Abstract
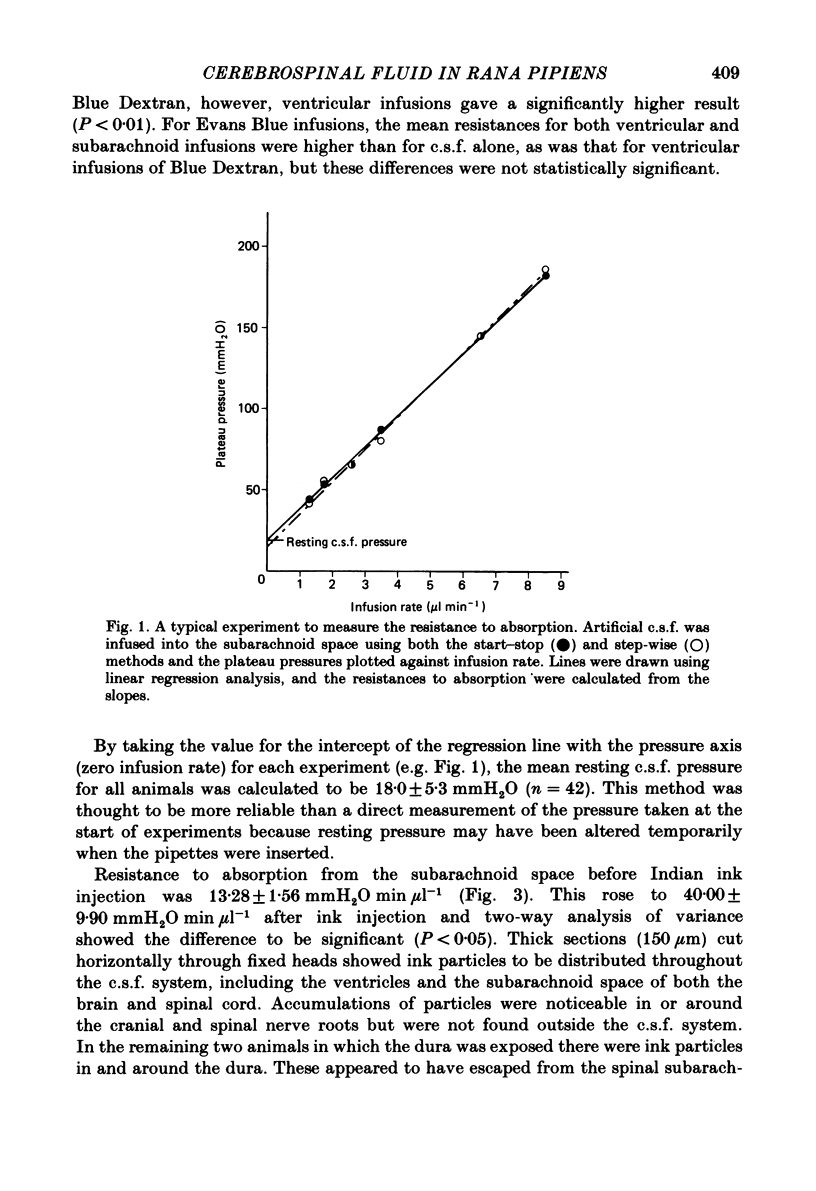
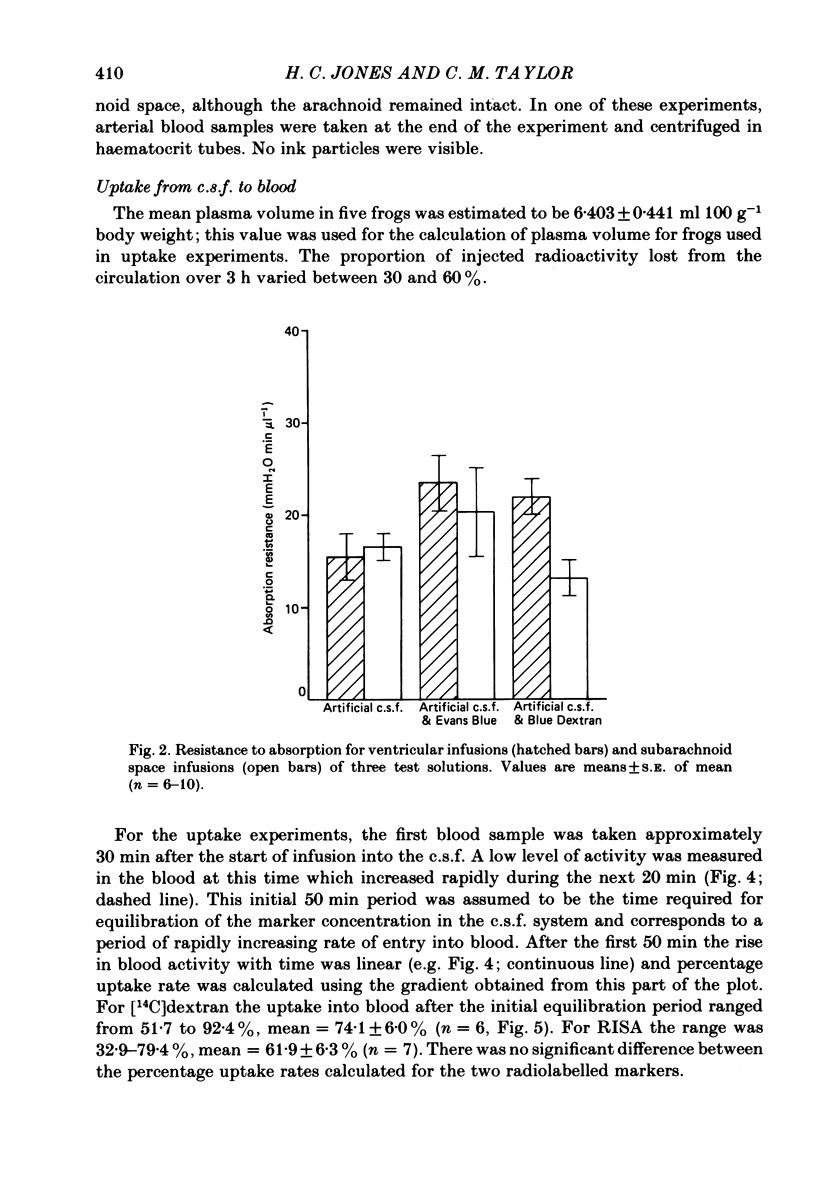
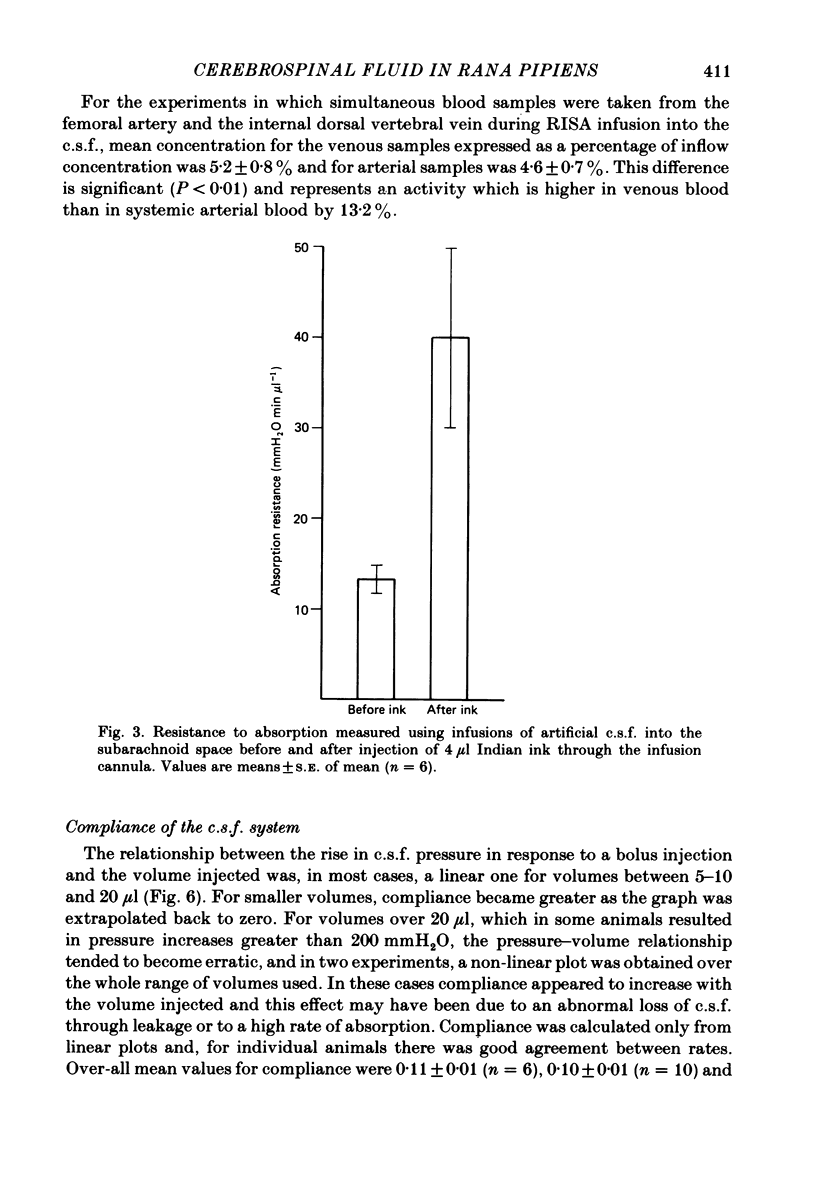
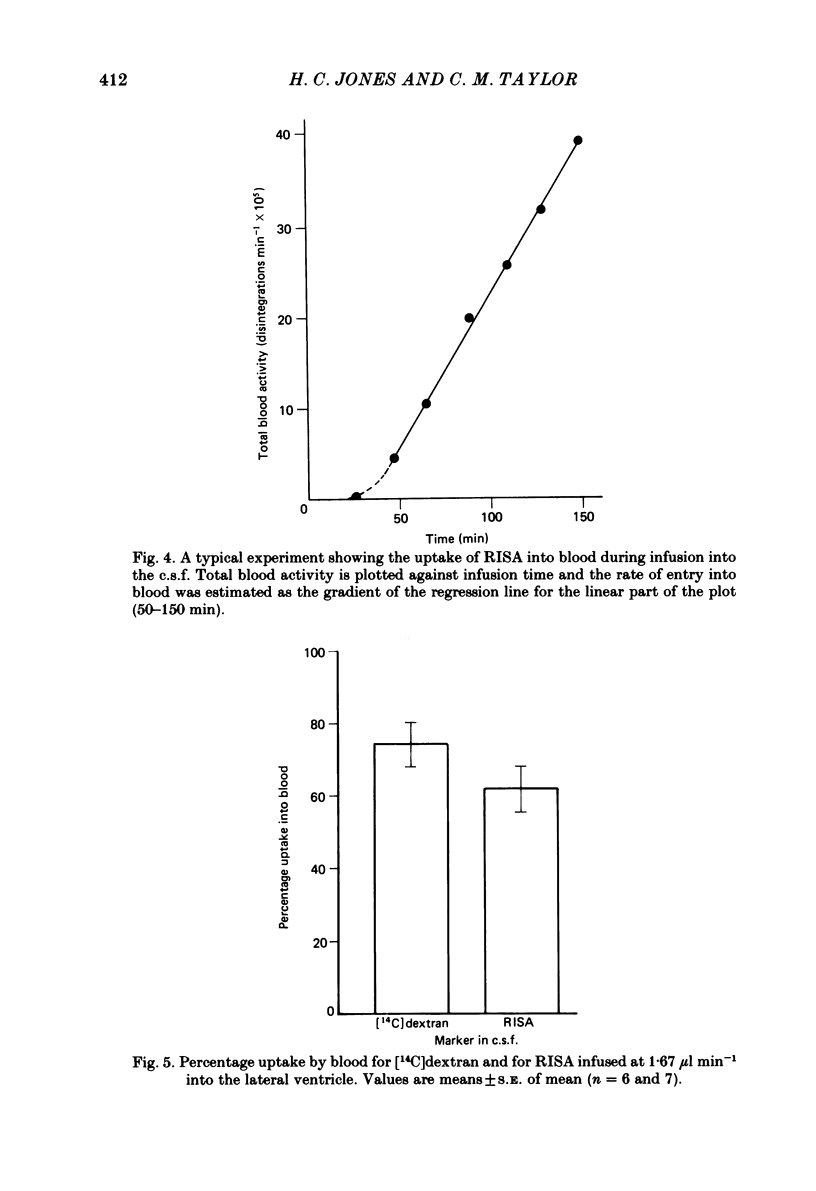
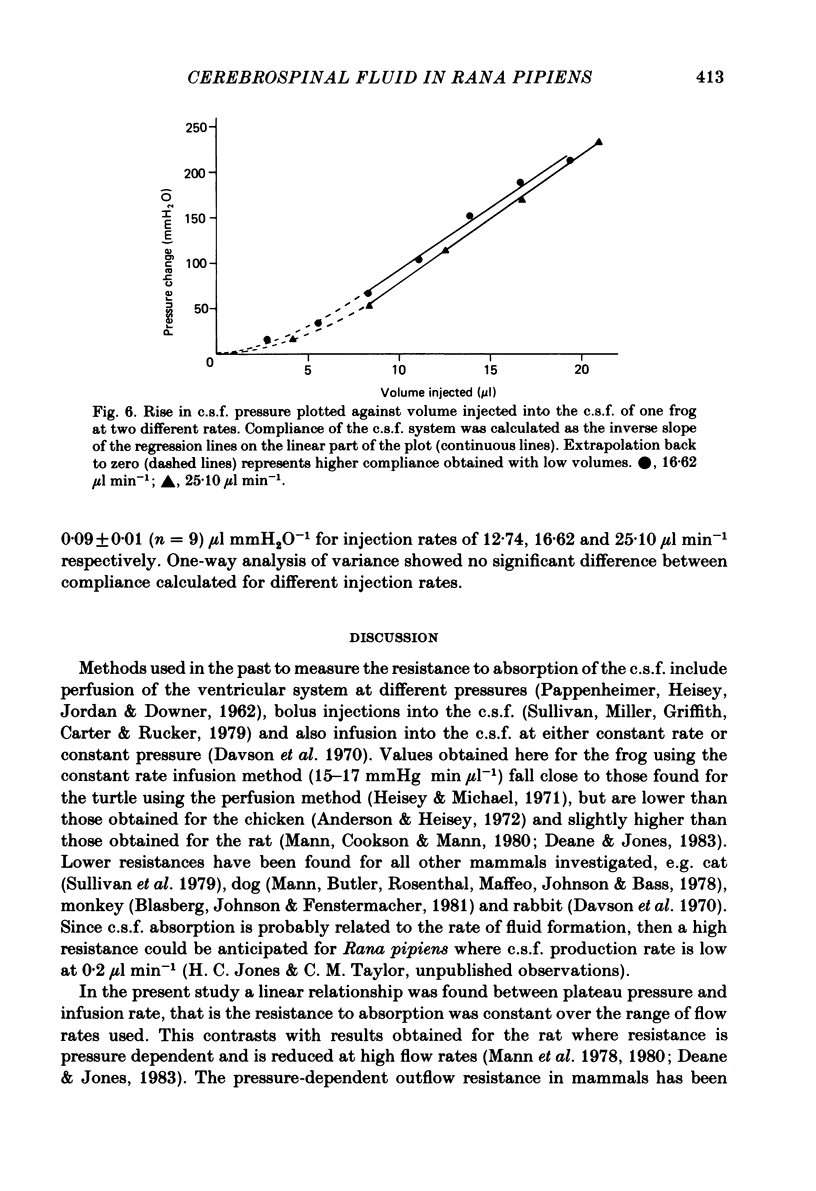
Experiments have been carried out to investigate the absorption of the cerebrospinal fluid (c.s.f.) and the intracranial compliance in an amphibian, Rana pipiens, using infusions into the c.s.f. system through glass micropipettes. Resistance to absorption of the c.s.f. was estimated by the constant rate infusion technique. Mean absorption resistance for infusions of artificial c.s.f. into the lateral ventricles and into the cerebral subarachnoid space were 15.48 and 16.52 mmH2O min microliter-1 respectively. This difference was not significant and it is concluded that the pores in the posterior tela situated in the roof of the fourth ventricle do not offer any resistance to the flow of c.s.f. out of the ventricles. The resistance to drainage of the c.s.f. in this amphibian is higher than that found for mammals. Mean resting c.s.f. pressure, estimated from the intercept of the regression line with the pressure axis at zero infusion rate was 18.0 mmH2O. Absorption resistance was measured from the cerebral subarachnoid space before and after injection of 4 microliter Indian ink solution. There was a 3-fold increase in resistance