Abstract
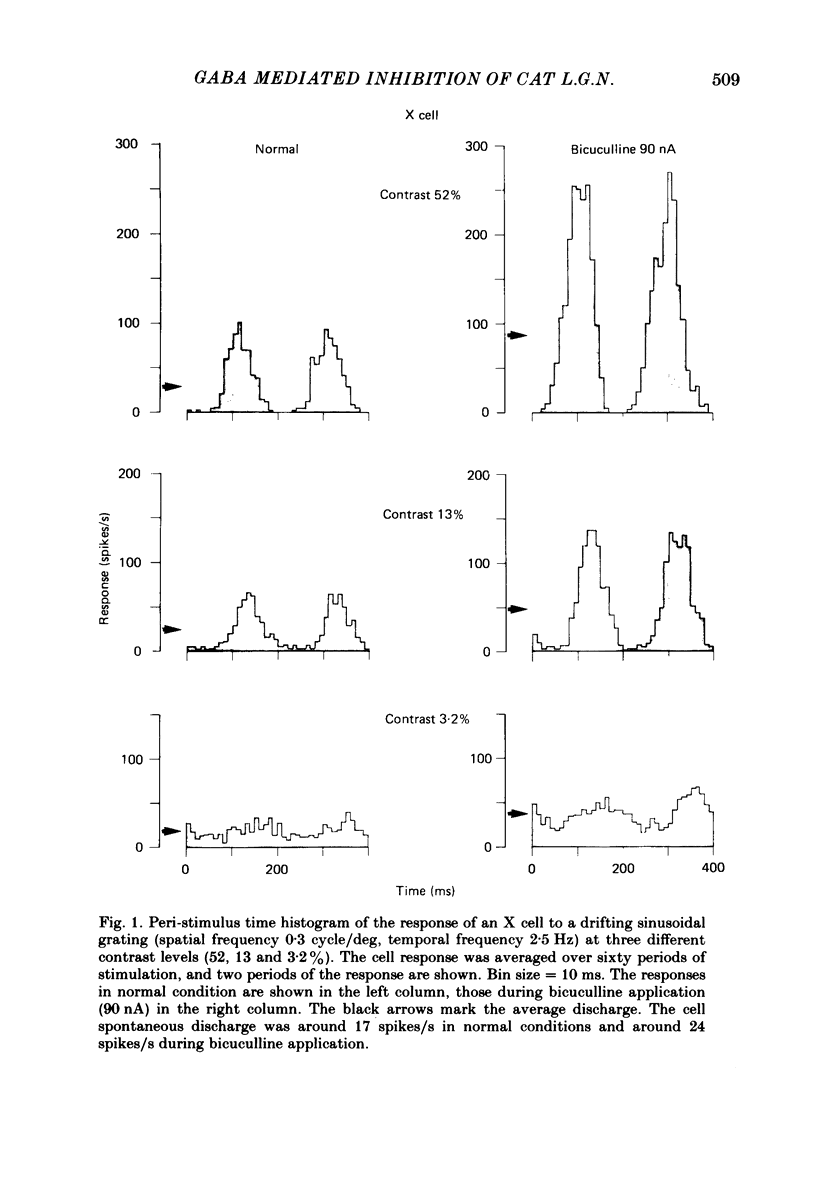
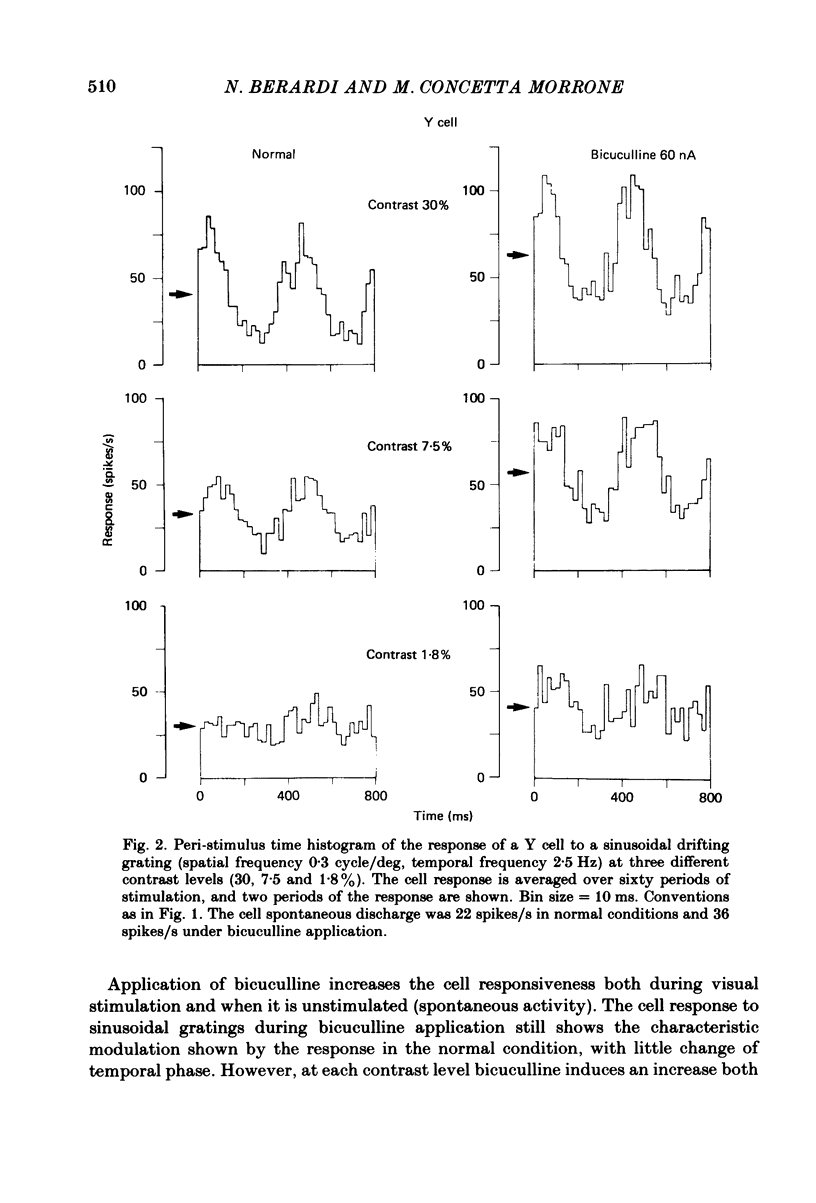
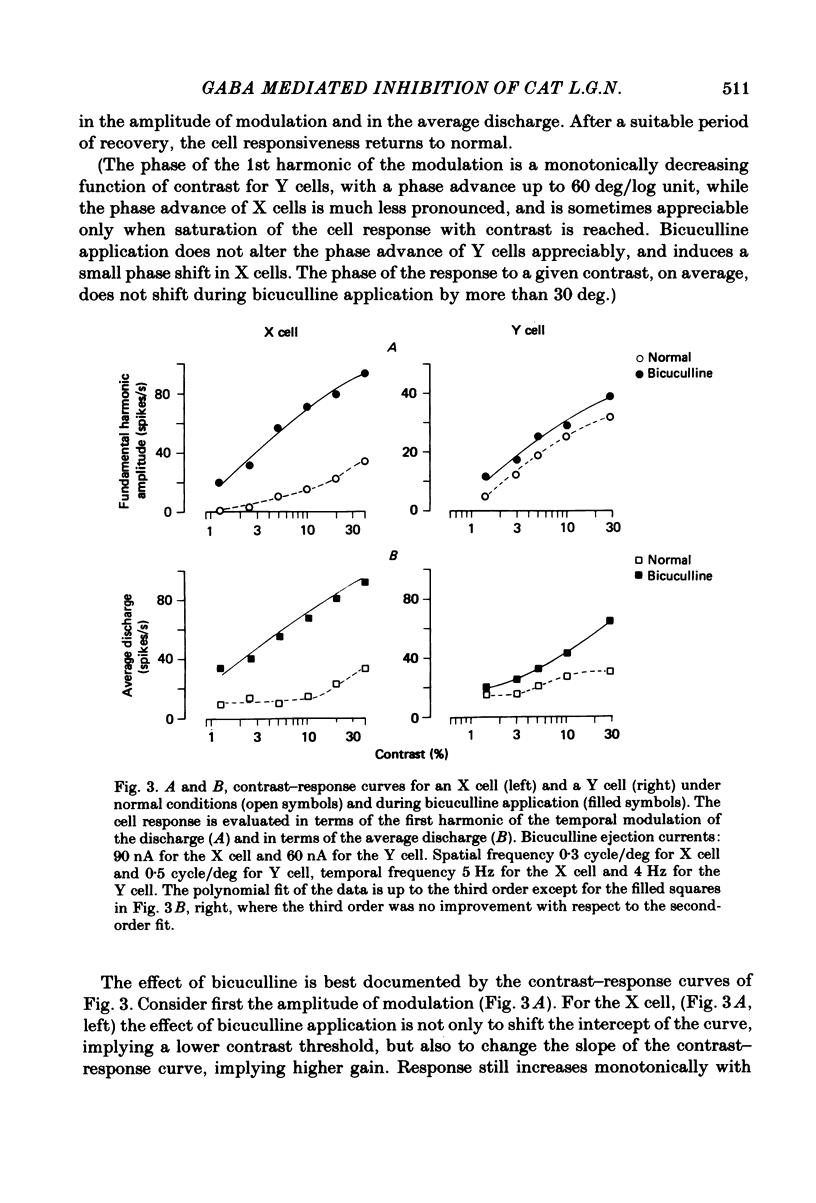
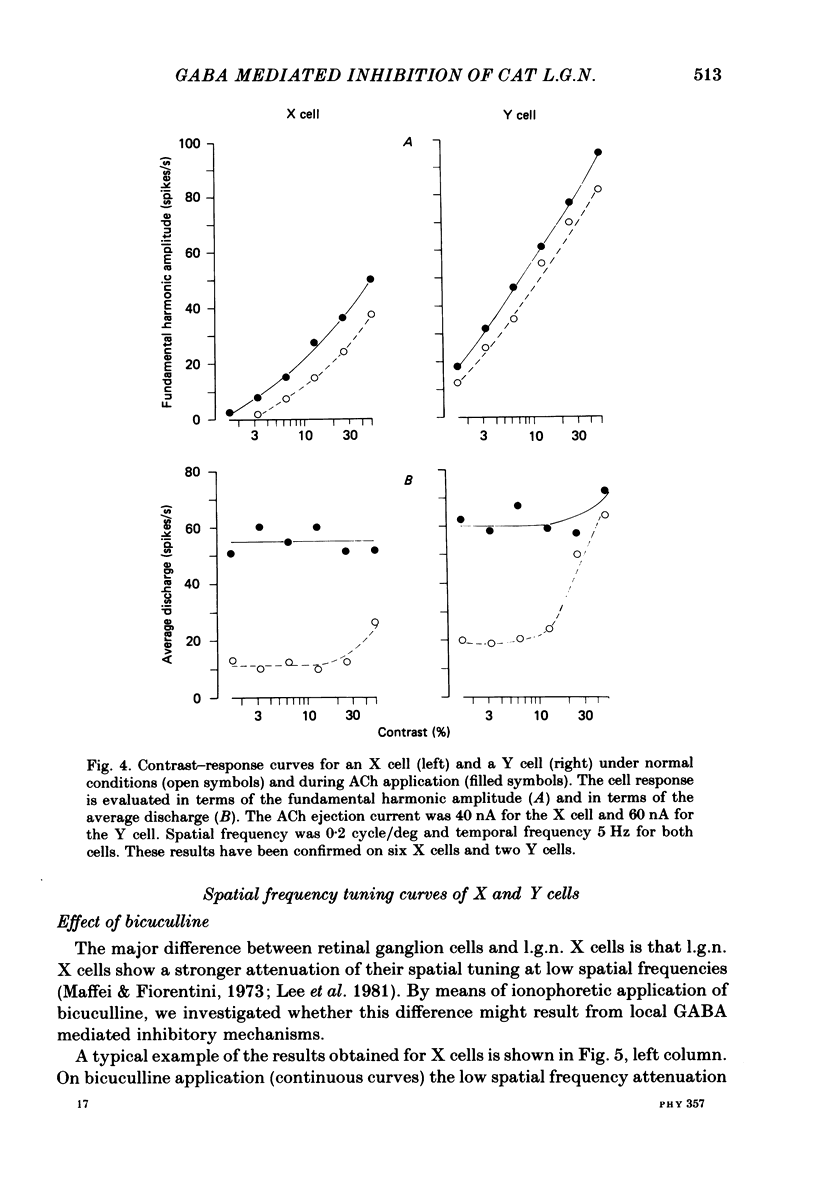
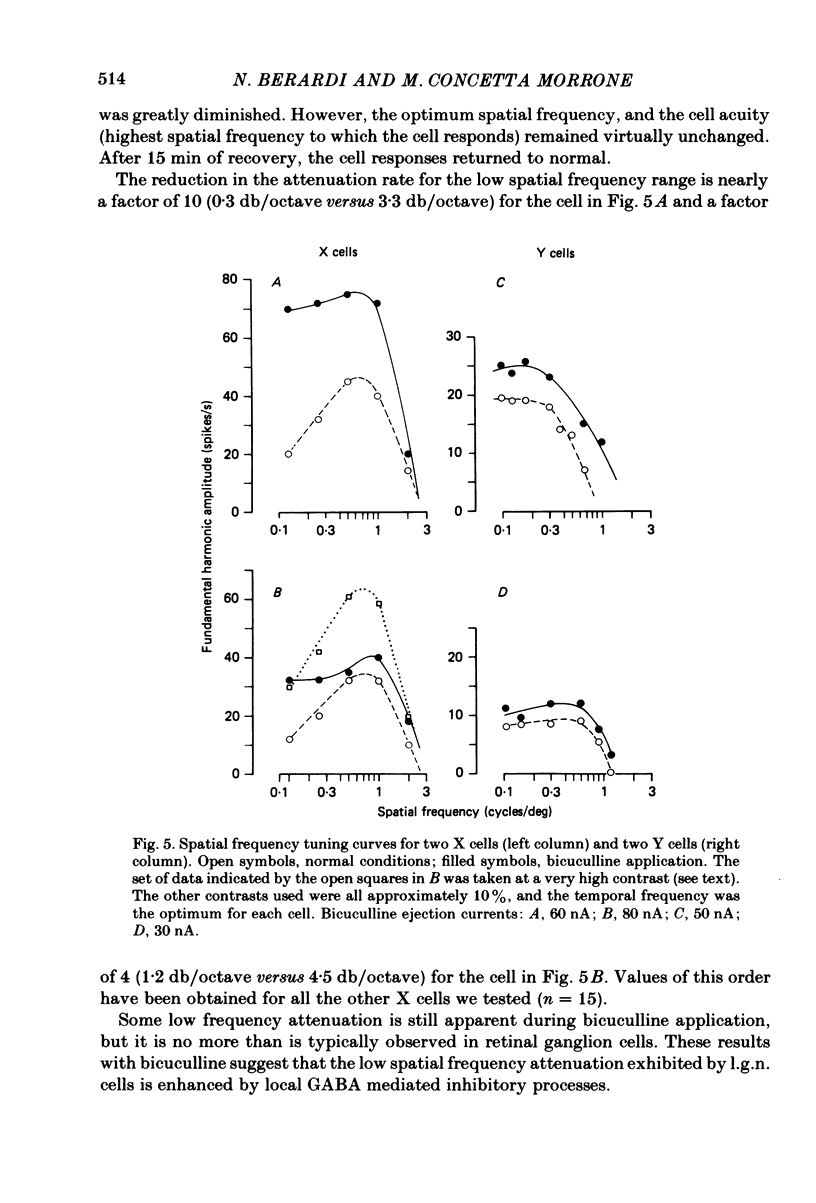
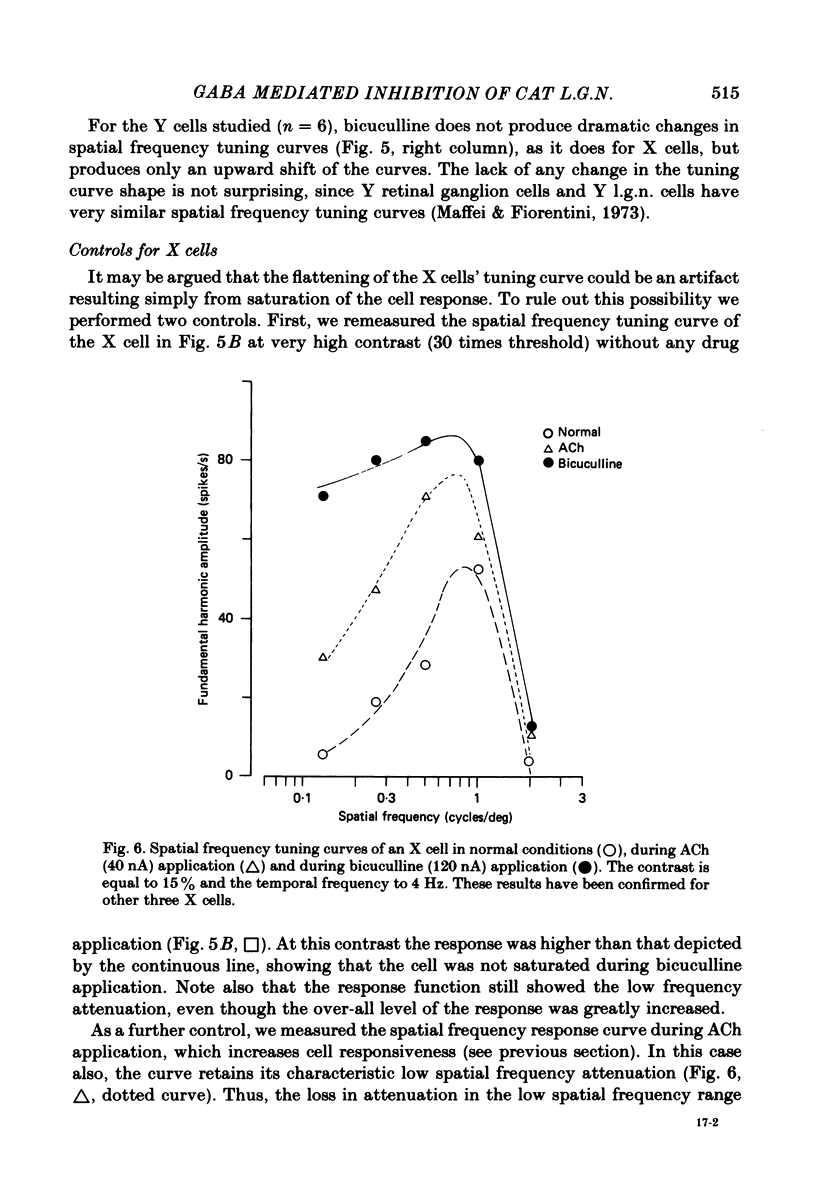
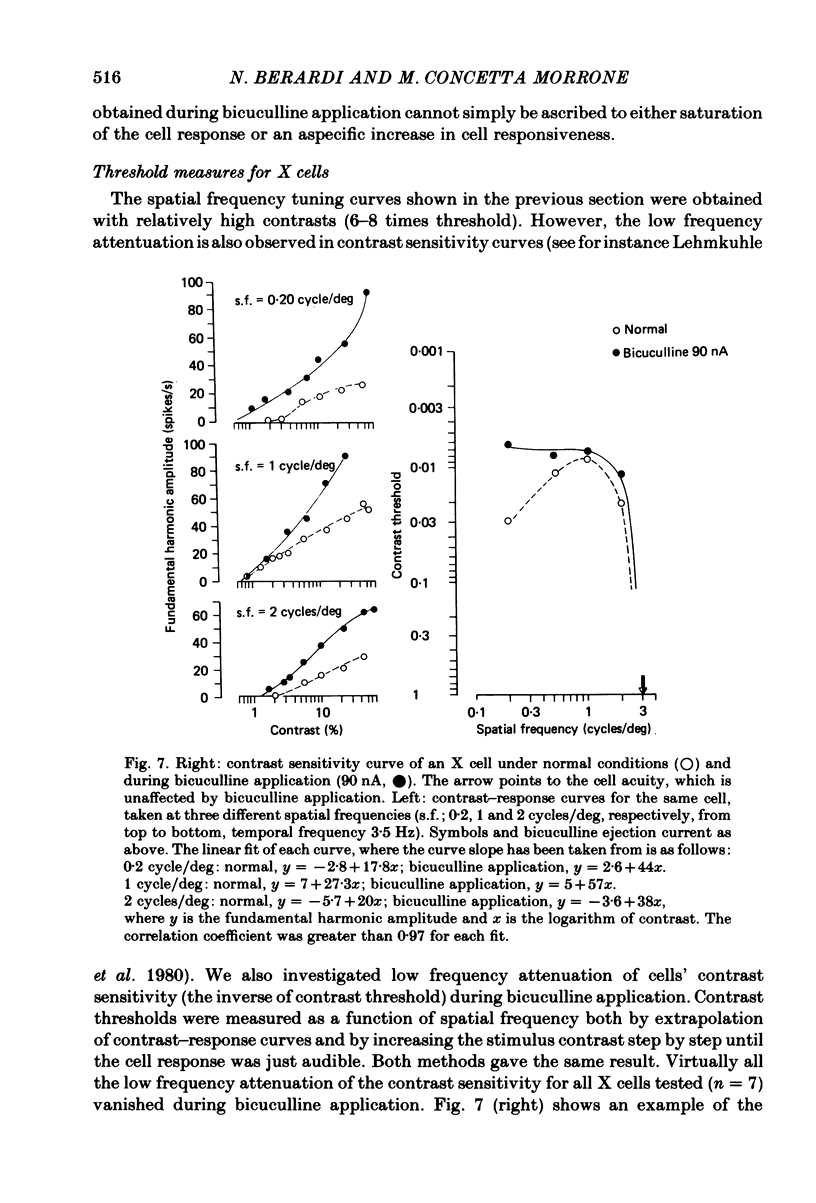
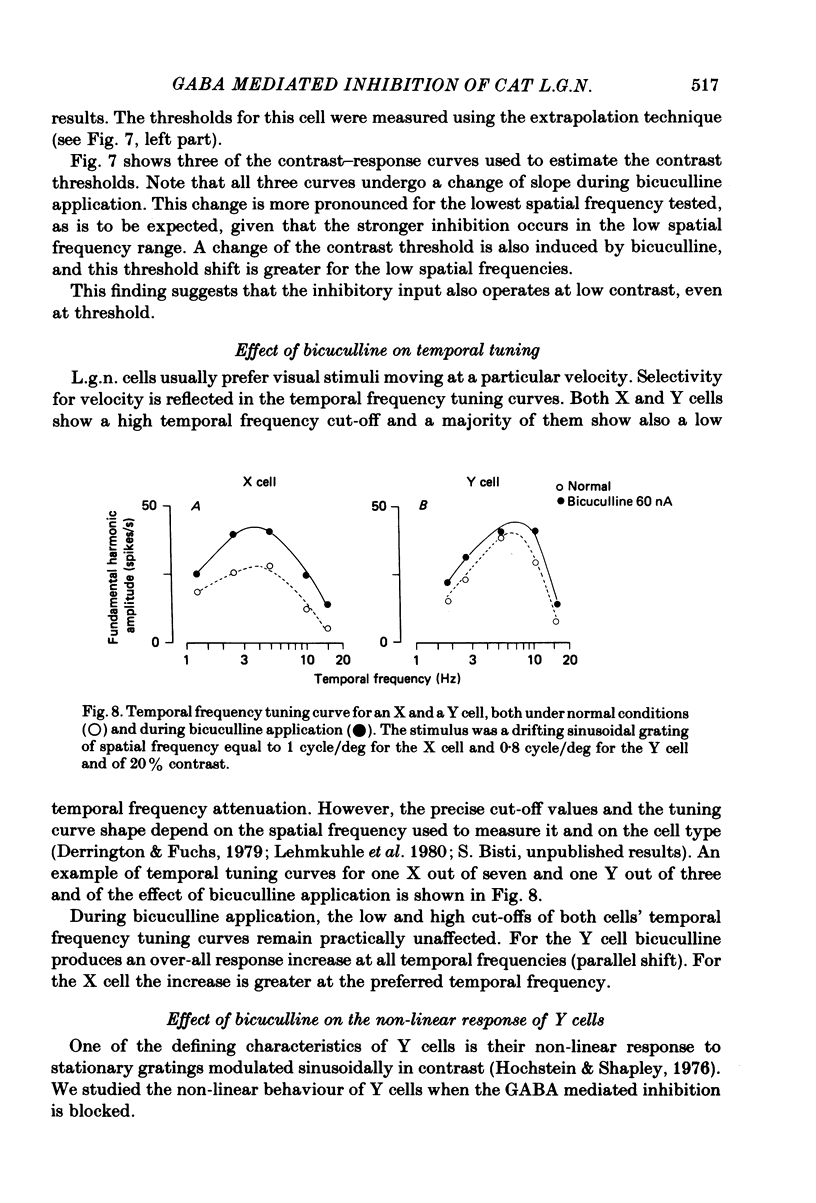
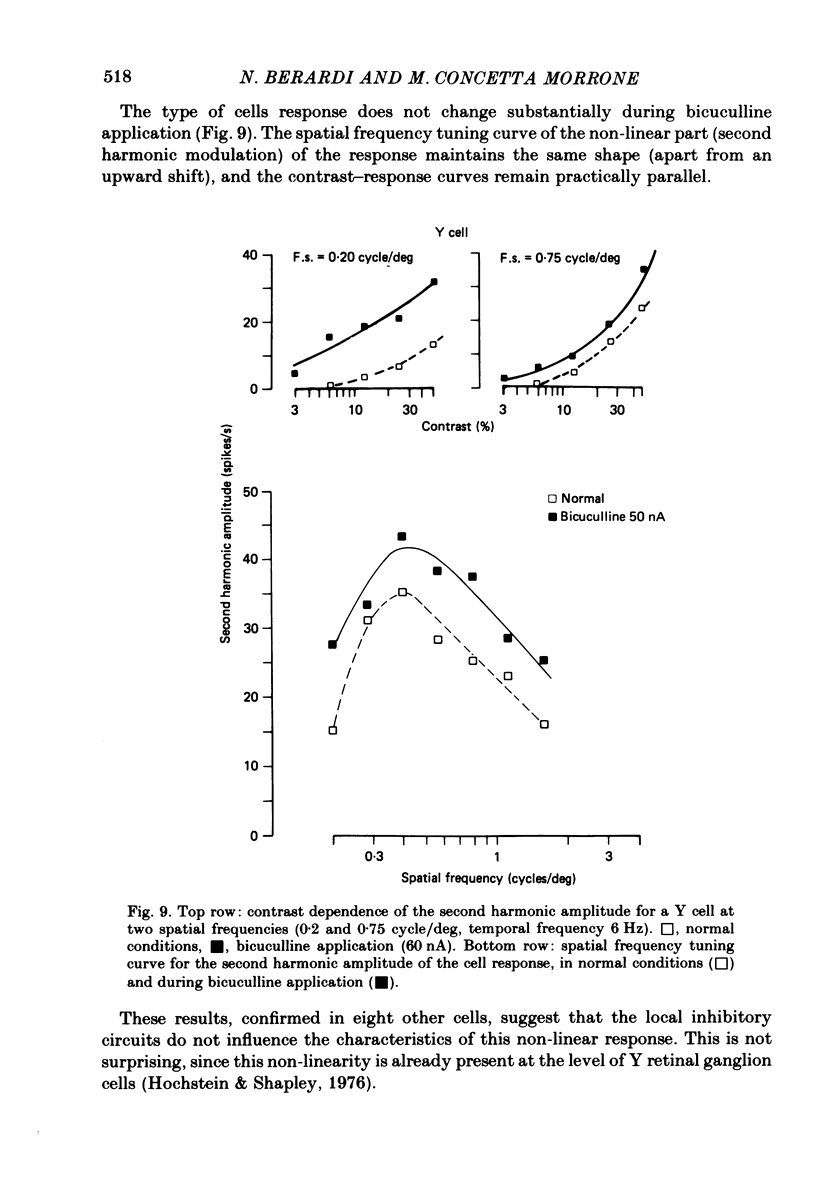
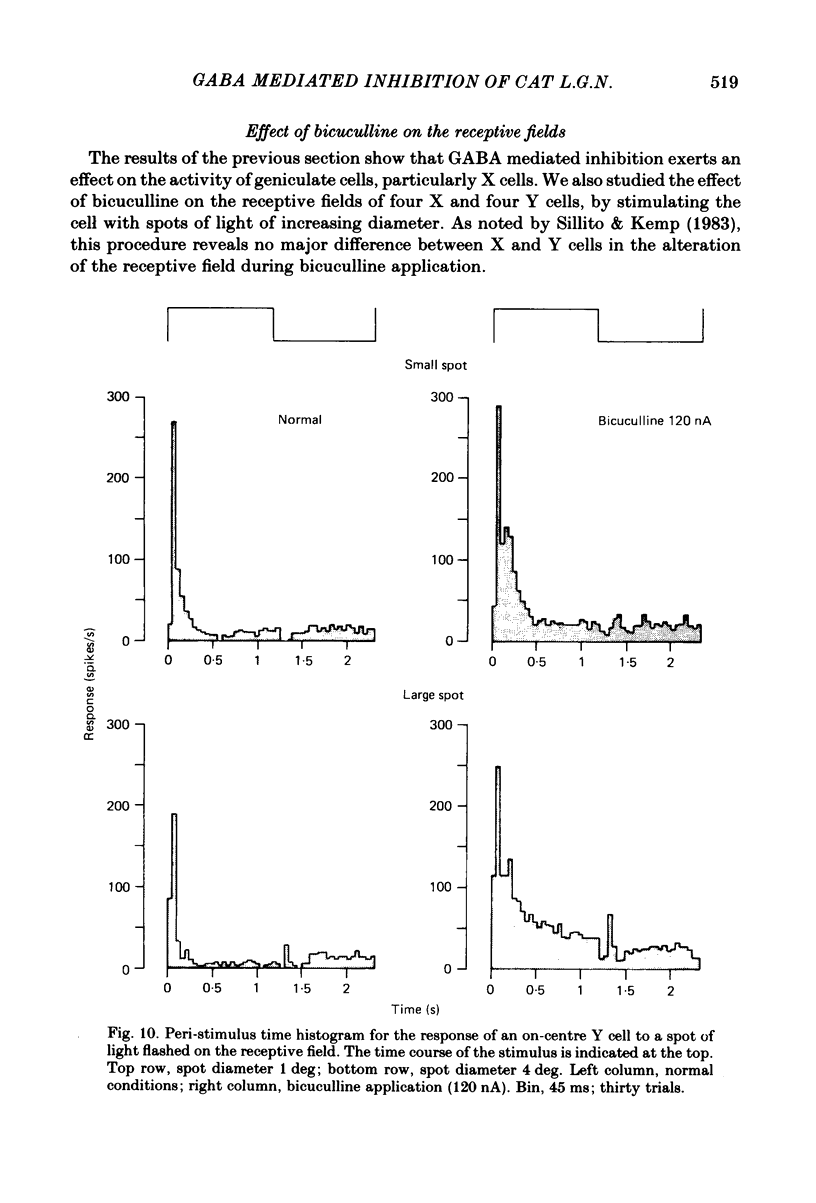
We studied the effect of local ionophoretic application of bicuculline on the response of cat lateral geniculate nucleus (laminae A) cells to stimulation by sinusoidal gratings and spots of light. Application of bicuculline produced an increase both of spontaneous and visually driven discharge of both X and Y cells. On stimulation by drifting sinusoidal gratings, the average discharge of both X and Y cells remained constant with increasing contrast under normal conditions. Application of bicuculline caused the average discharge to increase with contrast, indicating that the constancy of the average discharge was maintained by gamma-aminobutyric acid mediated inhibition. Under normal conditions, the amplitude of response modulation of both X and Y cells to sinusoidal grating stimulation increased monotonically with stimulus contrast. During bicuculline application, the slope of the contrast-response curve for X cells but not for Y cells