Abstract
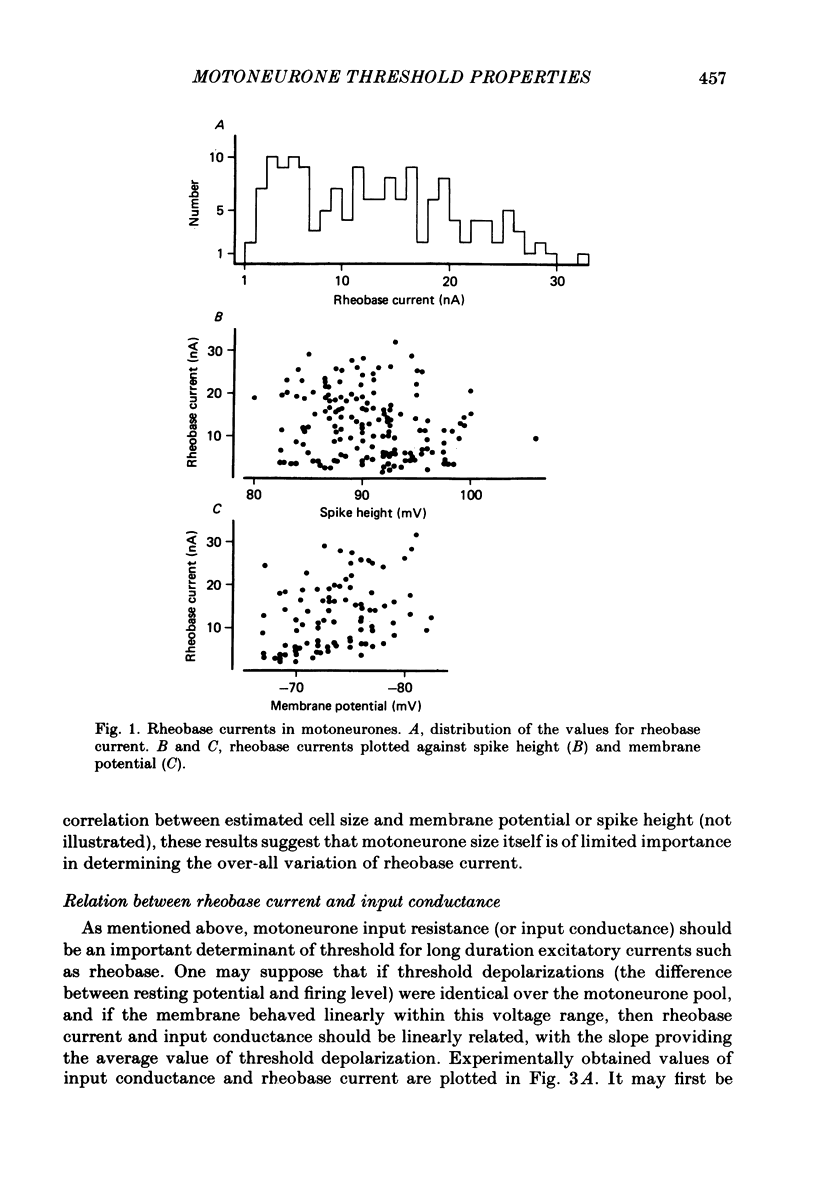
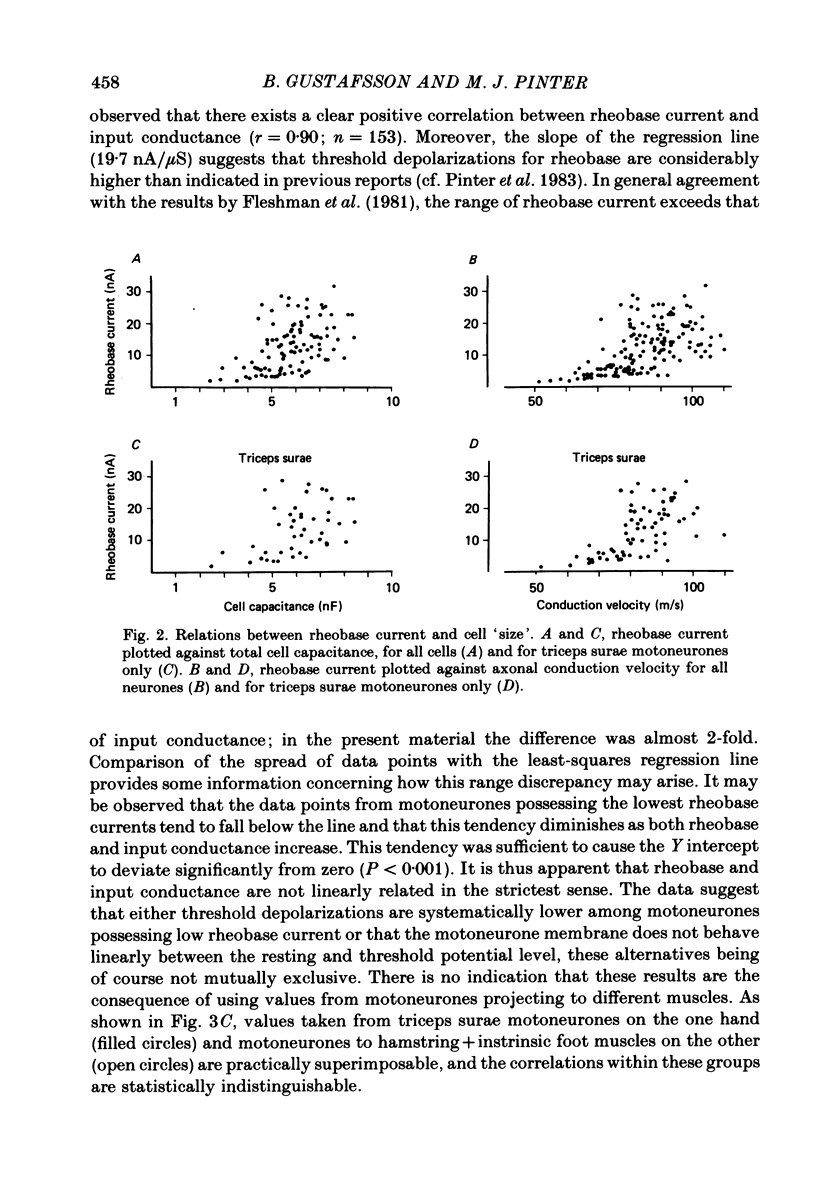
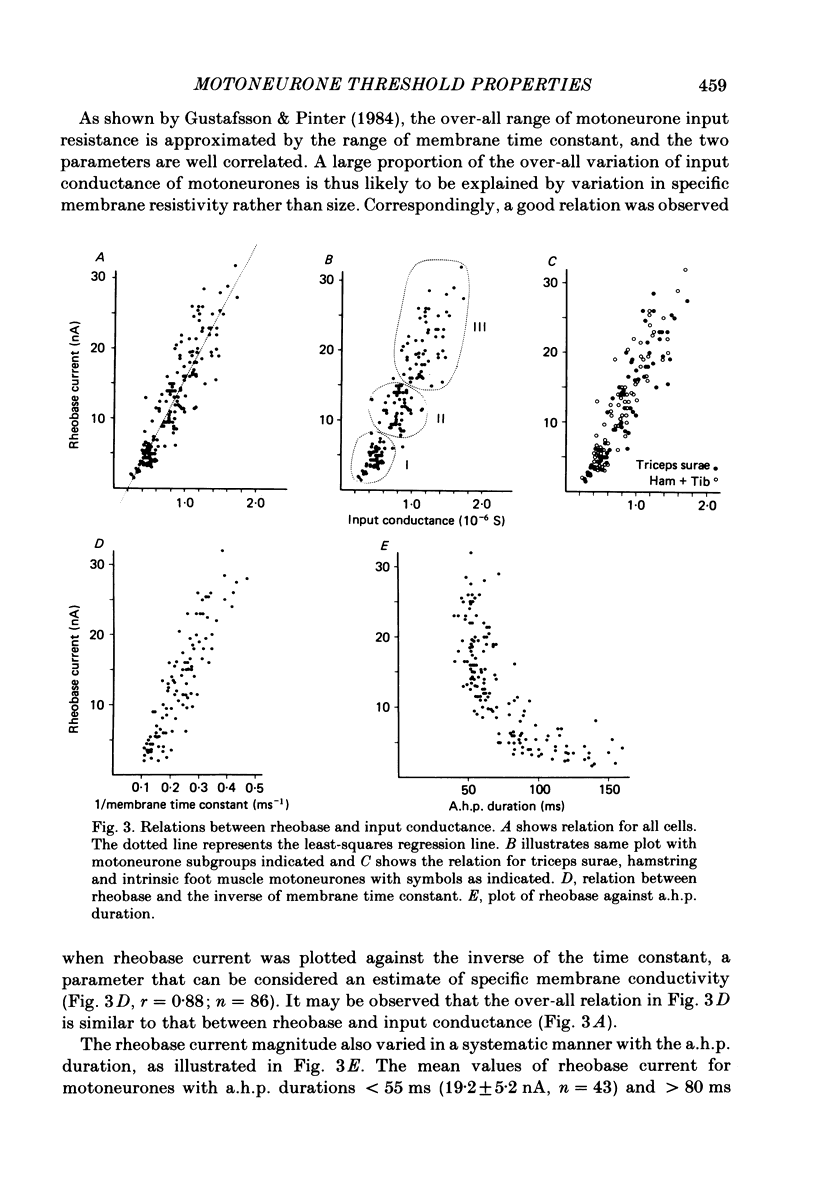
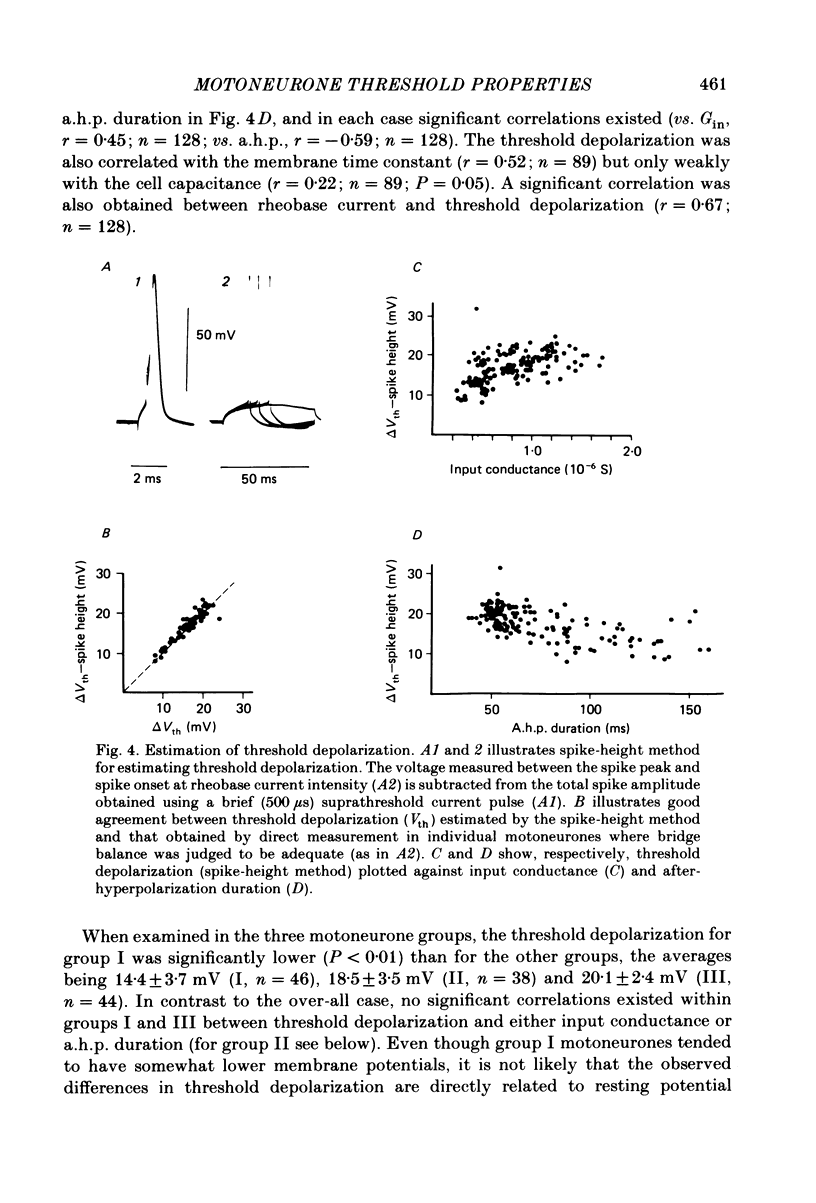
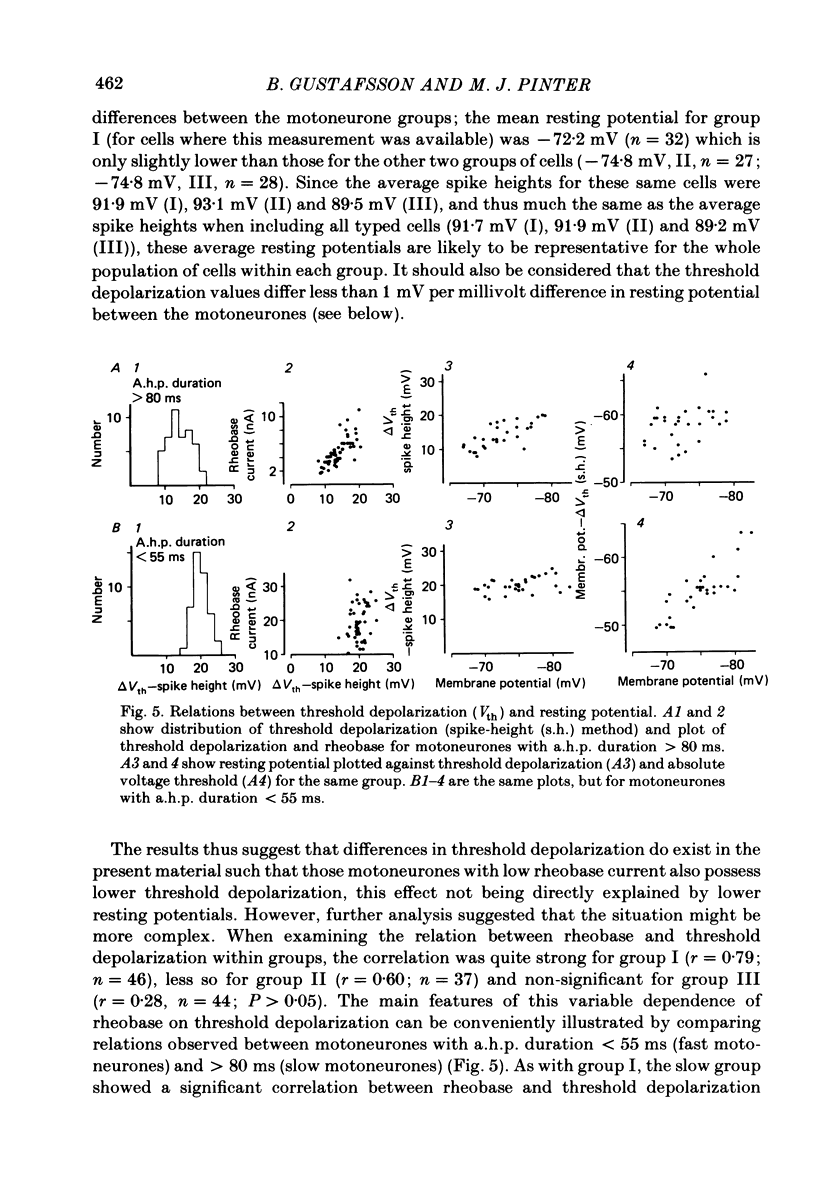
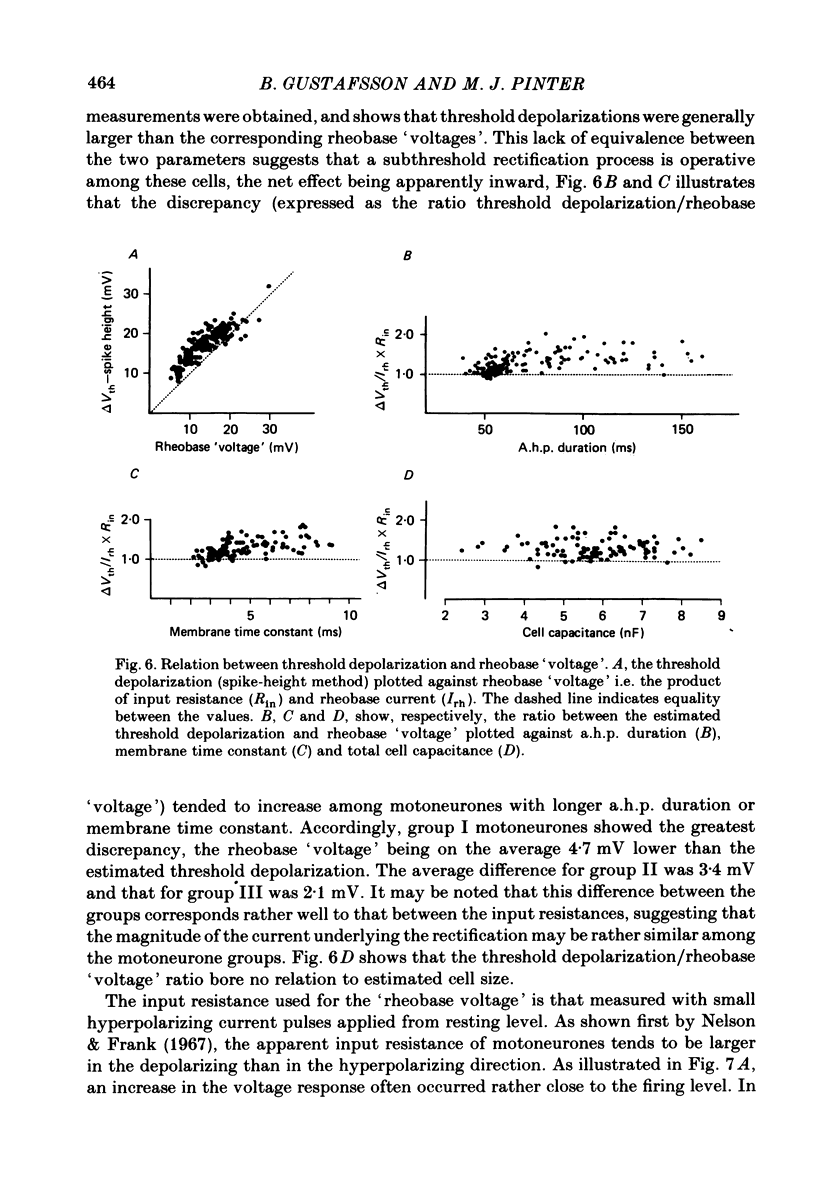
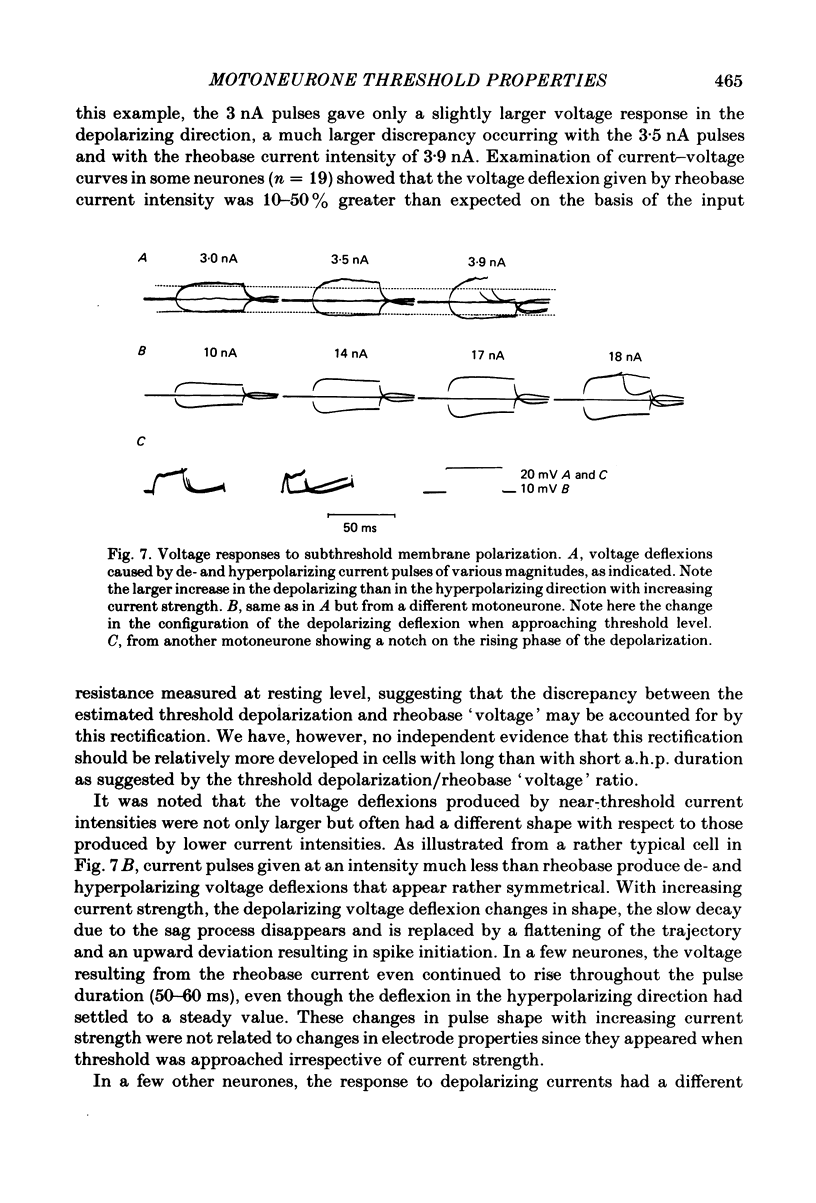
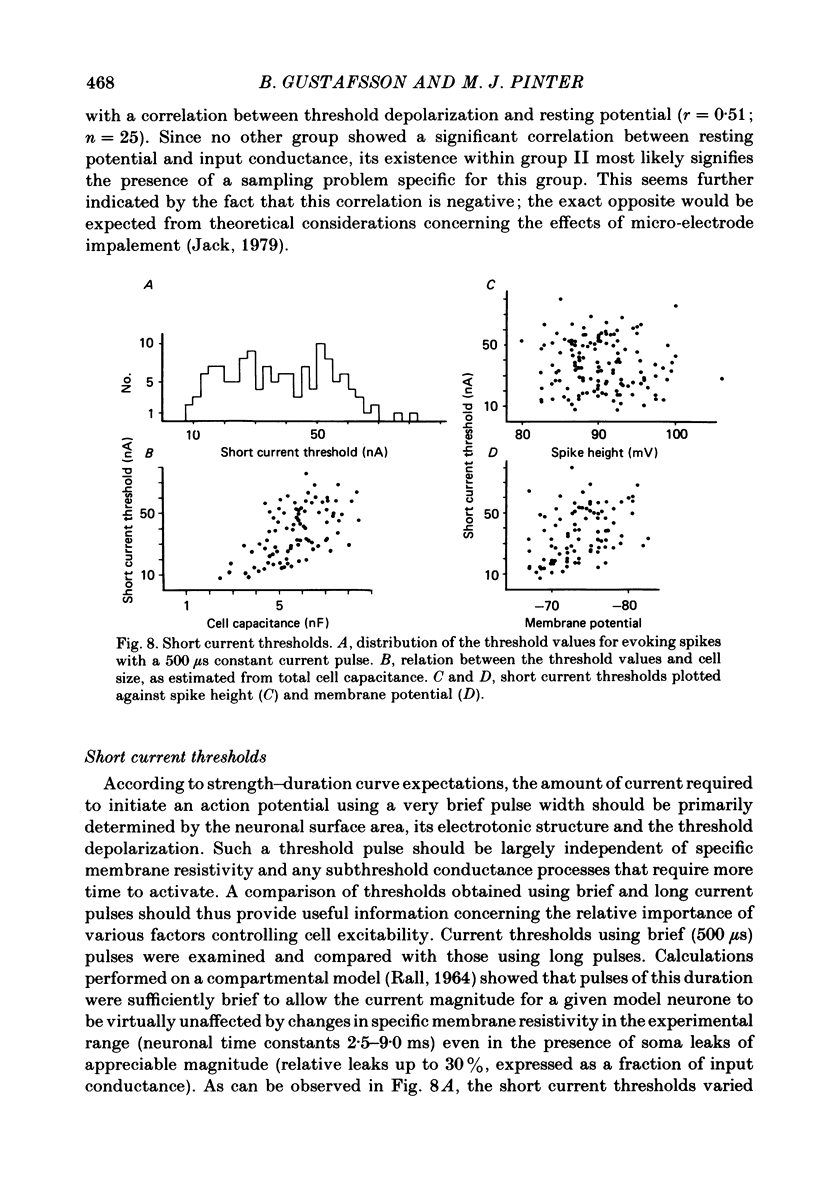
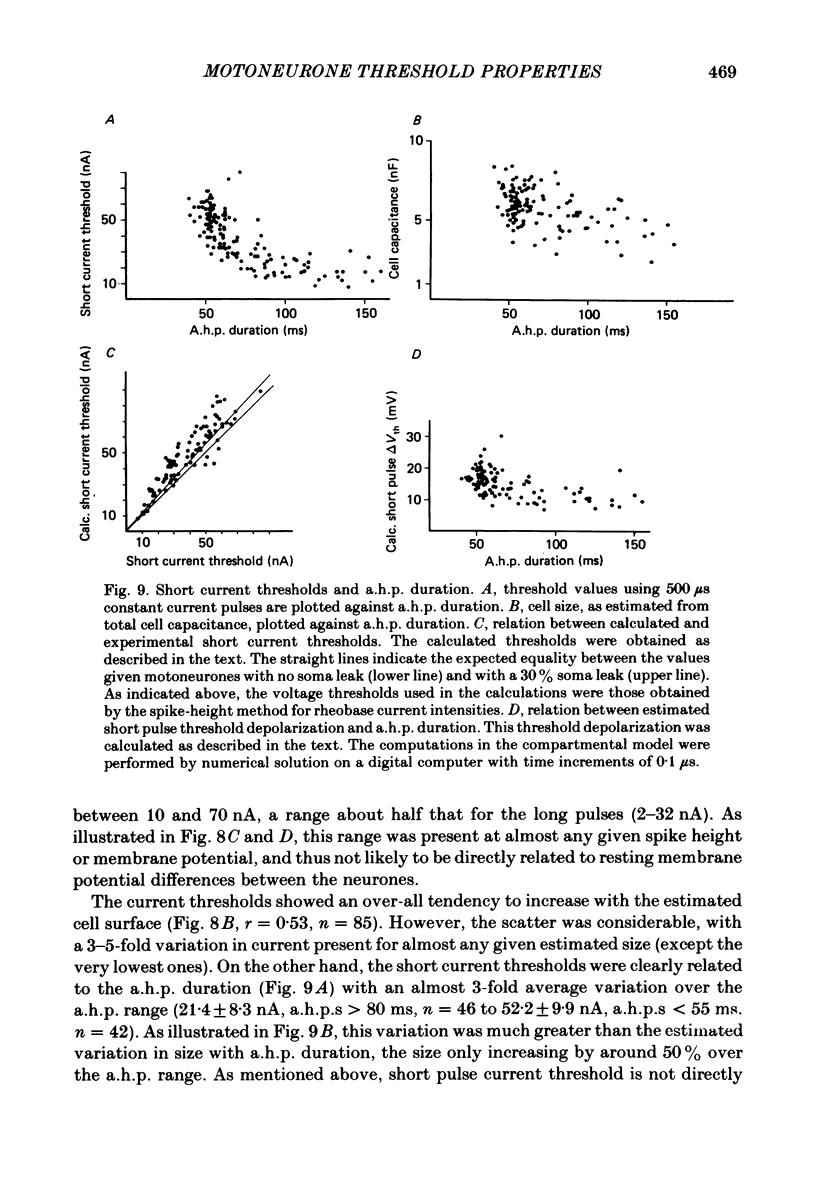
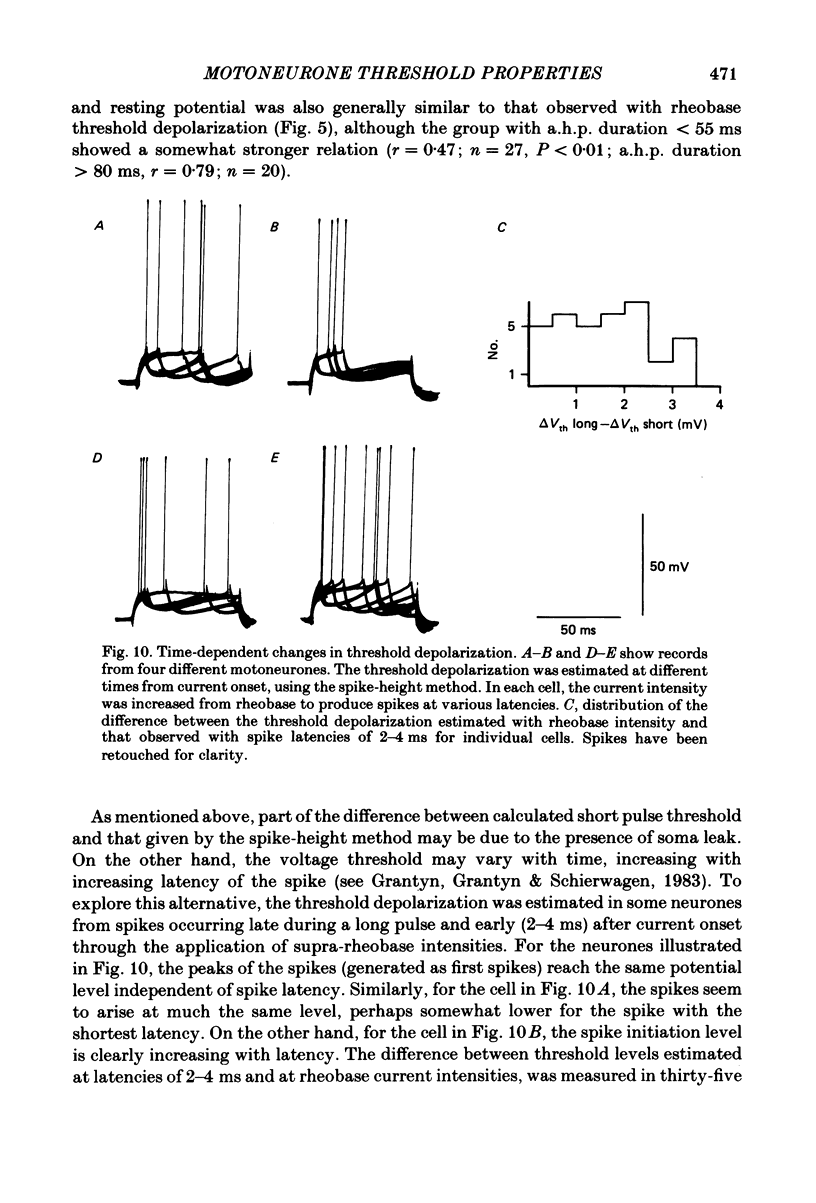
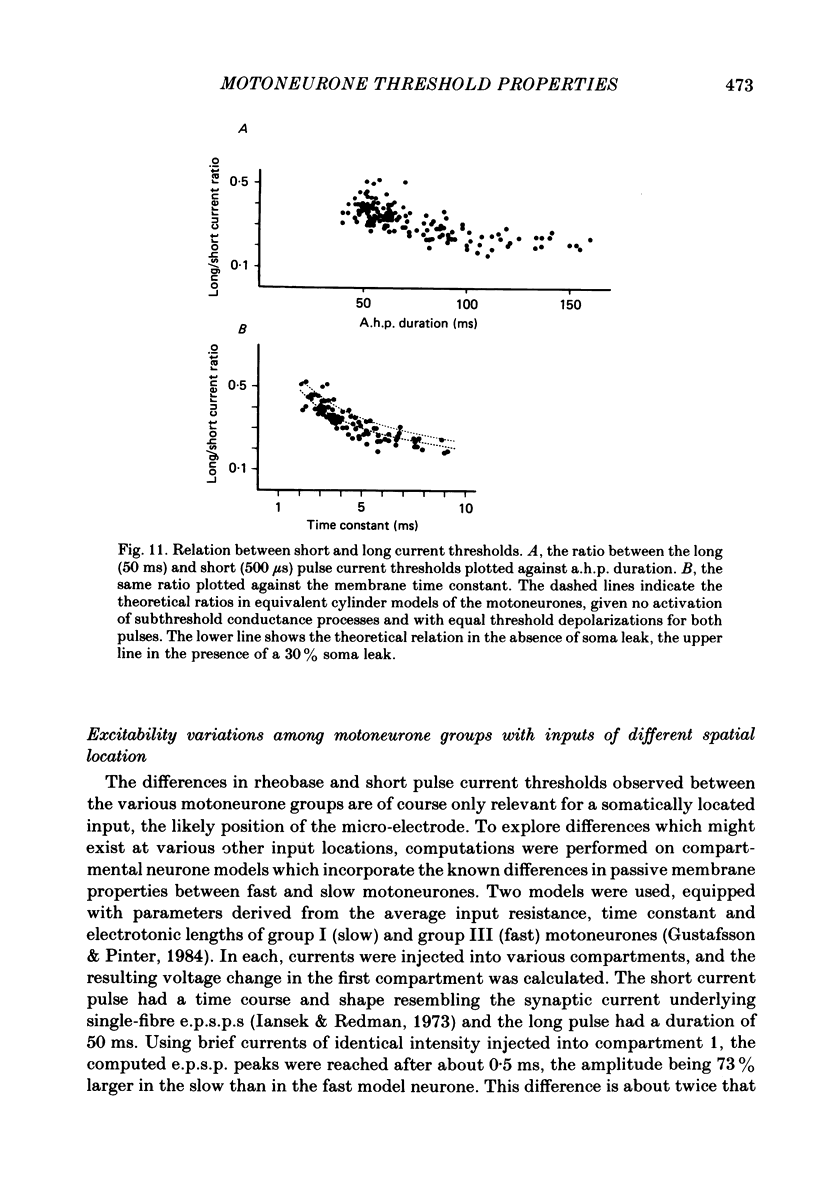
In anaesthetized cats, thresholds for long (rheobase) and brief duration current pulses have been obtained from spinal motoneurones and compared with other cell parameters and membrane properties. Rheobase showed only weak over-all relationships with conduction velocity and with cell size, estimated as the total capacitance of individual motoneuronal equivalent cylinders. Rheobase showed a clear tendency to vary inversely with after-hyperpolarization (a.h.p.) duration and