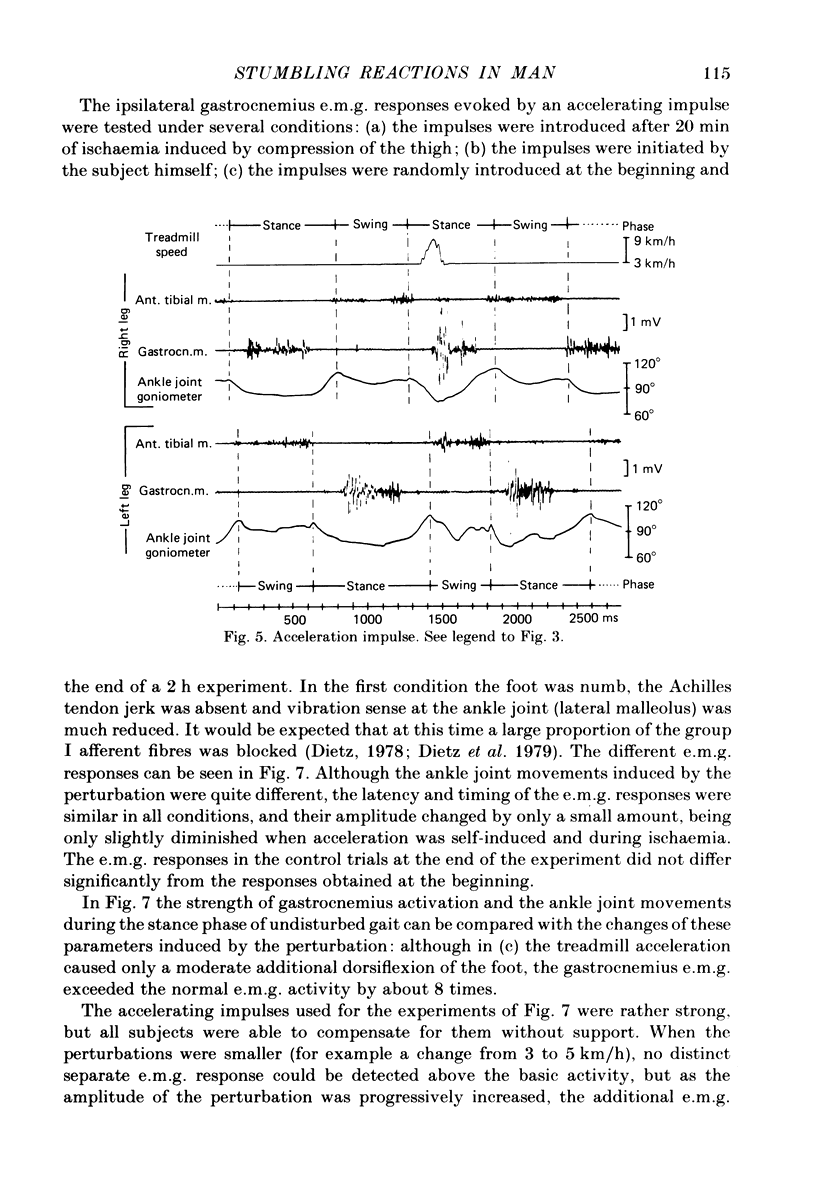
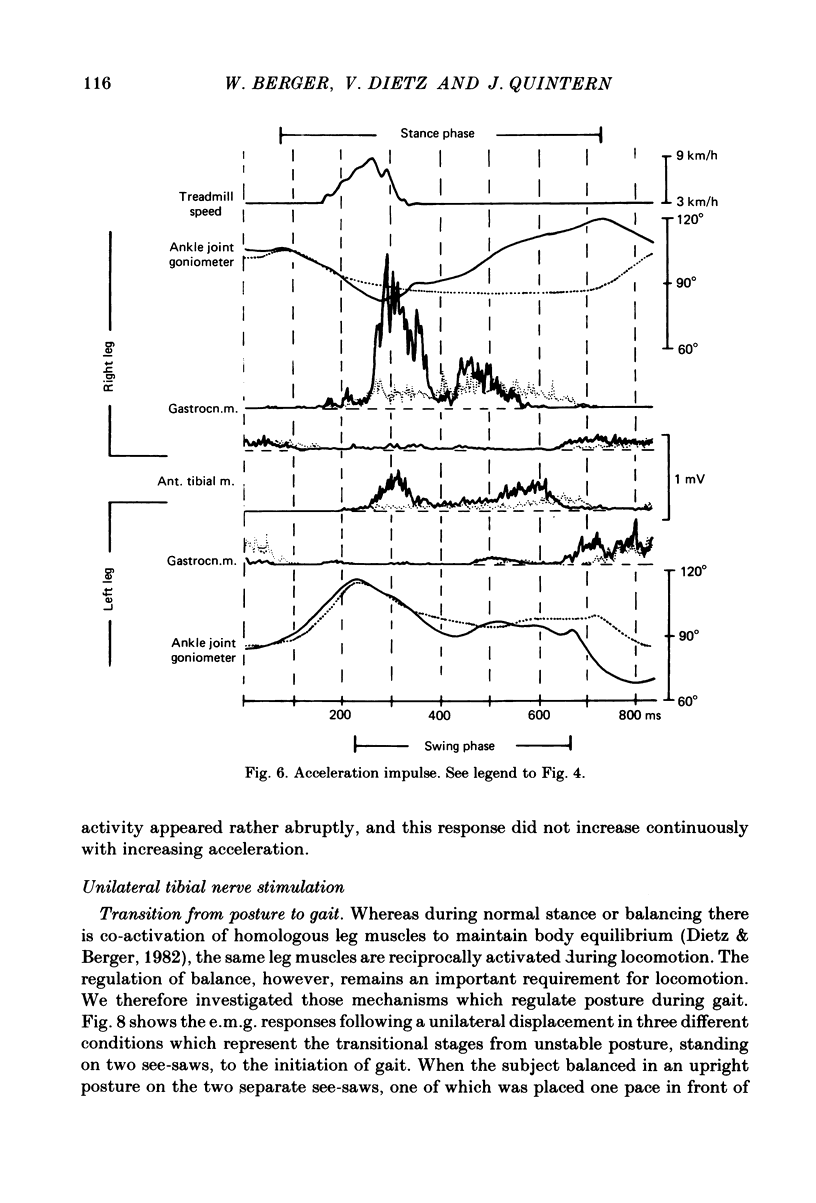
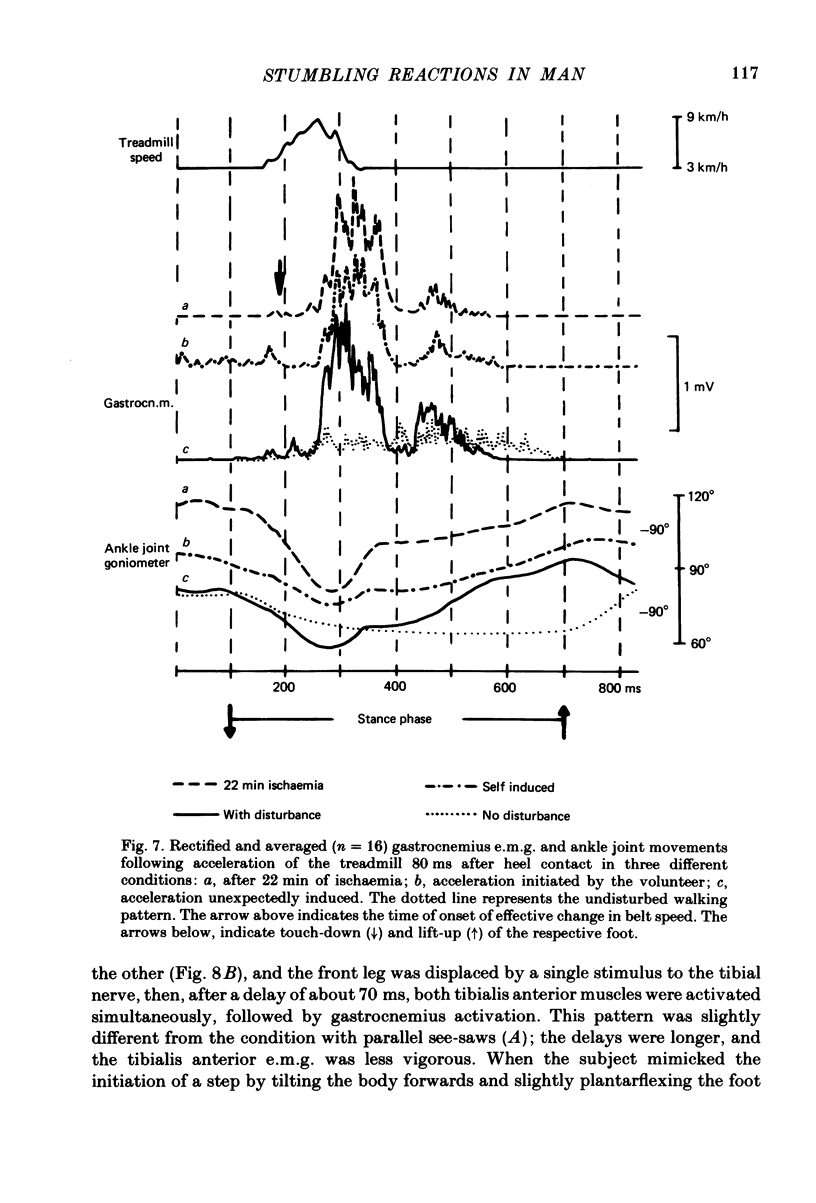
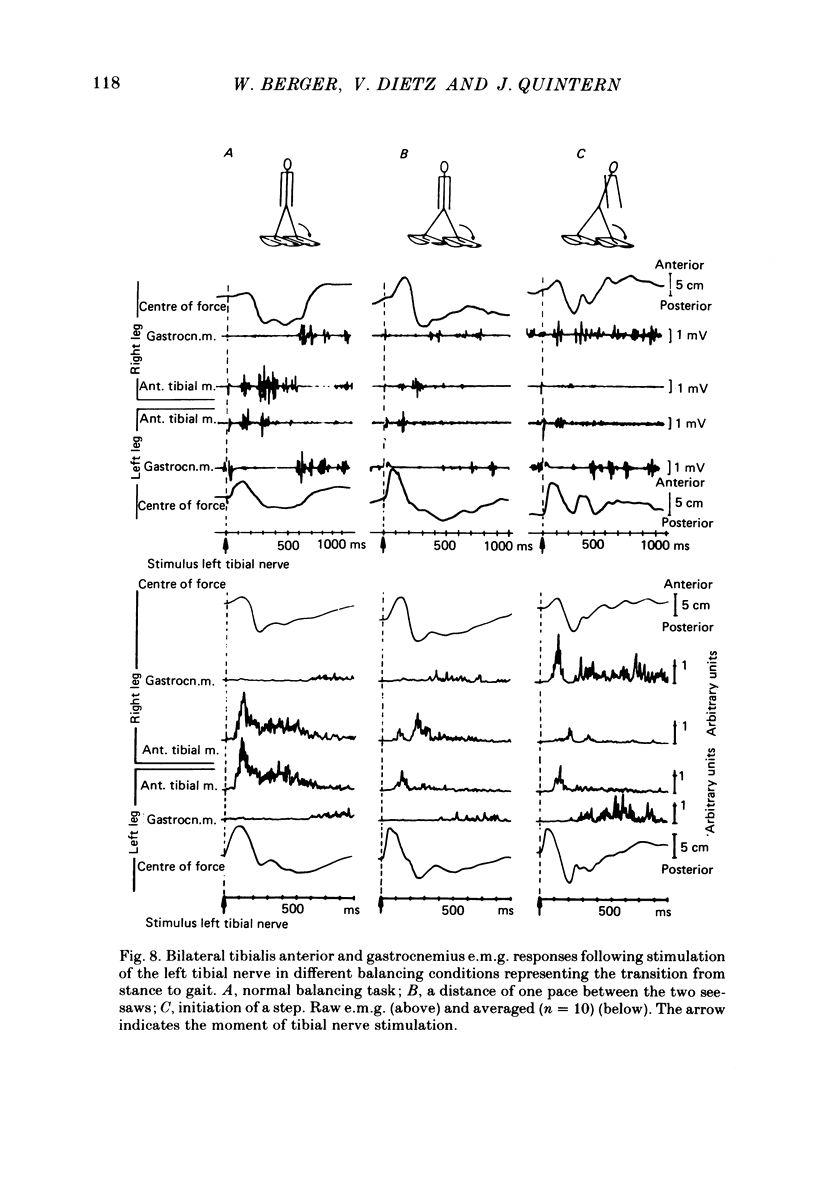
Abstract
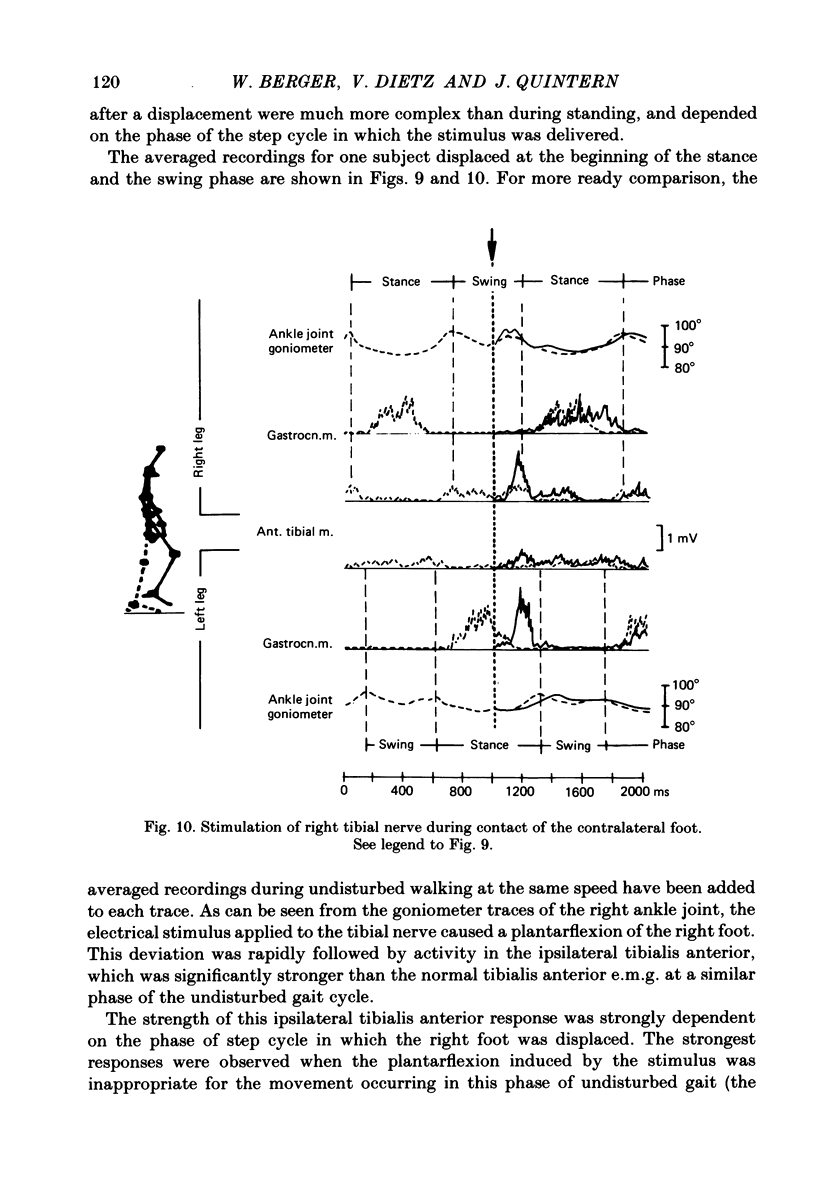
Electromyogram (e.m.g.) responses of lower leg muscles, and corresponding movements were studied following a perturbation of the limb during walking, produced by either (a) a randomly timed, short acceleration or decelerating impulse applied to the treadmill, or (b) a unilateral triceps surae contraction induced by tibial nerve stimulation. Bilateral e.m.g. responses following the perturbation were specific for the mode of perturbation and depended on the phase of the gait cycle in which the perturbation occurred. Treadmill deceleration evoked a bilateral tibialis anterior activation; acceleration evoked an ipsilateral gastrocnemius and contralateral tibialis anterior activation (latency in either condition and on both sides was 65-75 ms, duration about 150 ms). Tibial nerve stimulation at the beginning of a stance phase, was followed by an ipsilateral tibialis