increased, indicating that the inhibition which dampened the modulation of X cells (but not Y cells) was contrast dependent. Application of acetylcholine also increased the average discharge and the amplitude of modulation of the cell responses, but this increase did not depend on stimulus contrast. Under normal conditions, X but not Y cells showed an attenuation of response and an increase in contrast threshold to low spatial frequencies. This attenuation vanished during bicuculline application. The shape of Y-cell response curves was unaffected by bicuculline. Bicuculline had the same effect on the non-linear component of Y-cell response as on the linear component. Although bicuculline had a different effect on the response of X and Y cells to stimulation by gratings, it reduced the antagonistic surround of both X and Y cells to a similar extent (revealed by plotting the cell receptive fields with flashed spots of light).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlsen G., Grant K., Lindström S. Monosynaptic excitation of principal cells in the lateral geniculate nucleus by corticofugal fibers. Brain Res. 1982 Feb 25;234(2):454–458. doi: 10.1016/0006-8993(82)90886-1. [DOI] [PubMed] [Google Scholar]
- Ahlsén G., Lindström S., Sybirska E. Subcortical axon collaterals of principal cells in the lateral geniculate body of the cat. Brain Res. 1978 Nov 3;156(1):106–109. doi: 10.1016/0006-8993(78)90084-7. [DOI] [PubMed] [Google Scholar]
