Abstract
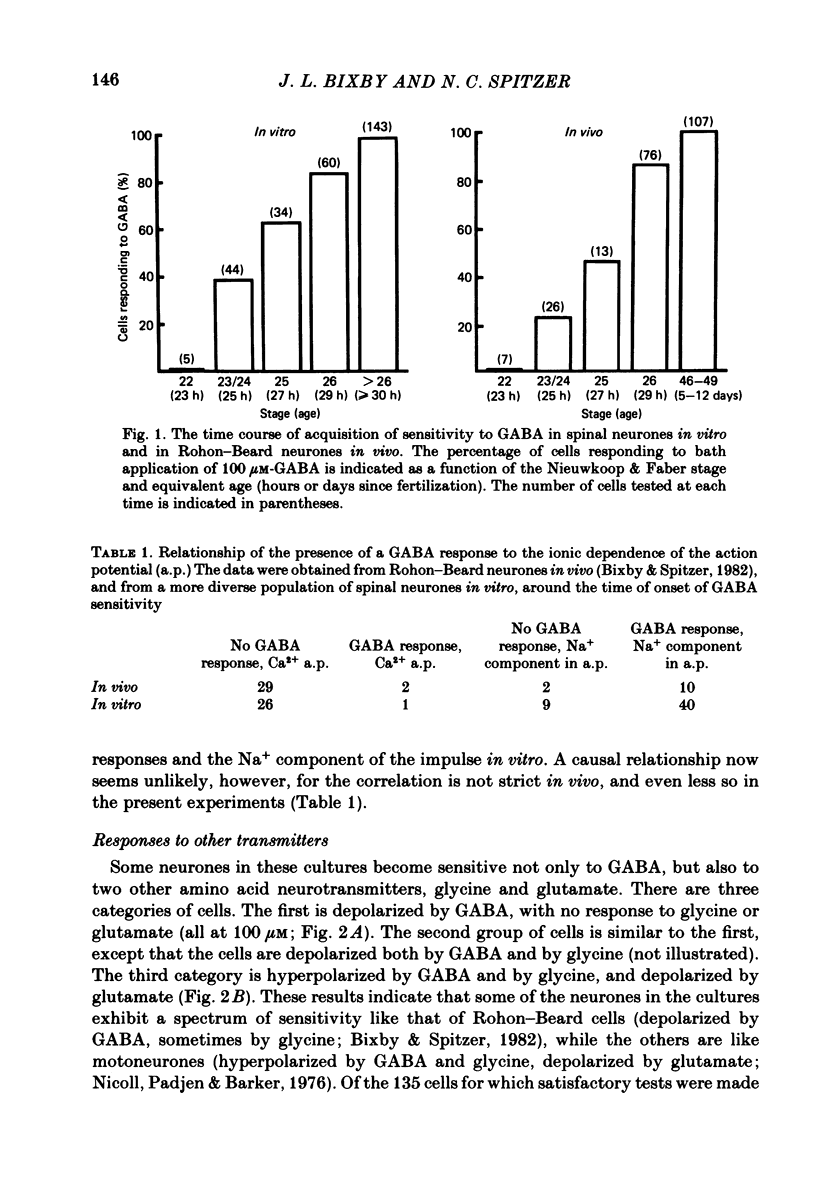
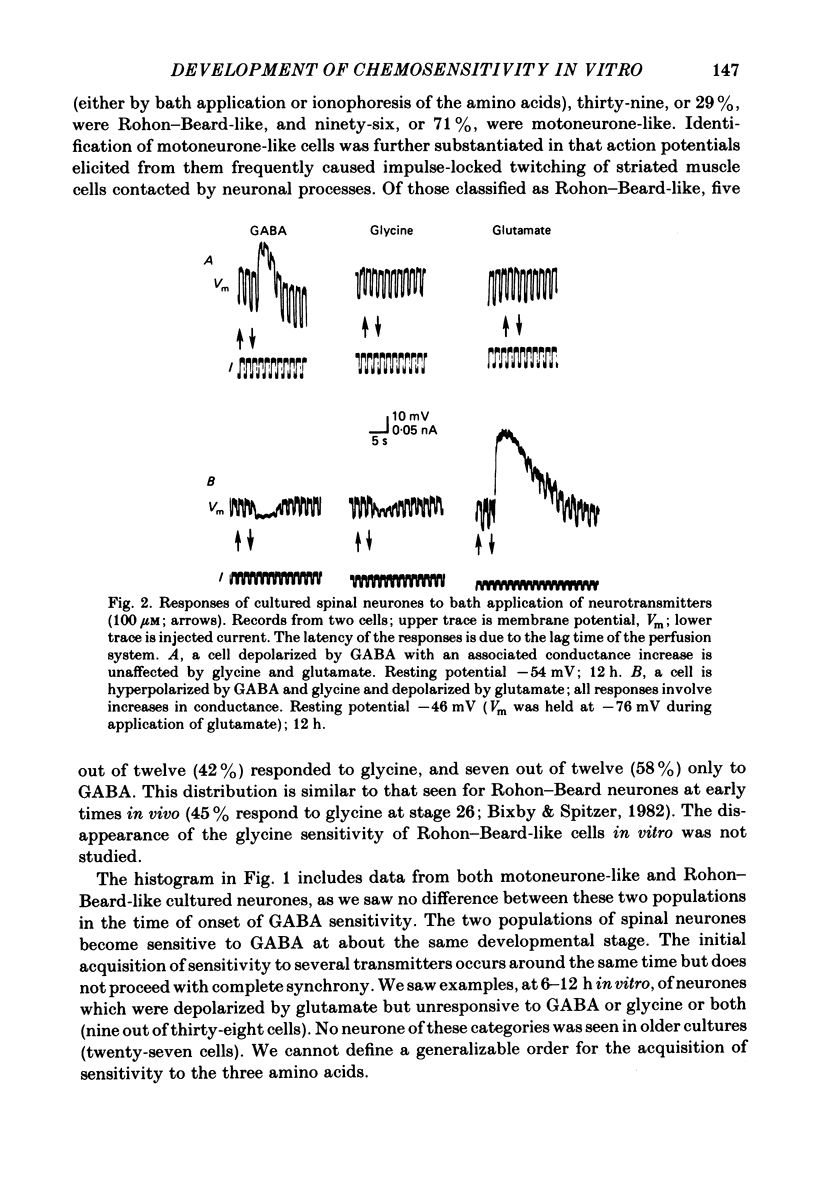
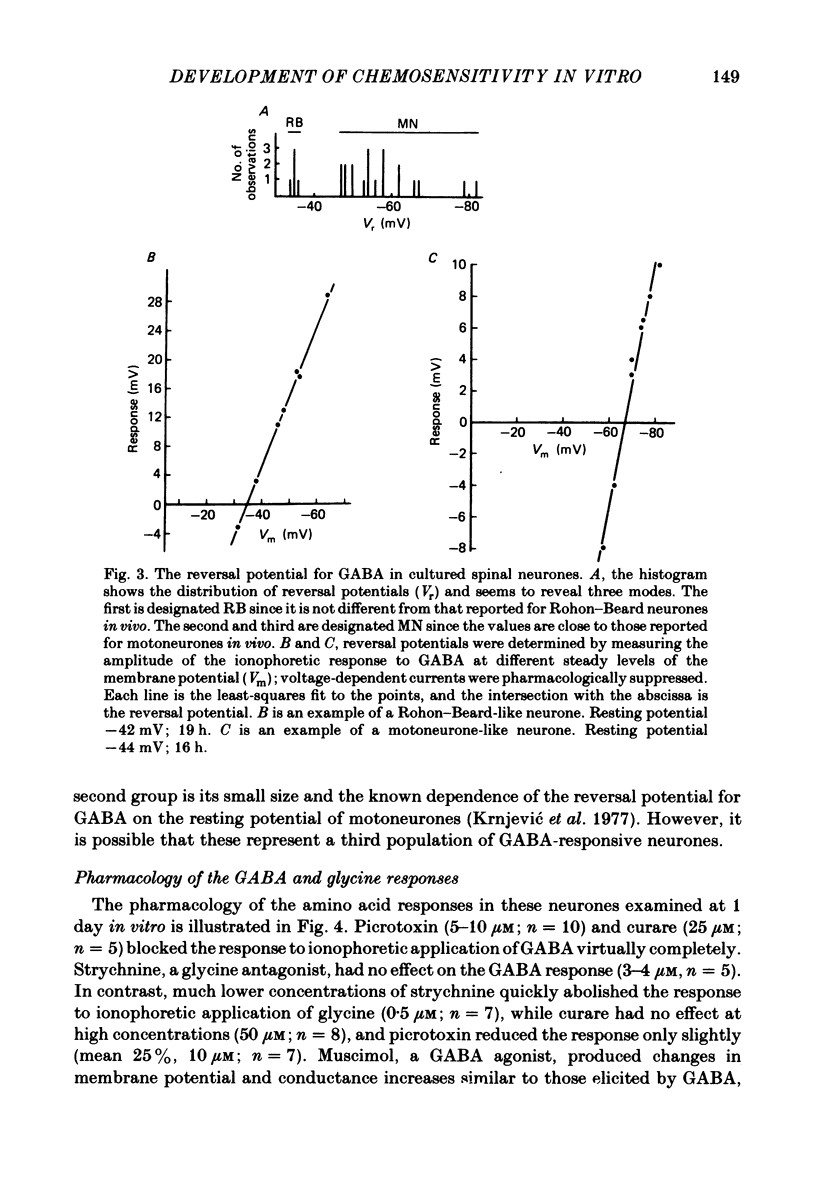
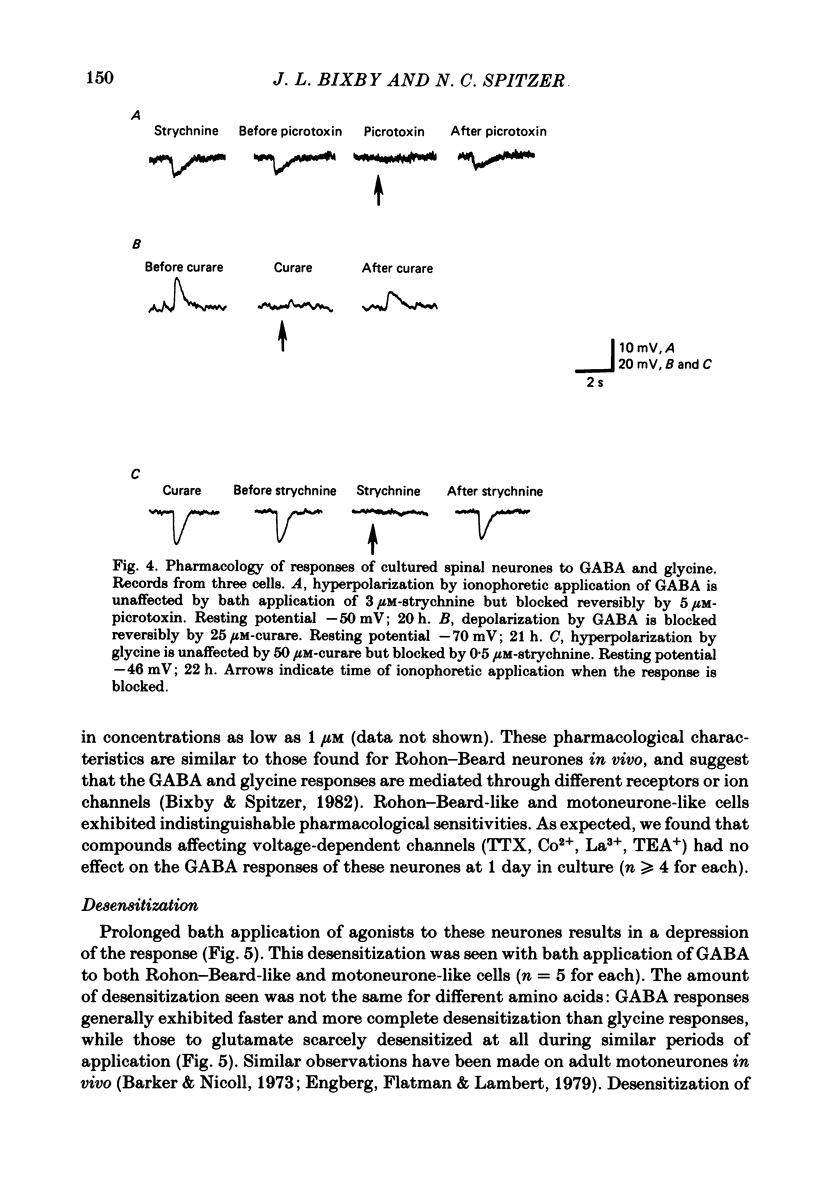
We have determined the time of onset and examined some of the properties of neurotransmitter sensitivity in Xenopus spinal neurones developing in dissociated cell culture. These cells are initially insensitive, but acquire responses to several agonists over a period of 6 h. Nearly one-third of the neurones were depolarized by gamma-aminobutyric acid (GABA) or by both GABA and glycine; these cells were not affected by glutamate. The reversal potential of the ionophoretic GABA response is -35 mV. These neurones are likely to be Rohon-Beard neurones. Roughly two-thirds of the neurones were depolarized by glutamate and hyperpolarized by GABA and by glycine. The reversal potential of the ionophoretic GABA response is -58 mV. These neurones are likely to include motoneurones. A quantitative measure of the sensitivity to a given GABA dose was obtained at early and intermediate stages of development. The mean 'sensitivity index' (ionophoretic sensitivity/input resistance) for both classes of neurones in vitro was initially the same as that seen in Rohon-Beard neurones