Abstract
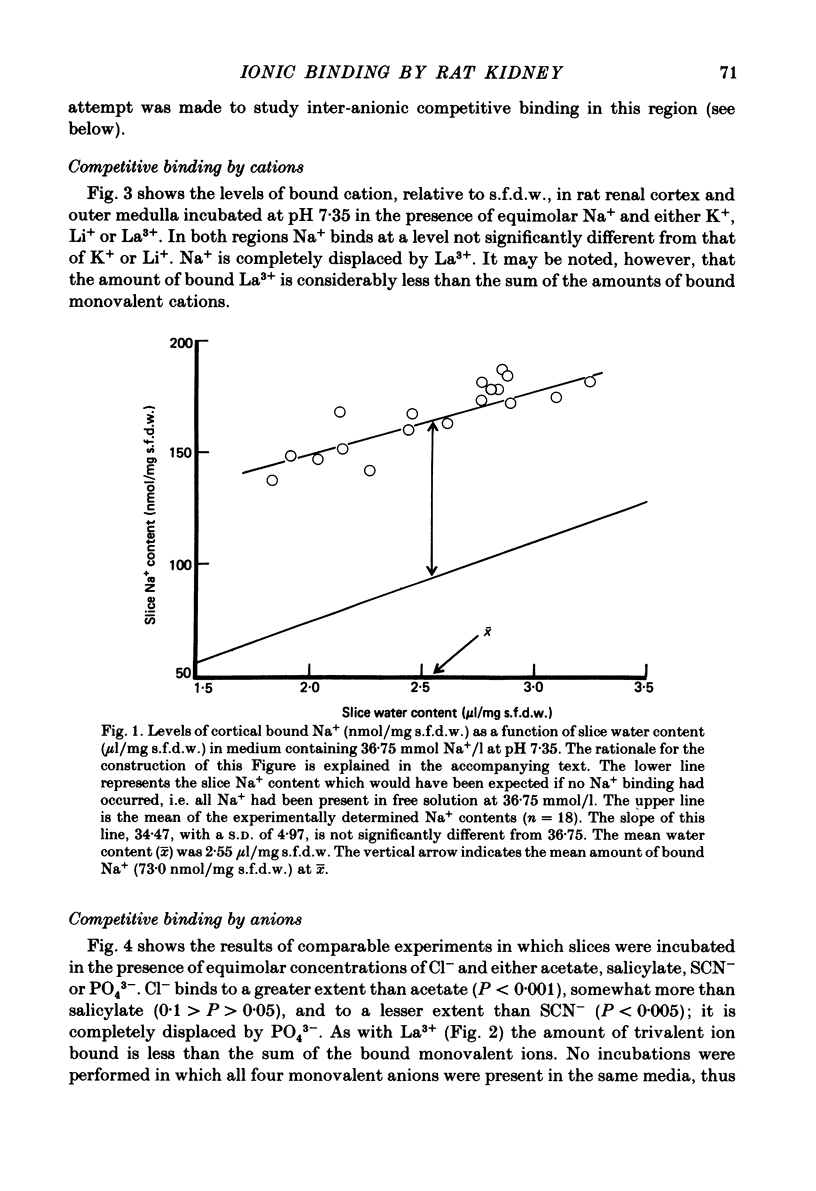
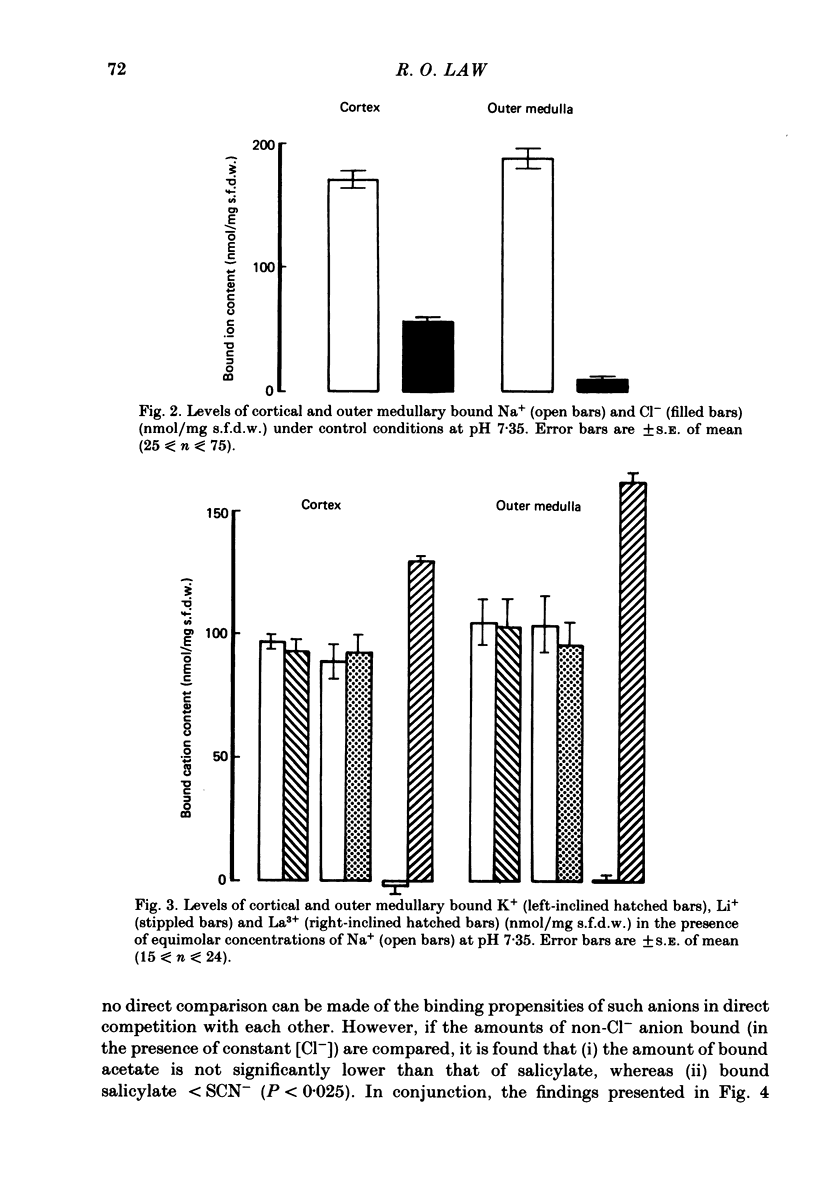
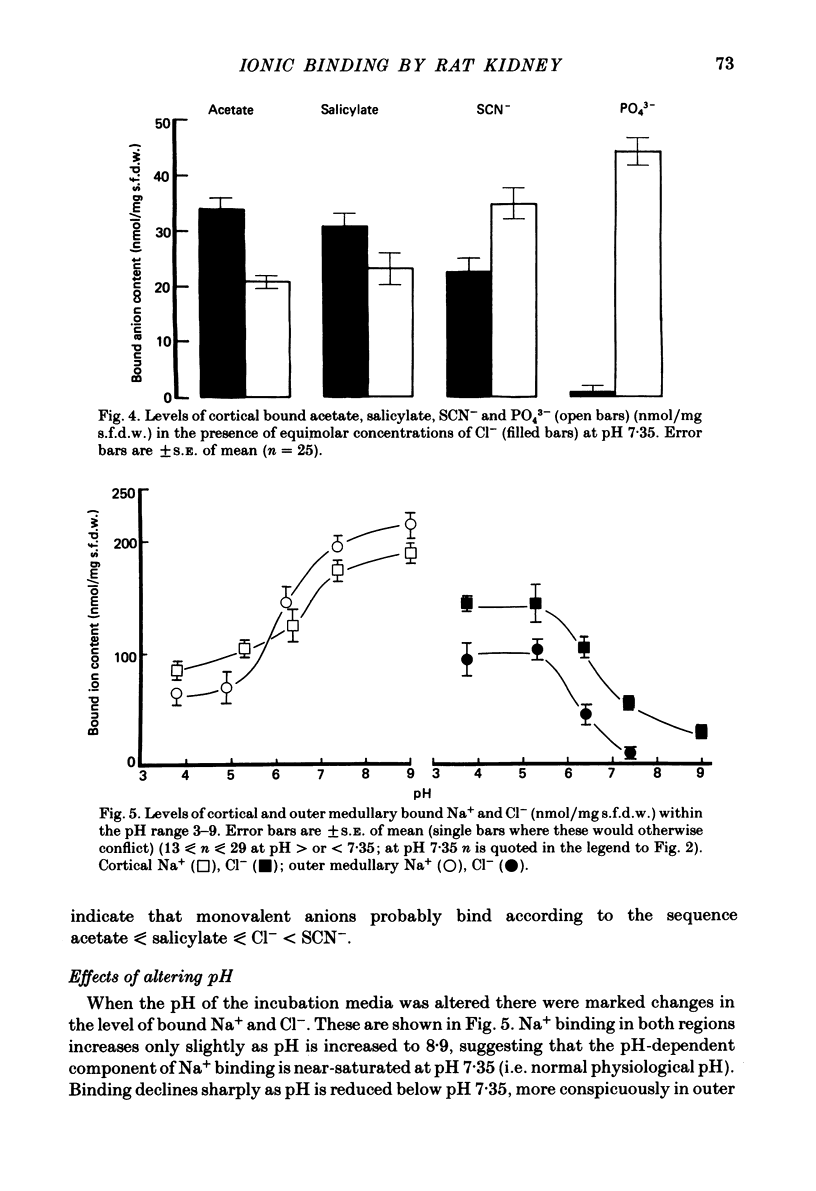
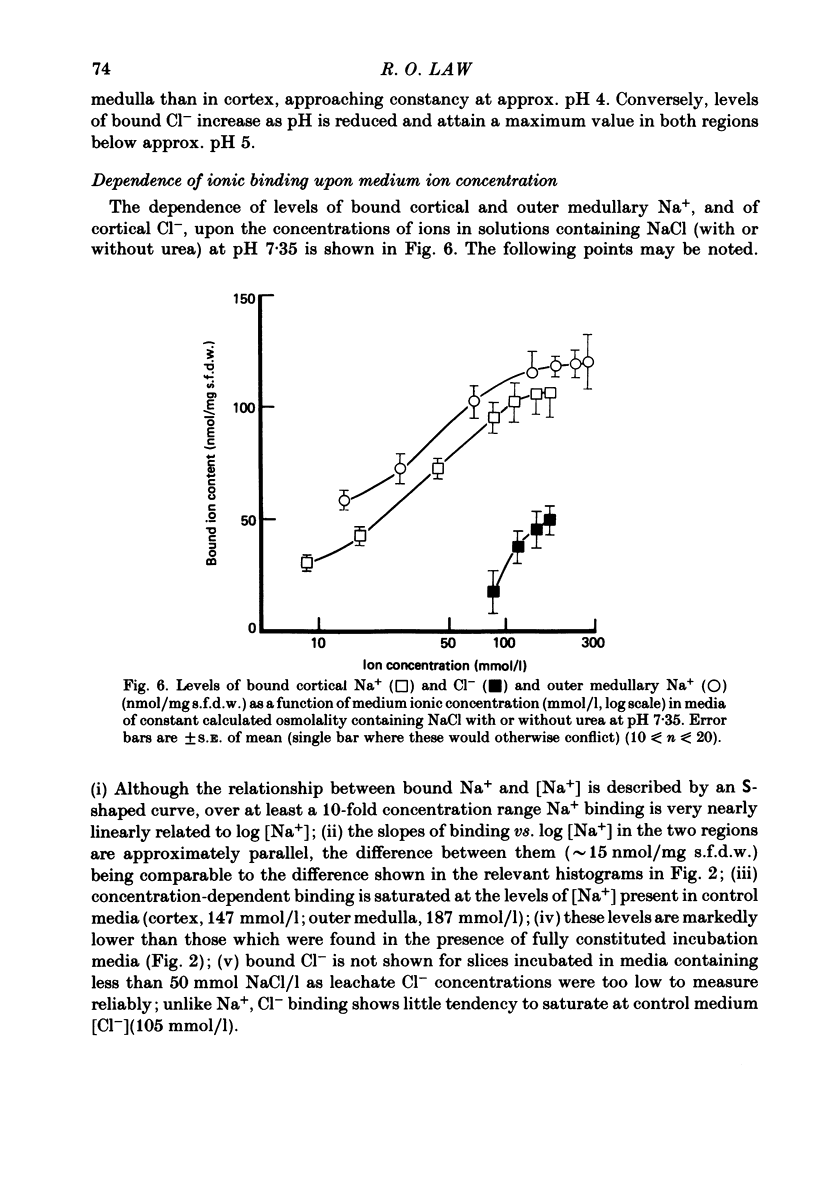
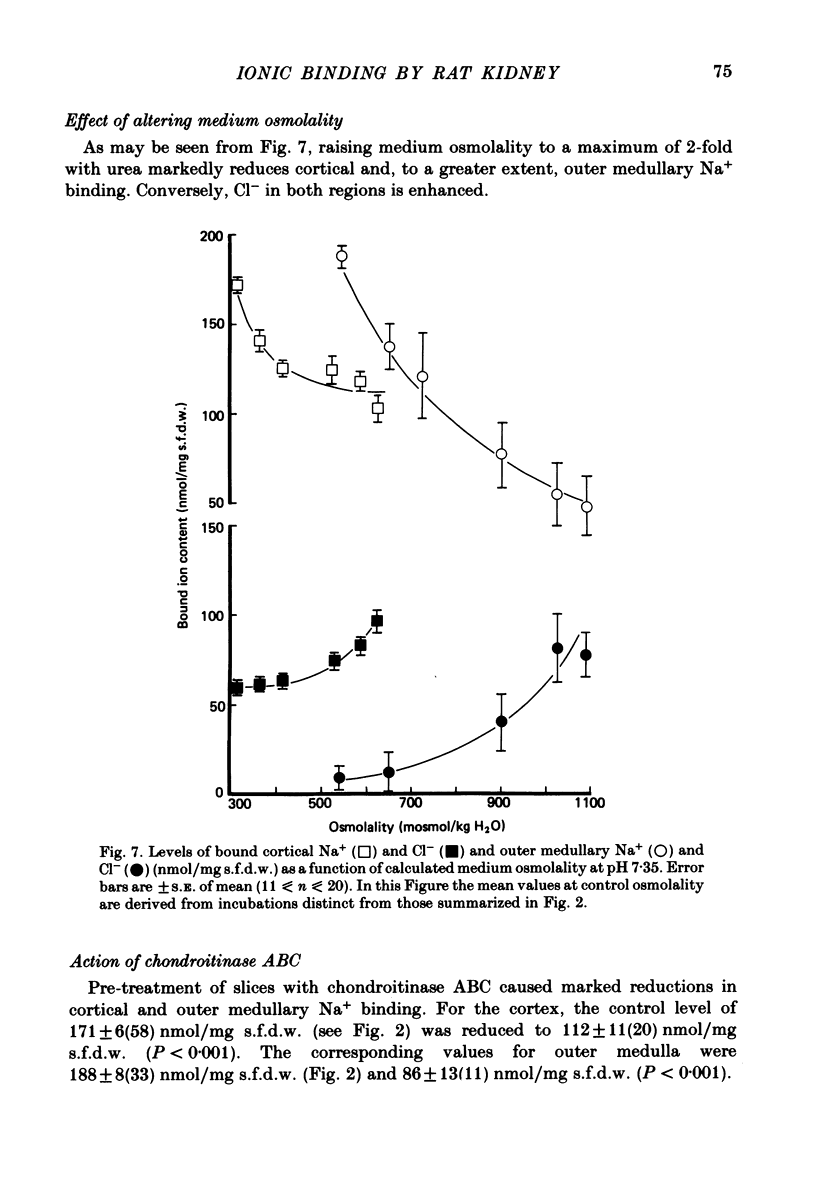
A study has been made of Na+ and Cl- binding in metabolically inhibited slices of rat renal cortex and outer medulla incubated in modified Krebs phosphate-bicarbonate Ringer solution. At pH 7.35 in control media (cortex, 147 mmol Na+/l, 105 mmol Cl-/l; outer medulla, 187 mmol Na+/l, 145 mmol Cl-/l) cortical slices bound (mean) 171 nmol Na+ and 56.7 nmol Cl-/mg solute-free dry weight; outer medullary slices bound 188 nmol Na+/mg and negligible amounts of Cl-. In both regions, Na+ was exchangeable on a 1:1 basis for K+ or Li+ in media containing equal concentrations of each cation: Na+ was completely displaced by La3+. In cortical slices in media containing equimolar Cl- and other monovalent anions, binding occurred according to the sequence acetate less than or equal to salicylate less than or equal to Cl- less than SCN-; Cl- was completely displaced by PO4(3-). When medium pH was lowered, Na+ binding was markedly reduced in both regions, whereas Cl- binding increased (and became significant in outer medulla). In NaCl solutions, Na+ binding capacity was saturated at control Na+ concentrations. When [Na+] was progressively reduced (iso-osmolality being maintained by addition of urea), bound Na+ in both regions was nearly linearly related to log medium [Na+]. Raising medium osmolality with urea caused decreased Na+ binding and increased Cl- binding in both regions. Na+ binding in both regions was significantly reduced by pre-treatment with chondroitinase ABC. Binding of both ions was independent of temperature within the range 2-37 degrees C. The possibility is raised that renal ionic binding might influence vectorial ion transport by affecting free ion activity in the region of the transporting cells.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLALOUF D., BER A., SHARON N. ACID MUCOPOLYSACCHARIDES IN RAT KIDNEYS. Biochim Biophys Acta. 1964 Nov 1;83:278–287. doi: 10.1016/0926-6526(64)90005-9. [DOI] [PubMed] [Google Scholar]
- Burg M. B. Isolated perfused tubule. Introduction: background and development of microperfusion technique. Kidney Int. 1982 Nov;22(5):417–424. doi: 10.1038/ki.1982.194. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Greene J. A. Regional distribution of acid mucopolysaccharides in the kidney. J Clin Invest. 1968 Sep;47(9):2125–2132. doi: 10.1172/JCI105898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Constantopoulos G., Louie M., Dekaban A. S. Acid mucopolysaccharides (glycosaminoglycans) in normal human kidneys and in kidneys of patients with mucopolysaccharidoses. Biochem Med. 1973 Jun;7(3):376–388. doi: 10.1016/0006-2944(73)90058-6. [DOI] [PubMed] [Google Scholar]
- Dani J. A., Sanchez J. A., Hille B. Lyotropic anions. Na channel gating and Ca electrode response. J Gen Physiol. 1983 Feb;81(2):255–281. doi: 10.1085/jgp.81.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARBER S. J., SCHUBERT M., SCHUSTER N. The binding of cations by chondroitin sulfate. J Clin Invest. 1957 Dec;36(12):1715–1722. doi: 10.1172/JCI103573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J. P., Solomon S. pH and temperature dependence of glutamine uptake, carbon dioxide and ammonia production in kidney slices from acidotic rats. J Physiol. 1981 Jul;316:251–261. doi: 10.1113/jphysiol.1981.sp013785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEADINGS V. E., RONDELL P. A., BOHR D. F. Bound soduium in arterv wall. Am J Physiol. 1960 Nov;199:783–787. doi: 10.1152/ajplegacy.1960.199.5.783. [DOI] [PubMed] [Google Scholar]
- Hai M. A., Thomas S. The time-course of changes in renal tissue composition during lysine vasopressin infusion in the rat. Pflugers Arch. 1969;310(4):297–317. doi: 10.1007/BF00587241. [DOI] [PubMed] [Google Scholar]
