Abstract
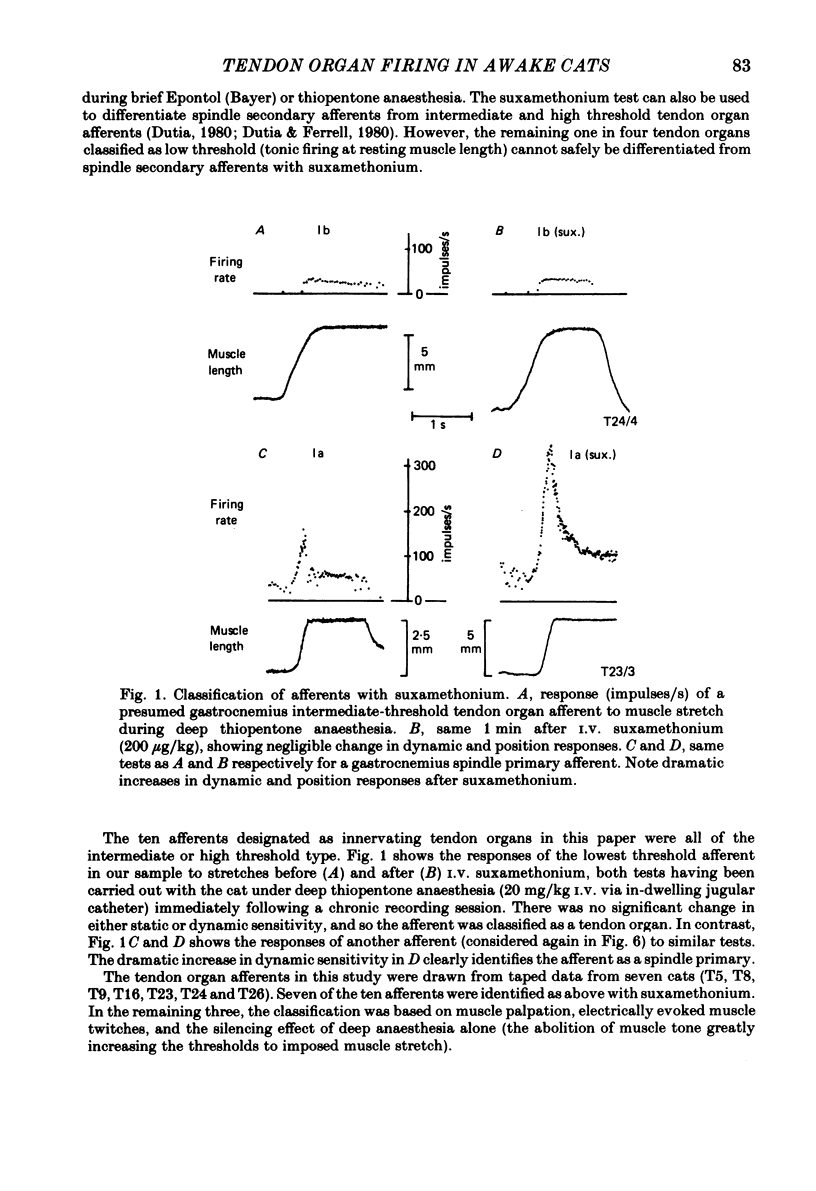
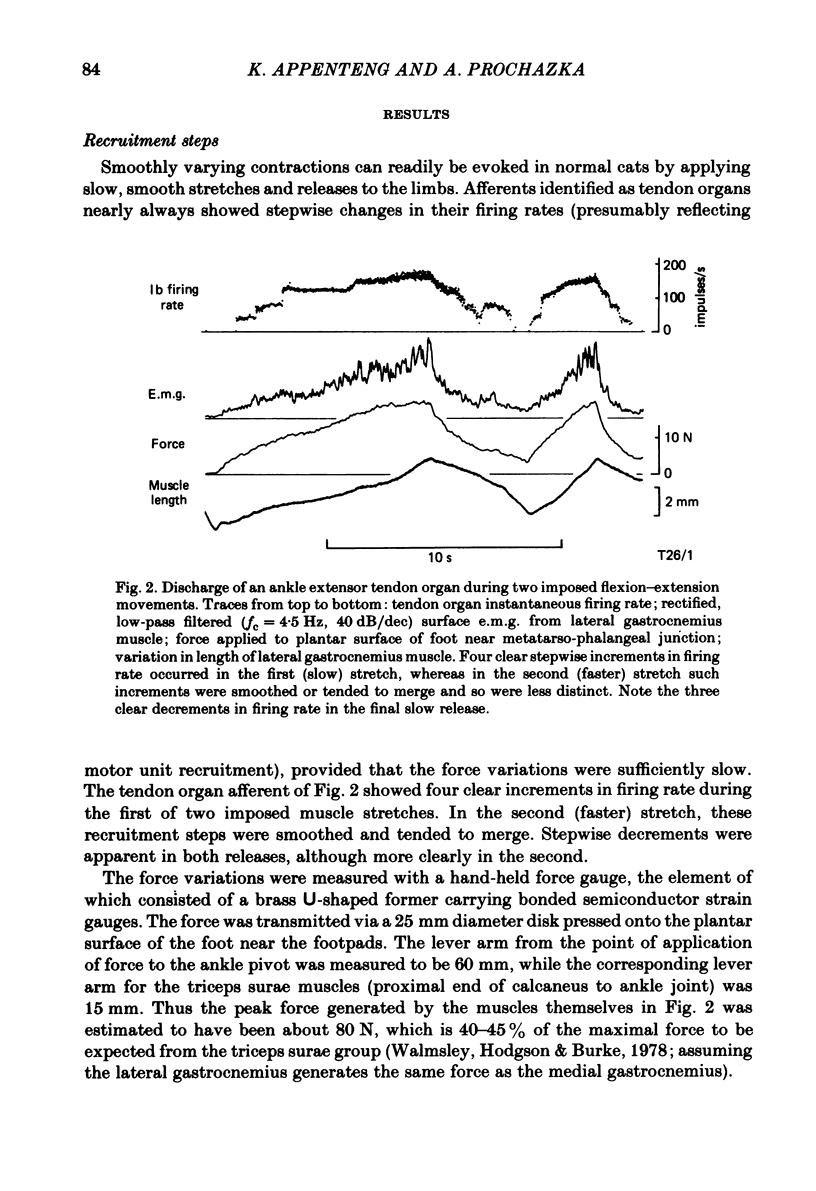
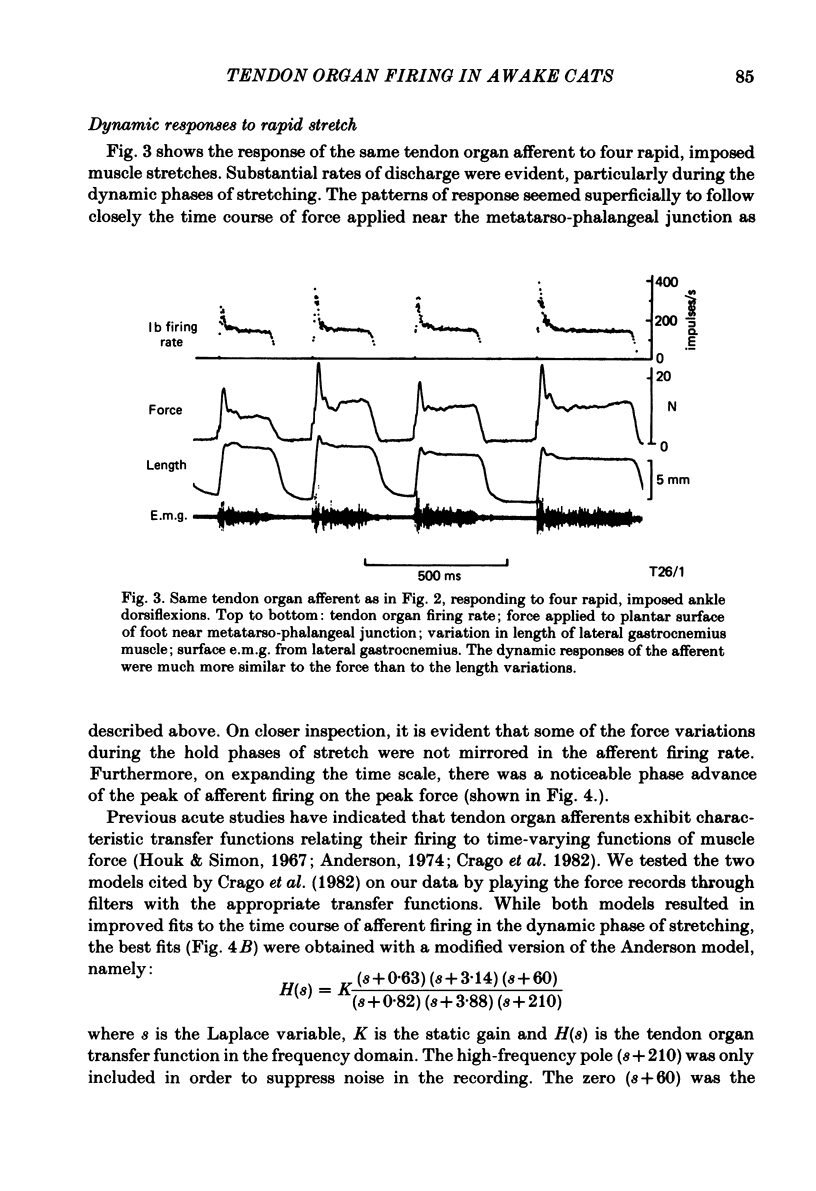
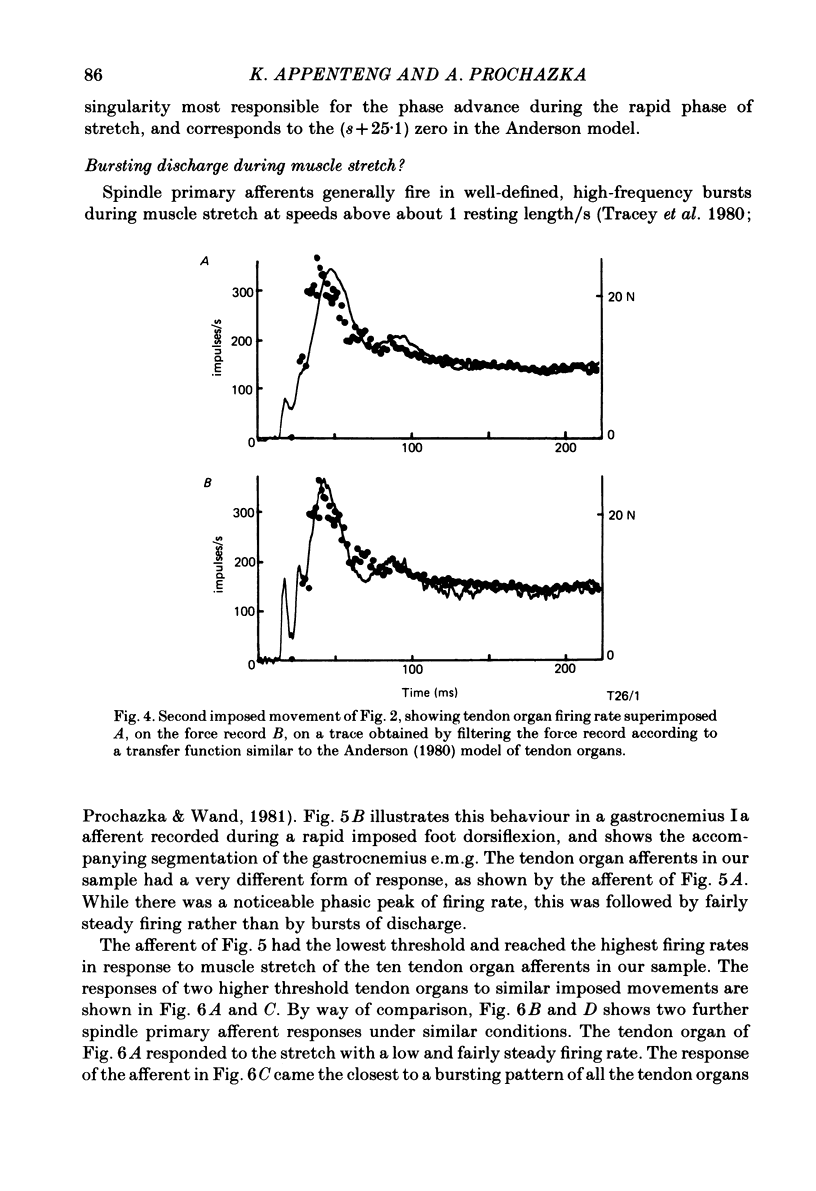
Recordings were obtained of the discharge of single tendon organ (Ib) and muscle spindle (Ia) afferents of the ankle extensor muscles during movement in normal cats. During very slow, smooth increases and decreases in muscle force, Ib afferents showed from one to five stepwise changes in firing rate, attributable to the recruitment of motor units inserting into the receptor capsule. These 'recruitment steps' in Ib firing rate became smoothed and tended to merge during faster variations in muscle force, and were rarely discernible in normal movements such as slow stepping. Rapid imposed stretches resulted in Ib firing patterns which fitted well a dynamic function of whole muscle force. Comparisons were made between the responses of Ib and Ia afferents during rapid, imposed muscle stretch. The segmentation of discharge typical of Ia afferents was not present in Ib afferents, despite segmentation of the e.m.g. of the receptor-bearing muscles. This would imply that Ib afferents exert a rapidly fluctuating reflex action against a relatively steady background of Ib input. Ankle