following ink injection. Two-way analysis of variance showed the difference to be significant (P less than 0.01) suggesting that the outflow sites can become partially blocked by particulate matter. During a continuous 3 h infusion of artificial c.s.f. containing [14C]dextran or [125I]-labelled human serum albumin (RISA) into the lateral ventricles, the mean percentage uptakes into the systemic circulation after the first 0.5 h of a 3 h period were 74.1 and 61.9% respectively. The difference is not significant. The rapid and high uptake into blood suggests there is a direct communication between c.s.f. and blood in amphibians. During continuous infusion of RISA into the lateral ventricles, simultaneous blood samples were taken from the femoral artery and the internal dorsal vertebral vein. Radioactivity was found to be 13.2% higher in venous samples. This suggests that at least some c.s.f. drainage takes place directly into the spinal venous system. Intracranial compliance was investigated by recording the peak pressure in response to a series of bolus injections of artificial c.s.f. into one lateral ventricle. Compliance was estimated to be 0.11, 0.10 and 0.09 microliter mmH2O-1 for injection rates of 12.74, 16.62 and 25.10 microliters min-1 respectively. The difference between these values is not significant. The results suggest that for injection volumes over 5 microliters the c.s.f. system behaves elastically.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. K., Heisley S. R. Clearance of molecules from cerebrospinal fluid in chickens. Am J Physiol. 1972 Mar;222(3):645–648. doi: 10.1152/ajplegacy.1972.222.3.645. [DOI] [PubMed] [Google Scholar]
- Blasberg R., Johnson D., Fenstermacher J. Absorption resistance of cerebrospinal fluid after subarachnoid hemorrhage in the monkey; effects of heparin. Neurosurgery. 1981 Dec;9(6):686–691. doi: 10.1227/00006123-198112000-00012. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Cole D. F. The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol. 1980 Feb;299:353–365. doi: 10.1113/jphysiol.1980.sp013129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury M. W., Westrop R. J. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983 Jun;339:519–534. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley J. B., Field E. J. The connexions of the spinal sub-arachnoid space with the lymphatic system. J Anat. 1948 Jul;82(Pt 3):153–166. [PMC free article] [PubMed] [Google Scholar]
- COURTICE F. C., SIMMONDS W. J. The removal of protein from the subarachnoid space. Aust J Exp Biol Med Sci. 1951 Jul;29(4):255–263. doi: 10.1038/icb.1951.30. [DOI] [PubMed] [Google Scholar]