- Hickling M. J., Barclay J. A., White S. J., White K. Isolation of sodium-complexing polypeptides from mammalian blood and cardiac muscle. Experientia. 1976 May 15;32(5):554–555. doi: 10.1007/BF01990156. [DOI] [PubMed] [Google Scholar]
- KREBS H. A. Body size and tissue respiration. Biochim Biophys Acta. 1950 Jan;4(1-3):249–269. doi: 10.1016/0006-3002(50)90032-1. [DOI] [PubMed] [Google Scholar]
- Law R. O., Rowen D. The influence of hyaluronidase on urinary and renal medullary composition following antidiuretic stimulus in the rat. J Physiol. 1981 Feb;311:341–354. doi: 10.1113/jphysiol.1981.sp013588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. Studies on the relationship between rat renal medullary cell volume and external anion concentration in hyperosmolal media. J Physiol. 1980 Oct;307:475–490. doi: 10.1113/jphysiol.1980.sp013448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. The inulin space, solute concentrations, and weight changes in rat renal medullary slices incubated in iso-osmolal media, and their modification during anoxia and hypothermia. J Physiol. 1975 May;247(1):37–54. doi: 10.1113/jphysiol.1975.sp010919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law R. O. Volume adjustment by renal medullary cells in hypo- and hyperosmolal solutions containing permeant and impermeant solutes. J Physiol. 