucleotides and their derivatives from cerebral tissues. Tetrodotoxin-resistant and calcium ion-requiring components. Biochem J. 1973 Dec;136(4):893–901. doi: 10.1042/bj1360893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. M., Straub R. W. Oxygen consumption and phosphate efflux in mammalian non-myelinated nerve fibres. J Physiol. 1980 Jul;304:109–121. doi: 10.1113/jphysiol.1980.sp013313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammons D. W., Henry H., Chilson O. P. Effect of salts on inhibition of chicken muscle adenosine monophosphate deaminase by phosphate esters and inorganic phosphate. Effect of 5,5'-dithiobis(2-nitrobenzoic acid)on enzyme activity and inhibition by 2,3-diphosphoglyceric acid. J Biol Chem. 1970 Apr 25;245(8):2109–2113. [PubMed] [Google Scholar]
- Schubert P., Reddington M., Kreutzberg G. W. On the possible role of adenosine as a modulatory messenger in the hippocampus and other regions of the CNS. Prog Brain Res. 1979;51:149–165. doi: 10.1016/S0079-6123(08)61303-5. [DOI] [PubMed] [Google Scholar]
- Schultz V., Lowenstein J. M. Purine nucleotide cycle. Evidence for the occurrence of the cycle in brain. J Biol Chem. 1976 Jan 25;251(2):485–492. [PubMed] [Google Scholar]
- Shimizu H., Creveling C. R., Daly J. Stimulated formation of adenosine 3',5'-cyclic phosphate in cerebral cortex: synergism between electrical activity and biogenic amines. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1033–1040. doi: 10.1073/pnas.65.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone T. W. Cell-membrane receptors for purines. Review. Biosci Rep. 1982 Feb;2(2):77–90. doi: 10.1007/BF01116173. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Physiological roles for adenosine and adenosine 5'-triphosphate in the nervous system. Neuroscience. 1981;6(4):523–555. doi: 10.1016/0306-4522(81)90145-7. [DOI] [PubMed] [Google Scholar]
- Sulakhe P. V., Phillis J. W. The release of 3H-adenosine and its derivatives from cat sensorimotor cortex. Life Sci. 1975 Aug 15;17(4):551–555. doi: 10.1016/0024-3205(75)90089-2. [DOI] [PubMed] [Google Scholar]
- Trams E. G. A proposal for the role of ecto-enzymes and adenylates in traumatic shock. J Theor Biol. 1980 Dec 7;87(3):609–621. doi: 10.1016/0022-5193(80)90239-8. [DOI] [PubMed] [Google Scholar]
- Trams E. G. Evidence for ATP action on the cell surface. Nature. 1974 Dec 6;252(5483):480–482. doi: 10.1038/252480a0. [DOI] [PubMed] [Google Scholar]
- Trams E. G., Lauter C. J. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974 Apr 29;345(2):180–197. doi: 10.1016/0005-2736(74)90257-0. [DOI] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. IV. Pasteur effect and Crabtree effect in ascites tumor cells. J Biol Chem. 1959 May;234(5):1036–1041. [PubMed] [Google Scholar]
- Yount R. G. ATP analogs. Adv Enzymol Relat Areas Mol Biol. 1975;43:1–56. doi: 10.1002/9780470122884.ch1. [DOI] [PubMed] [Google Scholar]
- Yushok W. D. Control mechanisms of adenine nucleotide metabolism of ascites tumor cells. J Biol Chem. 1971 Mar 25;246(6):1607–1617. [PubMed] [Google Scholar]


