Abstract
We studied the development of gamma-aminobutyric (GABA) mediated inhibitory processes of the lateral geniculate nucleus cells in kittens of various age groups, by measuring the effect of ionophoretic application of GABA and bicuculline on cell response to sinusoidal gratings. In young kittens (less than 30 days) we found very few Y cells only X cells and weakly responsive cells which fitted neither X nor Y classifications ('immature' cells). As with adult cells, GABA inhibits the visual response of young kitten cells. Simultaneous application of bicuculline restored responsiveness. The mean GABA current to silence cell response in young kittens was significantly higher than that obtained in adult cats. Application of bicuculline alone had little effect on young kitten X-cell responsiveness, either on the average discharge or on the amplitude of modulation to stimulation by sinusoidal gratings. For older kittens (40-45 days), bicuculline increased X-cell responsiveness, and the increase in responsiveness was dependent on stimulus contrast and spatial frequency. However, the increased responsiveness was less than that for adults. At 100 days the changes of slope of X-cell contrast-response curves during bicuculline application were similar to those observed for adult X cells. We conclude that, although GABA receptors may be present at 30 days, the GABA mediated inhibitory system does not begin to function until about 45 days and does not mature fully until about 100 days.
Full text
PDF



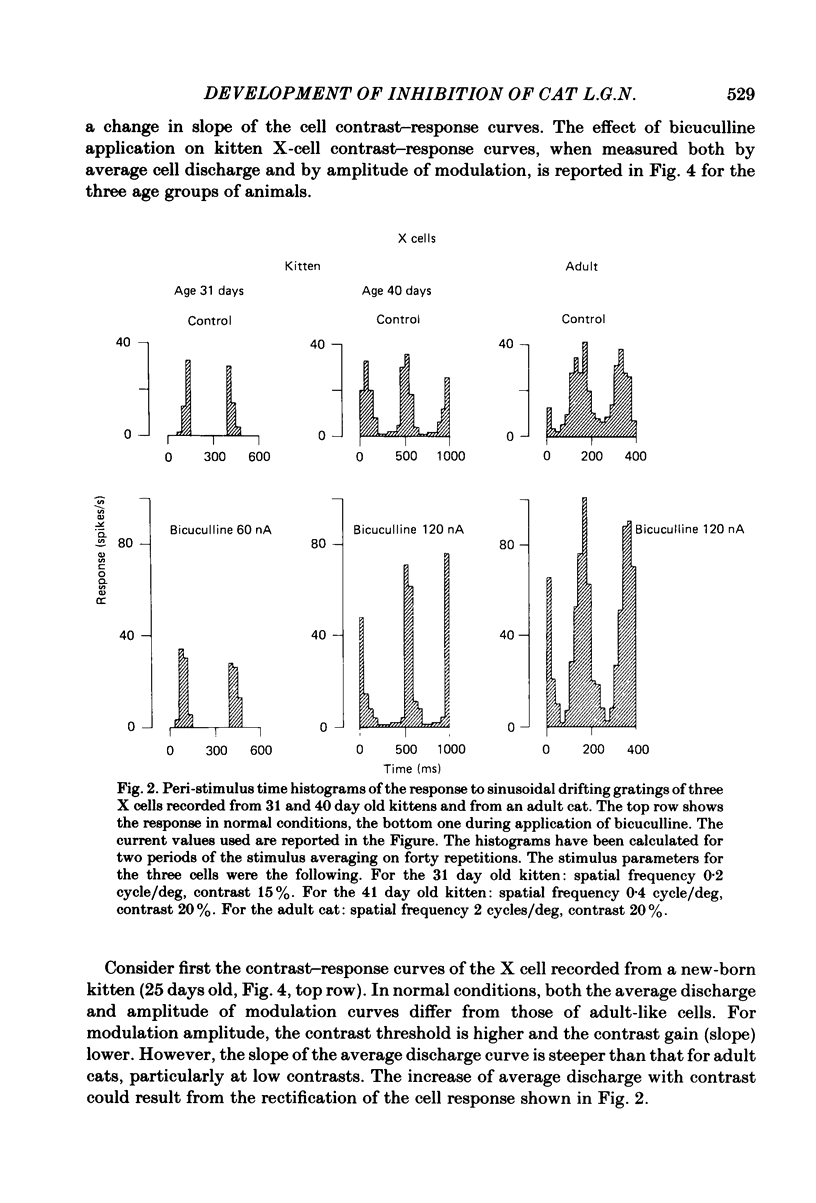



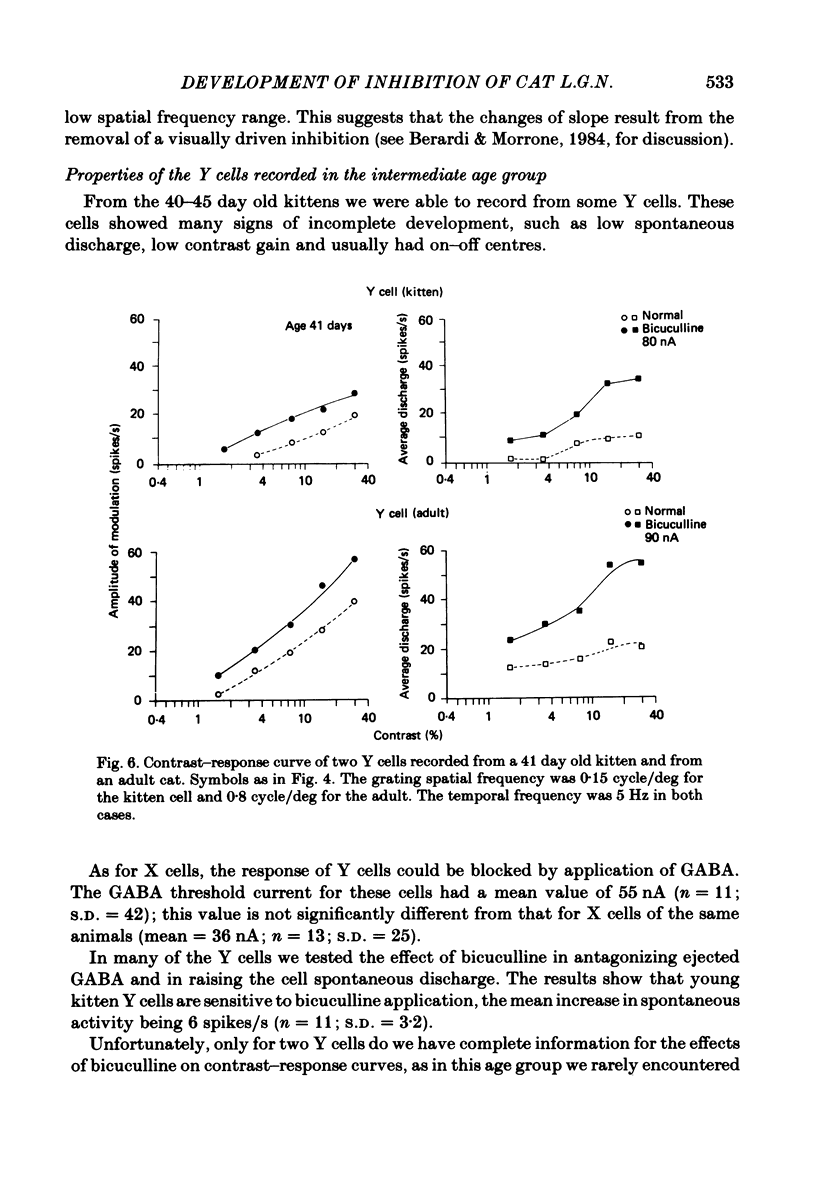




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrien J., Roffwarg H. P. The development of unit activity in the lateral geniculate nucleus of the kitten. Exp Neurol. 1974 Apr;43(1):261–275. doi: 10.1016/0014-4886(74)90145-9. [DOI] [PubMed] [Google Scholar]
- Berardi N., Morrone M. C. The role of gamma-aminobutyric acid mediated inhibition in the response properties of cat lateral geniculate nucleus neurones. J Physiol. 1984 Dec;357:505–523. doi: 10.1113/jphysiol.1984.sp015514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg B. G. The development of synapses in the visual system of the cat. J Comp Neurol. 1975 Mar 15;160(2):147–166. doi: 10.1002/cne.901600202. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Terbécis A. K. Bicuculline and thalamic inhibition. Exp Brain Res. 1972;16(2):210–218. doi: 10.1007/BF00233997. [DOI] [PubMed] [Google Scholar]