- Bisti S., Clement R., Maffei L., Mecacci L. Spatial frequency and orientation tuning curves of visual neurones in the cat: effects of mean luminance. Exp Brain Res. 1977 Mar 30;27(3-4):335–345. doi: 10.1007/BF00235508. [DOI] [PubMed] [Google Scholar]
- CURTIS D. R., DAVIS R. The excitation of lateral geniculate neurones by quaternary ammonium derivatives. J Physiol. 1963 Jan;165:62–82. doi: 10.1113/jphysiol.1963.sp007042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Simultaneous recording of input and output of lateral geniculate neurones. Nat New Biol. 1971 Jun 9;231(23):191–192. doi: 10.1038/newbio231191a0. [DOI] [PubMed] [Google Scholar]
- Cleland B. G., Dubin M. W., Levick W. R. Sustained and transient neurones in the cat's retina and lateral geniculate nucleus. J Physiol. 1971 Sep;217(2):473–496. doi: 10.1113/jphysiol.1971.sp009581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Terbécis A. K. Bicuculline and thalamic inhibition. Exp Brain Res. 1972;16(2):210–218. doi: 10.1007/BF00233997. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Fuchs A. F. Spatial and temporal properties of X and Y cells in the cat lateral geniculate nucleus. J Physiol. 1979 Aug;293:347–364. doi: 10.1113/jphysiol.1979.sp012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Peters A. The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1972 Mar;144(3):285–334. doi: 10.1002/cne.901440304. [DOI] [PubMed] [Google Scholar]