in vivo. This sensitivity index did not increase with time in culture to attain the value at intermediate stages in vivo. The development of chemosensitivity in Rohon-Beard-like neurones in these cultures resembles that of Rohon-Beard neurones in the spinal cord with respect to the time of onset of responses to GABA, the reversal potential, pharmacology and desensitization of these responses, and the spectrum of agonists to which they are sensitive. It differs in the absence of a developmental increase in sensitivity to GABA. The development of chemosensitivity in motoneurone-like neurones in these cultures parallels that of Rohon-Beard-like neurones, with respect to the time of onset and level of sensitivity, as well as susceptibility to pharmacological blockers. Several features of normal neurotransmitter sensitivity, like features of the action potential, differentiate in culture in the absence of normal cellular interactions.
Full text
PDF



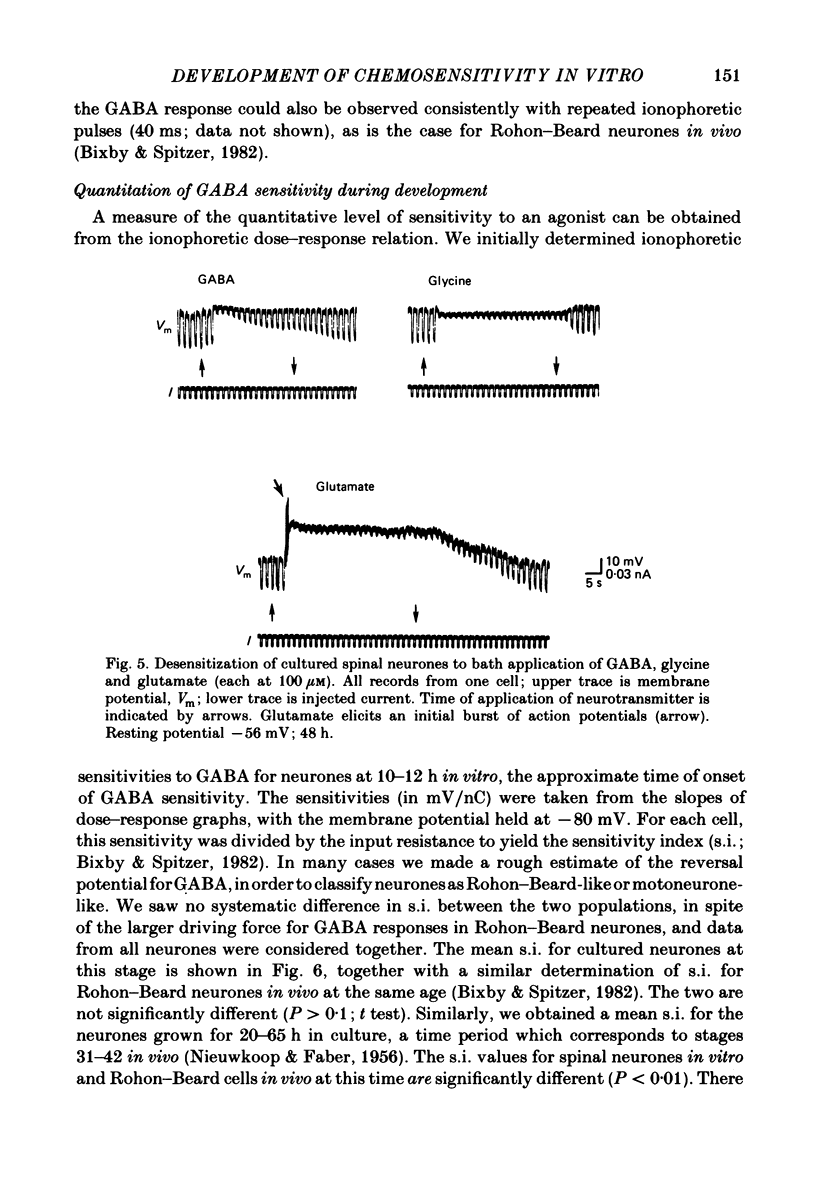
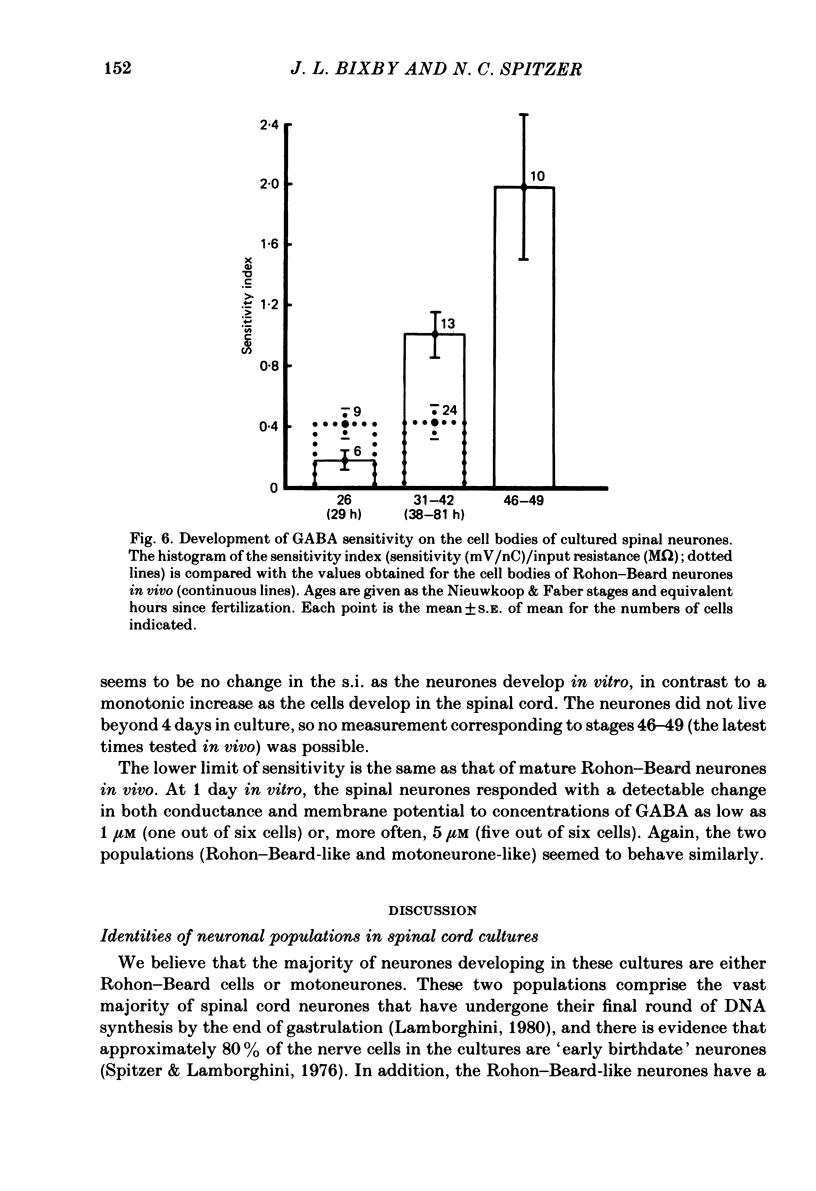



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccaglini P. I., Spitzer N. C. Developmental changes in the inward current of the action potential of Rohon-Beard neurones. J Physiol. 