was strongly correlated with the input conductance and with the inverse of the membrane time constant. However, the range of rheobase current exceeded that of input conductance by almost a factor of 2. Part of this range discrepancy arose because threshold depolarization tended to increase with rheobase current. Thus, among motoneurones grouped according to rheobase magnitude (three groups), those within the lowest rheobase group had threshold depolarizations about 6 mV on average lower than those within the highest rheobase group. Even though this difference was not directly related to resting potential differences between the groups, further analysis suggested that it may have arisen secondarily to impalement-induced depolarization. The finding that experimentally estimated threshold depolarizations in individual motoneurones were generally larger than those predicted by the product of input resistance and rheobase indicated that a subthreshold rectification process also contributed to the range of rheobase. The difference was largest in the low-rheobase group and smallest in the high-rheobase group. Because these differences were proportional to the differences in input resistance between the separate motoneurone groups, it is suggested that the magnitude of the current underlying the rectification process does not differ systematically among motoneurones. Within groups of motoneurones classified on the basis of rheobase or a.h.p. duration, significant correlations existed between rheobase current and input conductance. An analysis of variance indicated that even within such functional subgroups of motoneurones, rheobase was appreciably better correlated with membrane time constant than with estimated cell size. Although showing a range approximately half that of rheobase, the brief current threshold was similar to rheobase in its relations with total cell capacitance, a.h.p. duration and the inverse of membrane time constant.