- Daniels J. D., Pettigrew J. D., Norman J. L. Development of single-neuron responses in kitten's lateral geniculate nucleus. J Neurophysiol. 1978 Nov;41(6):1373–1393. doi: 10.1152/jn.1978.41.6.1373. [DOI] [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y., Stone J. Evidence of differential inhibitory influences on X- and Y-type relay cells in the cat's lateral geniculate nucleus. Brain Res. 1976 Aug 20;113(1):188–196. doi: 10.1016/0006-8993(76)90018-4. [DOI] [PubMed] [Google Scholar]
- Garey L. J., Fisken R. A., Powell T. P. Observations on the growth of cell sin the lateral geniculate nucleus of the cat. Brain Res. 1973 Mar 30;52:359–362. doi: 10.1016/0006-8993(73)90671-9. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Integrative action in the cat's lateral geniculate body. J Physiol. 1961 Feb;155:385–398. doi: 10.1113/jphysiol.1961.sp006635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaki D. I., Flynn J. T. Physiological properties of retinal ganglion cells of 3-week-old kittens. Vision Res. 1977 Feb;17(2):275–284. doi: 10.1016/0042-6989(77)90091-8. [DOI] [PubMed] [Google Scholar]
- Hamasaki D. I., Sutija V. G. Development of X- and Y-cells in kittens. Exp Brain Res. 1979 Mar 9;35(1):9–23. doi: 10.1007/BF00236781. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Tremain K. E. The development of spatial resolving power of lateral geniculate neurones in kittens. Exp Brain Res. 1978 Feb 15;31(2):193–206. doi: 10.1007/BF00237599. [DOI] [PubMed] [Google Scholar]
- Kalil R. Development of the dorsal lateral geniculate nucleus in the cat. J Comp Neurol. 1978 Nov 15;182(2):265–291. doi: 10.1002/cne.901820206. [DOI] [PubMed] [Google Scholar]
- Morgan R., Sillito A. M., Wolstencroft J. H. A pharmacological investigation of inhibition in the lateral geniculate nucleus. J Physiol. 1975 Mar;246(2):93P–94P. [PubMed] [Google Scholar]
- Norman J. L., Pettigrew J. D., Daniels J. D. Early development of X-cells in kitten lateral geniculate nucleus. Science. 1977 Oct 14;198(4313):202–204. doi: 10.1126/science.905824. [DOI] [PubMed] [Google Scholar]