- Cohen M. W., Gerschenfeld H. M., Kuffler S. W. Ionic environment of neurones and glial cells in the brain of an amphibian. J Physiol. 1968 Jul;197(2):363–380. doi: 10.1113/jphysiol.1968.sp008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserr H. F., Ostrach L. H. On the presence of subarachnoid fluid in the mudpuppy, Necturus maculosus. Comp Biochem Physiol A Comp Physiol. 1974 May 1;48(1):145–151. doi: 10.1016/0300-9629(74)90862-7. [DOI] [PubMed] [Google Scholar]
- Davson H., Hollingsworth G., Segal M. B. The mechanism of drainage of the cerebrospinal fluid. Brain. 1970;93(4):665–678. doi: 10.1093/brain/93.4.665. [DOI] [PubMed] [Google Scholar]
- Heisey S. R., Michael D. K. Cerebrospinal fluid formation and bulk absorption in the freshwater turtle. Exp Neurol. 1971 May;31(2):258–262. doi: 10.1016/0014-4886(71)90194-4. [DOI] [PubMed] [Google Scholar]
- JAYATILAKA D. P. ARACHNOID GRANULATIONS IN SHEEP. J Anat. 1965 Apr;99:315–327. [PMC free article] [PubMed] [Google Scholar]
- Jones H. C. Circulation of marker substances in the cerebrospinal fluid of an amphibian, Rana pipiens. Cell Tissue Res. 1980;211(2):317–330. doi: 10.1007/BF00236452. [DOI] [PubMed] [Google Scholar]
- Jones H. C. Continuity between the ventricular and subarachnoid cerebrospinal fluid in an amphibian, Rana pipiens. Cell Tissue Res. 1978 Dec 14;195(1):153–167. doi: 10.1007/BF00233683. [DOI] [PubMed] [Google Scholar]