- Fernald R., Chase R. An improved method for plotting retinal landmarks and focusing the eyes. Vision Res. 1971 Jan;11(1):95–96. doi: 10.1016/0042-6989(71)90207-0. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Lin C. S., Stanford L. R., Sherman S. M. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981 Jul;46(1):80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Stone J. Evidence of differential inhibitory influences on X- and Y-type relay cells in the cat's lateral geniculate nucleus. Brain Res. 1976 Aug 20;113(1):188–196. doi: 10.1016/0006-8993(76)90018-4. [DOI] [PubMed] [Google Scholar]
- Geisert E. E., Langsetmo A., Spear P. D. Influence of the cortico-geniculate pathway on response properties of cat lateral geniculate neurons. Brain Res. 1981 Mar 16;208(2):409–415. doi: 10.1016/0006-8993(81)90568-0. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. A study of Golgi preparations from the dorsal lateral geniculate nucleus of the adult cat. J Comp Neurol. 1966 Sep;128(1):21–50. doi: 10.1002/cne.901280104. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond P. Contrasts in spatial organization of receptive fields at geniculate and retinal levels: centre, surround and outer surround. J Physiol. 1973 Jan;228(1):115–137. doi: 10.1113/jphysiol.1973.sp010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Quantitative analysis of retinal ganglion cell classifications. J Physiol. 1976 Nov;262(2):237–264. doi: 10.1113/jphysiol.1976.sp011594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover D. B., Jacobowitz D. M. Neurochemical and histochemical studies of the effect of a lesion of the nucleus cuneiformis on the cholinergic innervation of discrete areas of the rat brain. Brain Res. 1979 Jul 6;170(1):113–122. doi: 10.1016/0006-8993(79)90944-2. [DOI] [PubMed] [Google Scholar]
- Houser C. R., Vaughn J. E., Barber R. P., Roberts E. GABA neurons are the major cell type of the nucleus reticularis thalami. Brain Res. 1980 Nov 3;200(2):341–354. doi: 10.1016/0006-8993(80)90925-7. [DOI] [PubMed] [Google Scholar]
- Kaplan E., Marcus S., So Y. T. Effects of dark adaptation on spatial and temporal properties of receptive fields in cat lateral geniculate nucleus. J Physiol. 1979 Sep;294:561–580. doi: 10.1113/jphysiol.1979.sp012946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp J. A., Sillito A. M. The nature of the excitatory transmitter mediating X and Y cell inputs to the cat dorsal lateral geniculate nucleus. J Physiol. 