1977 Sep;271(1):93–117. doi: 10.1113/jphysiol.1977.sp011992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker J. L., Nicoll R. A. The pharmacology and ionic dependency of amino acid responses in the frog spinal cord. J Physiol. 1973 Jan;228(2):259–277. doi: 10.1113/jphysiol.1973.sp010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L., Spitzer N. C. Enkephalin reduces calcium action potentials in Rohon-Beard neurons in vivo. J Neurosci. 1983 May;3(5):1014–1018. doi: 10.1523/JNEUROSCI.03-05-01014.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bixby J. L., Spitzer N. C. The appearance and development of chemosensitivity in Rohon-Beard neurones of the Xenopus spinal cord. J Physiol. 1982 Sep;330:513–536. doi: 10.1113/jphysiol.1982.sp014356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair L. A. The timing of protein synthesis required for the development of the sodium action potential in embryonic spinal neurons. J Neurosci. 1983 Jul;3(7):1430–1436. doi: 10.1523/JNEUROSCI.03-07-01430.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Hösli L., Johnston G. A., Johnston I. H. The hyperpolarization of spinal motoneurones by glycine and related amino acids. Exp Brain Res. 1968;5(3):235–258. doi: 10.1007/BF00238666. [DOI] [PubMed] [Google Scholar]