- Jones H. C. Fenestration of the epithelium lining the roof of the fourth cerebral ventricle in amphibia. Cell Tissue Res. 1979 Apr 30;198(1):129–136. doi: 10.1007/BF00234840. [DOI] [PubMed] [Google Scholar]
- Löfgren J., von Essen C., Zwetnow N. N. The pressure-volume curve of the cerebrospinal fluid space in dogs. Acta Neurol Scand. 1973;49(5):557–574. doi: 10.1111/j.1600-0404.1973.tb01330.x. [DOI] [PubMed] [Google Scholar]
- Mann J. D., Butler A. B., Johnson R. N., Bass N. H. Clearance of macromolecular and particulate substances from the cerebrospinal fluid system of the rat. J Neurosurg. 1979 Mar;50(3):343–348. doi: 10.3171/jns.1979.50.3.0343. [DOI] [PubMed] [Google Scholar]
- Mann J. D., Butler A. B., Rosenthal J. E., Maffeo C. J., Johnson R. N., Bass N. H. Regulation of intracranial pressure in rat, dog, and man. Ann Neurol. 1978 Feb;3(2):156–165. doi: 10.1002/ana.410030212. [DOI] [PubMed] [Google Scholar]
- Marmarou A., Shulman K., Rosende R. M. A nonlinear analysis of the cerebrospinal fluid system and intracranial pressure dynamics. J Neurosurg. 1978 Mar;48(3):332–344. doi: 10.3171/jns.1978.48.3.0332. [DOI] [PubMed] [Google Scholar]
- McComb J. G., Davson H., Hyman S., Weiss M. H. Cerebrospinal fluid drainage as influenced by ventricular pressure in the rabbit. J Neurosurg. 1982 Jun;56(6):790–797. doi: 10.3171/jns.1982.56.6.0790. [DOI] [PubMed] [Google Scholar]