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROCK L. G., COOMBS J. S., ECCLES J. C. The recording of potentials from motoneurones with an intracellular electrode. J Physiol. 1952 Aug;117(4):431–460. doi: 10.1113/jphysiol.1952.sp004759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Dum R. P., Fleshman J. W., Glenn L. L., Lev-Tov A., O'Donovan M. J., Pinter M. J. A HRP study of the relation between cell size and motor unit type in cat ankle extensor motoneurons. J Comp Neurol. 1982 Jul 20;209(1):17–28. doi: 10.1002/cne.902090103. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Nelson P. G. Accommodation to current ramps in motoneurons of fast and slow twitch motor units. Int J Neurosci. 1971 Jun;1(6):347–356. doi: 10.3109/00207457109146983. [DOI] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W., Friedman W. A. Rheobase, input resistance, and motor-unit type in medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1326–1338. doi: 10.1152/jn.1981.46.6.1326. [DOI] [PubMed] [Google Scholar]
- Fleshman J. W., Munson J. B., Sypert G. W. Homonymous projection of individual group Ia-fibers to physiologically characterized medial gastrocnemius motoneurons in the cat. J Neurophysiol. 1981 Dec;46(6):1339–1348. doi: 10.1152/jn.1981.46.6.1339. [DOI] [PubMed] [Google Scholar]
- Galvan M. A transient outward current in rat sympathetic neurones. Neurosci Lett. 1982 Aug 31;31(3):295–300. doi: 10.1016/0304-3940(82)90036-2. [DOI] [PubMed] [Google Scholar]
- Grantyn R., Grantyn A., Schierwagen A. Passive membrane properties, afterpotentials and repetitive firing of superior colliculus neurons studied in the anesthetized cat. Exp Brain Res. 1983;50(2-3):377–391. doi: 10.1007/BF00239204. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Pinter M. J. Relations among passive electrical properties of lumbar alpha-motoneurones of the cat. J Physiol. 1984 Nov;356:401–431. doi: 10.1113/jphysiol.1984.sp015473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., OLSON C. B. RELATIONS BETWEEN STRUCTURE AND FUNCTION IN THE DESIGN OF SKELETAL MUSCLES. J Neurophysiol. 1965 May;28:581–598. doi: 10.1152/jn.1965.28.3.581. [DOI] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Harrison P. J. The relationship between the distribution of motor unit mechanical properties and the forces due to recruitment and to rate coding for the generation of muscle force. Brain Res. 1983 Apr 4;264(2):311–315. doi: 10.1016/0006-8993(83)90831-4. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A., Schwartzkroin P. A. Anomalous inward rectification in hippocampal neurons. J Neurophysiol. 1979 May;42(3):889–895. doi: 10.1152/jn.1979.42.3.889. [DOI] [PubMed] [Google Scholar]
- Iansek R., Redman S. J. The amplitude, time course and charge of unitary excitatory post-synaptic potentials evoked in spinal motoneurone dendrites. J Physiol. 1973 Nov;234(3):665–688. doi: 10.1113/jphysiol.1973.sp010366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Oshima T. Electrical behaviour of the motoneurone membrane during intracellularly applied current steps. J Physiol. 1965 Oct;180(3):607–635. doi: 10.1113/jphysiol.1965.sp007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNO M. Excitability following antidromic activation in spinal motoneurones supplying red muscles. J Physiol. 1959 Dec;149:374–393. doi: 10.1113/jphysiol.1959.sp006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Zwaagstra B. Input conductance axonal conduction velocity and cell size among hindlimb motoneurones of the cat. Brain Res. 1981 Jan 12;204(2):311–326. doi: 10.1016/0006-8993(81)90591-6. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973 Apr;230(2):359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Pinter M. J., Curtis R. L., Hosko M. J. Voltage threshold and excitability among variously sized cat hindlimb motoneurons. J Neurophysiol. 1983 Sep;50(3):644–657. doi: 10.1152/jn.1983.50.3.644. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Factors influencing motoneuron rhythmic firing: results from a voltage-clamp study. J Neurophysiol. 1982 Oct;48(4):875–890. doi: 10.1152/jn.1982.48.4.875. [DOI] [PubMed] [Google Scholar]
- Schwindt P. C., Crill W. E. Properties of a persistent inward current in normal and TEA-injected motoneurons. J Neurophysiol. 1980 Jun;43(6):1700–1724. doi: 10.1152/jn.1980.43.6.1700. [DOI] [PubMed] [Google Scholar]
- Ulfhake B., Kellerth J. O. Does alpha-motoneurone size correlate with motor unit type in cat triceps surae? Brain Res. 1982 Nov 18;251(2):201–209. doi: 10.1016/0006-8993(82)90738-7. [DOI] [PubMed] [Google Scholar]