1982 Feb;323:377–391. doi: 10.1113/jphysiol.1982.sp014078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz K. E., Webb S. V., Sherman S. M. Electrophysiological classification of X- and Y-cells in the cats lateral geniculate nucleus. Vision Res. 1978;18(9):1261–1264. doi: 10.1016/0042-6989(78)90112-8. [DOI] [PubMed] [Google Scholar]
- LeVay S., Ferster D. Proportion of interneurons in the cat's lateral geniculate nucleus. Brain Res. 1979 Mar 23;164:304–308. doi: 10.1016/0006-8993(79)90026-x. [DOI] [PubMed] [Google Scholar]
- Lee B. B., Elepfandt A., Virsu V. Phase of responses to moving sinusoidal gratings in cells of cat retina and lateral geniculate nucleus. J Neurophysiol. 1981 May;45(5):807–817. doi: 10.1152/jn.1981.45.5.807. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle S., Kratz K. E., Mangel S. C., Sherman S. M. Spatial and temporal sensitivity of X- and Y-cells in dorsal lateral geniculate nucleus of the cat. J Neurophysiol. 1980 Feb;43(2):520–541. doi: 10.1152/jn.1980.43.2.520. [DOI] [PubMed] [Google Scholar]
- Lennie P. Parallel visual pathways: a review. Vision Res. 1980;20(7):561–594. doi: 10.1016/0042-6989(80)90115-7. [DOI] [PubMed] [Google Scholar]
- Lindström S. Synaptic organization of inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Brain Res. 1982 Feb 25;234(2):447–453. doi: 10.1016/0006-8993(82)90885-x. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. Retinogeniculate convergence and analysis of contrast. J Neurophysiol. 