extensor Ib firing during stepping was characterized by feeble firing during the swing phase and substantial, smoothly modulated firing during the stance phase. Taken together with previous chronic recordings, the data support the view that the ensemble of Ib afferents from a muscle signals a dynamic, non-linear function of whole muscle force over a wide range of normal movement.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. H. Dynamic characteristics of Golgi tendon organs. Brain Res. 1974 Mar 8;67(3):531–537. doi: 10.1016/0006-8993(74)90501-0. [DOI] [PubMed] [Google Scholar]
- Crago P. E., Houk J. C., Rymer W. Z. Sampling of total muscle force by tendon organs. J Neurophysiol. 1982 Jun;47(6):1069–1083. doi: 10.1152/jn.1982.47.6.1069. [DOI] [PubMed] [Google Scholar]
- Dutia M. B., Ferrell W. R. The effect of suxamethonium on the response to stretch of Golgi tendon organs in the cat. J Physiol. 1980 Sep;306:511–518. doi: 10.1113/jphysiol.1980.sp013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Stauffer E. K., Nemeth W. C., Stuart D. G. The cat step cycle; responses of muscle spindles and tendon organs to passive stretch within the locomotor range. Brain Res. 1973 Sep 28;60(1):35–54. doi: 10.1016/0006-8993(73)90849-4. [DOI] [PubMed] [Google Scholar]