1975 May;247(1):55–70. doi: 10.1113/jphysiol.1975.sp010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Macknight A. D., Mason D. R., Rose R. C., Sherman B. Ions and water in the epithelial cells of rabbit descending colon. J Physiol. 1982 Dec;333:111–123. doi: 10.1113/jphysiol.1982.sp014442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. The effect of lanthanum ions on acetylcholine in frog muscle. J Physiol. 1980 Dec;309:199–214. doi: 10.1113/jphysiol.1980.sp013504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K. Acidic glycosaminoglycans in human kidney tissue. Clin Chim Acta. 1975 Sep 1;63(2):157–169. doi: 10.1016/0009-8981(75)90158-8. [DOI] [PubMed] [Google Scholar]
- Palatý V., Gustafson B., Friedman S. M. Sodium binding in the arterial wall. Can J Physiol Pharmacol. 1969 Sep;47(9):763–770. doi: 10.1139/y69-129. [DOI] [PubMed] [Google Scholar]
- Pressler M. L., Elharrar V., Bailey J. C. Effects of extracellular calcium ions, verapamil, and lanthanum on active and passive properties of canine cardiac purkinje fibers. Circ Res. 1982 Nov;51(5):637–651. doi: 10.1161/01.res.51.5.637. [DOI] [PubMed] [Google Scholar]