- Rusoff A. C., Dubin M. W. Development of receptive-field properties of retinal ganglion cells in kittens. J Neurophysiol. 1977 Sep;40(5):1188–1198. doi: 10.1152/jn.1977.40.5.1188. [DOI] [PubMed] [Google Scholar]
- SCHADE O. H., Sr Optical and photoelectric analog of the eye. J Opt Soc Am. 1956 Sep;46(9):721–739. doi: 10.1364/josa.46.000721. [DOI] [PubMed] [Google Scholar]
- Sherman S. M., Spear P. D. Organization of visual pathways in normal and visually deprived cats. Physiol Rev. 1982 Apr;62(2):738–855. doi: 10.1152/physrev.1982.62.2.738. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Singer W., Bedworth N. Inhibitory interaction between X and Y units in the cat lateral geniculate nucleus. Brain Res. 1973 Jan 30;49(2):291–307. doi: 10.1016/0006-8993(73)90424-1. [DOI] [PubMed] [Google Scholar]
- Singer W., Creutzfeldt O. D. Reciprocal lateral inhibition of on- and off-center neurones in the lateral geniculate body of the cat. Exp Brain Res. 1970;10(3):311–330. doi: 10.1007/BF00235054. [DOI] [PubMed] [Google Scholar]
- Singer W., Pöppel E., Creutzfeldt O. Inhibitory interaction in the cat's lateral geniculate nucleus. Exp Brain Res. 1972;14(2):210–226. doi: 10.1007/BF00234800. [DOI] [PubMed] [Google Scholar]
- Sterling P., Davis T. L. Neurons in cat lateral geniculate nucleus that concentrate exogenous [3H]-gamma-aminobutyric acid (GABA). J Comp Neurol. 1980 Aug 15;192(4):737–749. doi: 10.1002/cne.901920408. [DOI] [PubMed] [Google Scholar]
- Winfield D. A., Headon M. P., Powell T. P. Postnatal development of the synaptic organisation of the lateral geniculate nucleus in the kitten with unilateral eyelid closure. Nature. 1976 Oct 14;263(5578):591–594. doi: 10.1038/263591a0. [DOI] [PubMed] [Google Scholar]
- Winfield D. A., Hiorns R. W., Powell T. P. A quantitative electron-microscopical study of the postnatal development of the lateral geniculate nucleus in normal kittens and in kittens with eyelid suture. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1179):211–234. doi: 10.1098/rspb.1980.0130. [DOI] [PubMed] [Google Scholar]
- Winfield D. A., Powell T. P. An electron-microscopical study of the postnatal development of the lateral geniculate nucleus in the normal kitten and after eyelid suture. Proc R Soc Lond B Biol Sci. 1980 Nov 19;210(1179):197–210. doi: 10.1098/rspb.1980.0129. [DOI] [PubMed] [Google Scholar]