- Hagbarth K. E., Hägglund J. V., Wallin E. U., Young R. R. Grouped spindle and electromyographic responses to abrupt wrist extension movements in man. J Physiol. 1981 Mar;312:81–96. doi: 10.1113/jphysiol.1981.sp013617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Jankowska E., Johannisson T. Shared reflex pathways of group I afferents of different cat hind-limb muscles. J Physiol. 1983 May;338:113–128. doi: 10.1113/jphysiol.1983.sp014664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J., Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967 May;30(3):466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- Houk J., Simon W. Responses of Golgi tendon organs to forces applied to muscle tendon. J Neurophysiol. 1967 Nov;30(6):1466–1481. doi: 10.1152/jn.1967.30.6.1466. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol. 1979 Mar;42(2):420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Loeb G. E. Somatosensory unit input to the spinal cord during normal walking. Can J Physiol Pharmacol. 1981 Jul;59(7):627–635. doi: 10.1139/y81-097. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Hulliger M. Muscle afferent function and its significance for motor control mechanisms during voluntary movements in cat, monkey, and man. Adv Neurol. 1983;39:93–132. [PubMed] [Google Scholar]
- Prochazka A., Wand P. Tendon organ discharge during voluntary movements in cats. J Physiol. 1980 Jun;303:385–390. doi: 10.1113/jphysiol.1980.sp013293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol. 1976 Sep;39(5):1090–1104. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- Tracey D. J., Walmsley B., Brinkman J. 'Long-loop' reflexes can be obtained in spinal monkeys. Neurosci Lett. 1980 May 15;18(1):59–65. doi: 10.1016/0304-3940(80)90213-x. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B. Afferent discharge from human muscle spindles in non-contracting muscles. Steady state impulse frequency as a function of joint angle. Acta Physiol Scand. 1974 Feb;90(2):303–318. doi: 10.1111/j.1748-1716.1974.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Vallbo A. B. Slowly adapting muscle receptors in man. Acta Physiol Scand. 1970 Mar;78(3):315–333. doi: 10.1111/j.1748-1716.1970.tb04667.x. [DOI] [PubMed] [Google Scholar]
- Walmsley B., Hodgson J. A., Burke R. E. Forces produced by medial gastrocnemius and soleus muscles during locomotion in freely moving cats. J Neurophysiol. 1978 Sep;41(5):1203–1216. doi: 10.1152/jn.1978.41.5.1203. [DOI] [PubMed] [Google Scholar]