- Engberg I., Flatman J. A., Lambert J. D. The actions of excitatory amino acids on motoneurones in the feline spinal cord. J Physiol. 1979 Mar;288:227–261. [PMC free article] [PubMed] [Google Scholar]
- Fambrough D., Rash J. E. Development of acetylcholine sensitivity during myogenesis. Dev Biol. 1971 Sep;26(1):55–68. doi: 10.1016/0012-1606(71)90107-2. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., O'Shea M., McCaman R., Spitzer N. C. Embryonic development of identified neurons: temporal pattern of morphological and biochemical differentiation. Science. 1979 Jun 15;204(4398):1219–1222. doi: 10.1126/science.36661. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Spitzer N. C. Embryonic development of identified neurones: differentiation from neuroblast to neurone. Nature. 1979 Jul 19;280(5719):208–214. doi: 10.1038/280208a0. [DOI] [PubMed] [Google Scholar]
- Goodman C. S., Spitzer N. C. The development of electrical properties of identified neurones in grasshopper embryos. J Physiol. 1981;313:385–403. doi: 10.1113/jphysiol.1981.sp013672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J. Embryonic growth and innervation of rat skeletal muscles. III. Neural regulation of junctional and extra-junctional acetylcholine receptor clusters. Philos Trans R Soc Lond B Biol Sci. 1981 Jul 16;293(1065):287–314. doi: 10.1098/rstb.1981.0078. [DOI] [PubMed] [Google Scholar]