1972 Jan;35(1):65–72. doi: 10.1152/jn.1972.35.1.65. [DOI] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A. The visual cortex as a spatial frequency analyser. Vision Res. 1973 Jul;13(7):1255–1267. doi: 10.1016/0042-6989(73)90201-0. [DOI] [PubMed] [Google Scholar]
- Marr D. Early processing of visual information. Philos Trans R Soc Lond B Biol Sci. 1976 Oct 19;275(942):483–519. doi: 10.1098/rstb.1976.0090. [DOI] [PubMed] [Google Scholar]
- Marr D., Hildreth E. Theory of edge detection. Proc R Soc Lond B Biol Sci. 1980 Feb 29;207(1167):187–217. doi: 10.1098/rspb.1980.0020. [DOI] [PubMed] [Google Scholar]
- Morgan R., Sillito A. M., Wolstencroft J. H. A pharmacological investigation of inhibition in the lateral geniculate nucleus. J Physiol. 1975 Mar;246(2):93P–94P. [PubMed] [Google Scholar]
- Phillis J. W., Tebecis A. K., York D. H. A study of cholinoceptive cells in the lateral geniculate nucleus. J Physiol. 1967 Oct;192(3):695–713. doi: 10.1113/jphysiol.1967.sp008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller P. H. Central connections of the retinal ON and OFF pathways. Nature. 1982 Jun 17;297(5867):580–583. doi: 10.1038/297580a0. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A., Berardi N. The cholinergic influence on the function of the cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Dec 5;280(2):299–307. doi: 10.1016/0006-8993(83)90059-8. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Singer W., Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res. 1973 Jan 30;49(2):291–307. doi: 10.1016/0006-8993(73)90424-1. [DOI] [PubMed] [Google Scholar]
- Singer W. Control of thalamic transmission by corticofugal and ascending reticular pathways in the visual system. Physiol Rev. 1977 Jul;57(3):386–420. doi: 10.1152/physrev.1977.57.3.386. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Singer W., Pöppel E., Creutzfeldt O. Inhibitory interaction in the cat's lateral geniculate nucleus. Exp Brain Res. 1972;14(2):210–226. doi: 10.1007/BF00234800. [DOI] [PubMed] [Google Scholar]
- So Y. T., Shapley R. Spatial tuning of cells in and around lateral geniculate nucleus of the cat: X and Y relay cells and perigeniculate interneurons. J Neurophysiol. 1981 Jan;45(1):107–120. doi: 10.1152/jn.1981.45.1.107. [DOI] [PubMed] [Google Scholar]
- Sterling P., Davis T. L. Neurons in cat lateral geniculate nucleus that concentrate exogenous [3H]-gamma-aminobutyric acid (GABA). J Comp Neurol. 1980 Aug 15;192(4):737–749. doi: 10.1002/cne.901920408. [DOI] [PubMed] [Google Scholar]
- Virsu V., Lee B. B., Creutzfeldt O. D. Dark adaptation and receptive field organisation of cells in the cat lateral geniculate nucleus. Exp Brain Res. 1977 Jan 18;27(1):35–50. doi: 10.1007/BF00234823. [DOI] [PubMed] [Google Scholar]