anterior activation; during the swing phase it was followed by an ipsilateral tibialis anterior and contralateral gastrocnemius activation (latency about 90 ms, duration about 100 ms). These patterns differed from the response seen after a unilateral displacement during static standing, which evoked a bilateral tibialis anterior activation. These early responses were in most cases followed by late ipsilateral responses, but the e.m.g. pattern of the next step cycle was usually unchanged, or affected only at its onset. The e.m.g. responses were unaltered by ischaemic nerve blockade of group I afferents, by training effects or by pre-warning of the onset of perturbation (randomly or self-induced). Despite the different e.m.g. responses following a perturbation during gait, the same basic functional mechanism was obviously at work: the early ipsilateral response achieved a repositioning of the displaced foot and leg, while the early contralateral and late ipsilateral responses provided compensation for body displacement. It is suggested that the e.m.g. responses may be mediated predominantly by peripheral information from group II and group III afferents, which modulate the basic motor pattern of spinal interneuronal circuits underlying the respective motor task.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnet M., Gurfinkel S., Lipchits M. J., Popov K. E. Central programming of lower limb muscular activity in the standing man. Agressologie. 1976;17(SPECNO):35–42. [PubMed] [Google Scholar]
- Dietz V. Analysis of the electrical muscle activity during maximal contraction and the influence of ischaemia. J Neurol Sci. 1978 Jul;37(3):187–197. doi: 10.1016/0022-510x(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Dietz V., Berger W. Interlimb coordination of posture in patients with spastic paresis. Impaired function of spinal reflexes. Brain. 1984 Sep;107(Pt 3):965–978. doi: 10.1093/brain/107.3.965. [DOI] [PubMed] [Google Scholar]
- Dietz V., Berger W. Spinal coordination of bilateral leg muscle activity during balancing. Exp Brain Res. 1982;47(2):172–176. doi: 10.1007/BF00239376. [DOI] [PubMed] [Google Scholar]