- Rowen D., Law R. O. Renal medullary hexosamine content following antidiuresis and water-loading in the rat. Effects of antisera against rat urinary and testicular hyaluronidase. Pflugers Arch. 1981 May;390(2):152–155. doi: 10.1007/BF00590198. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H. Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol. 1979 May;81(2):316–335. doi: 10.1083/jcb.81.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton D. J., Hunt S., Huckerby T. N. The glycosaminoglycans of pig colonic wall connective tissue. Biochim Biophys Acta. 1983 May 25;757(2):219–225. doi: 10.1016/0304-4165(83)90112-5. [DOI] [PubMed] [Google Scholar]
- Treherne J. E., Schofield P. K., Lane N. J. Physiological and ultrastructural evidence for an extracellular anion matrix in the central nervous system of an insect (Periplaneta americana). Brain Res. 1982 Sep 16;247(2):255–267. doi: 10.1016/0006-8993(82)91250-1. [DOI] [PubMed] [Google Scholar]
- Villamil M. F., Rettori V., Barajas L., Kleeman C. R. Extracellular space and the ionic distribution in the isolated arterial wall. Am J Physiol. 1968 May;214(5):1104–1112. doi: 10.1152/ajplegacy.1968.214.5.1104. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Diamond J. M. Anion selectivity in biological systems. Physiol Rev. 1977 Jan;57(1):109–156. doi: 10.1152/physrev.1977.57.1.109. [DOI] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