- Krnjević K., Puil E., Werman R. GABA and glycine actions on spinal motoneurons. Can J Physiol Pharmacol. 1977 Jun;55(3):658–669. doi: 10.1139/y77-090. [DOI] [PubMed] [Google Scholar]
- Lamborghini J. E. Rohon-beard cells and other large neurons in Xenopus embryos originate during gastrulation. J Comp Neurol. 1980 Jan 15;189(2):323–333. doi: 10.1002/cne.901890208. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Padjen A., Barker J. L. Analysis of amino acid responses on frog motoneurones. Neuropharmacology. 1976 Jan;15(1):45–53. doi: 10.1016/0028-3908(76)90096-4. [DOI] [PubMed] [Google Scholar]
- Ritchie A. K., Fambrough D. M. Ionic properties of the acetylcholine receptor in cultured rat myotubes. J Gen Physiol. 1975 Jun;65(6):751–767. doi: 10.1085/jgp.65.6.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Hildebrand J. G. Acetylcholine and its metabolic enzymes in developing antennae of the moth, Manduca sexta. Dev Biol. 1976 Aug;52(1):105–120. doi: 10.1016/0012-1606(76)90011-7. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Hildebrand J. G. Origin and morphogenesis of sensory neurons in an insect antenna. Dev Biol. 1976 Jul 15;51(2):300–319. doi: 10.1016/0012-1606(76)90145-7. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Hildebrand J. G. Structure and development of antennae in a moth, Manduca sexta. Dev Biol. 1976 Jul 15;51(2):280–299. [PubMed] [Google Scholar]
- Schweitzer E. S., Sanes J. R., Hildebrand J. G. Ontogeny of electroantennogram responses in the moth, Manduca sexta. J Insect Physiol. 1976;22(7):955–960. doi: 10.1016/0022-1910(76)90078-0. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C., Lamborghini J. E. The development of the action potential mechanism of amphibian neurons isolated in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer N. C. Voltage- and stage-dependent uncoupling of Rohon-Beard neurones during embryonic development of Xenopus tadpoles. J Physiol. 1982 Sep;330:145–162. doi: 10.1113/jphysiol.1982.sp014334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard A. L. Electrical excitability of outgrowing neurites of embryonic neurones in cultures of dissociated neural plate of Xenopus laevis. J Physiol. 1980 Apr;301:115–128. doi: 10.1113/jphysiol.1980.sp013193. [DOI] [PMC free article] [PubMed] [Google Scholar]