- Dietz V., Quintern J., Berger W. Corrective reactions to stumbling in man: functional significance of spinal and transcortical reflexes. Neurosci Lett. 1984 Feb 10;44(2):131–135. doi: 10.1016/0304-3940(84)90070-3. [DOI] [PubMed] [Google Scholar]
- Dietz V., Quintern J., Berger W. Electrophysiological studies of gait in spasticity and rigidity. Evidence that altered mechanical properties of muscle contribute to hypertonia. Brain. 1981 Sep;104(3):431–449. doi: 10.1093/brain/104.3.431. [DOI] [PubMed] [Google Scholar]
- Dietz V., Schmidtbleicher D., Noth J. Neuronal mechanisms of human locomotion. J Neurophysiol. 1979 Sep;42(5):1212–1222. doi: 10.1152/jn.1979.42.5.1212. [DOI] [PubMed] [Google Scholar]
- Duysens J., Pearson K. G. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp Brain Res. 1976 Jan 26;24:245–255. doi: 10.1007/BF00235013. [DOI] [PubMed] [Google Scholar]
- Forssberg H., Grillner S., Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975 Feb 21;85(1):103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol. 1979 Jul;42(4):936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Gottlieb G. L., Agarwal G. C. Response to sudden torques about ankle in man: myotatic reflex. J Neurophysiol. 1979 Jan;42(1 Pt 1):91–106. doi: 10.1152/jn.1979.42.1.91. [DOI] [PubMed] [Google Scholar]
- Morin C., Katz R., Mazieres L., Pierrot-Deseilligny E. Comparison of soleus H reflex facilitation at the onset of soleus contractions produced voluntarily and during the stance phase of human gait. Neurosci Lett. 1982 Nov 16;33(1):47–53. doi: 10.1016/0304-3940(82)90128-8. [DOI] [PubMed] [Google Scholar]
- Nashner L. M. Balance adjustments of humans perturbed while walking. J Neurophysiol. 1980 Oct;44(4):650–664. doi: 10.1152/jn.1980.44.4.650. [DOI] [PubMed] [Google Scholar]
- Nashner L. M. Fixed patterns of rapid postural responses among leg muscles during stance. Exp Brain Res. 1977 Oct 24;30(1):13–24. doi: 10.1007/BF00237855. [DOI] [PubMed] [Google Scholar]
- Nashner L. M., Woollacott M., Tuma G. Organization of rapid responses to postural and locomotor-like perturbations of standing man. Exp Brain Res. 1979 Aug 1;36(3):463–476. doi: 10.1007/BF00238516. [DOI] [PubMed] [Google Scholar]