- PROCKOP L. D., SCHANKER L. S., BRODIE B. B. Passage of lipid-insoluble substances from cerebrospinal fluid to blood. J Pharmacol Exp Ther. 1962 Mar;135:266–270. [PubMed] [Google Scholar]
- SIMMONDS W. J. The absorption of labelled erythrocytes from the subarachnoid space in rabbits. Aust J Exp Biol Med Sci. 1953 Feb;31(1):77–83. doi: 10.1038/icb.1953.10. [DOI] [PubMed] [Google Scholar]
- Sahar A., Hochwald G. M., Ransohoff J. Passage of cerebrospinal fluid into cranial venous sinuses in normal and experimental hydrocephalic cats. Exp Neurol. 1970 Jul;28(1):113–122. doi: 10.1016/0014-4886(70)90166-4. [DOI] [PubMed] [Google Scholar]
- Sullivan H. G., Miller J. D., Griffith R. L., 3rd, Carter W., Jr, Rucker S. Bolous versus steady-state infusion for determination of CSF outflow resistance. Ann Neurol. 1979 Mar;5(3):228–238. doi: 10.1002/ana.410050304. [DOI] [PubMed] [Google Scholar]
- Tornheim P. A., Foltz F. M. Circulation of cerebrospinal fluid in the bullfrog, Rana catesbiana. Anat Rec. 1979 Jul;194(3):389–403. doi: 10.1002/ar.1091940306. [DOI] [PubMed] [Google Scholar]
- WELCH K., POLLAY M. Perfusion of particles through arachnoid villi of the monkey. Am J Physiol. 1961 Oct;201:651–654. doi: 10.1152/ajplegacy.1961.201.4.651. [DOI] [PubMed] [Google Scholar]


