Abstract
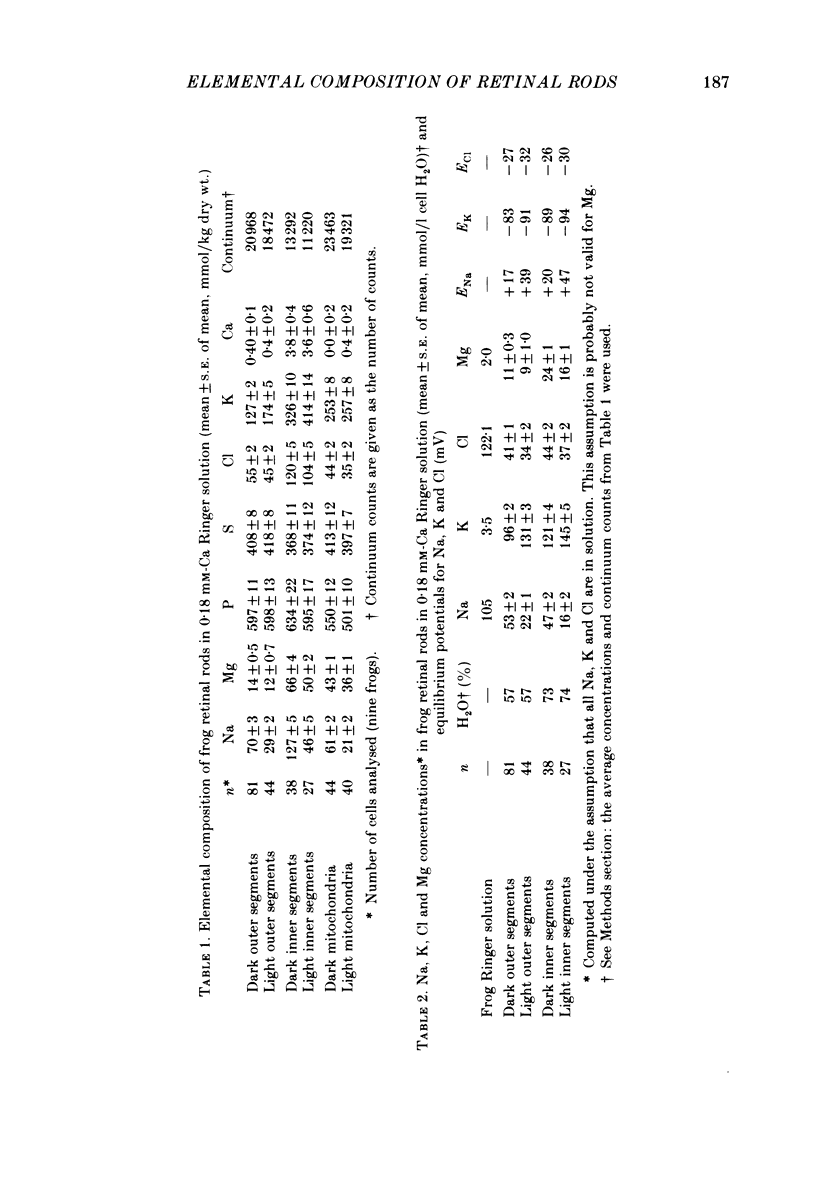
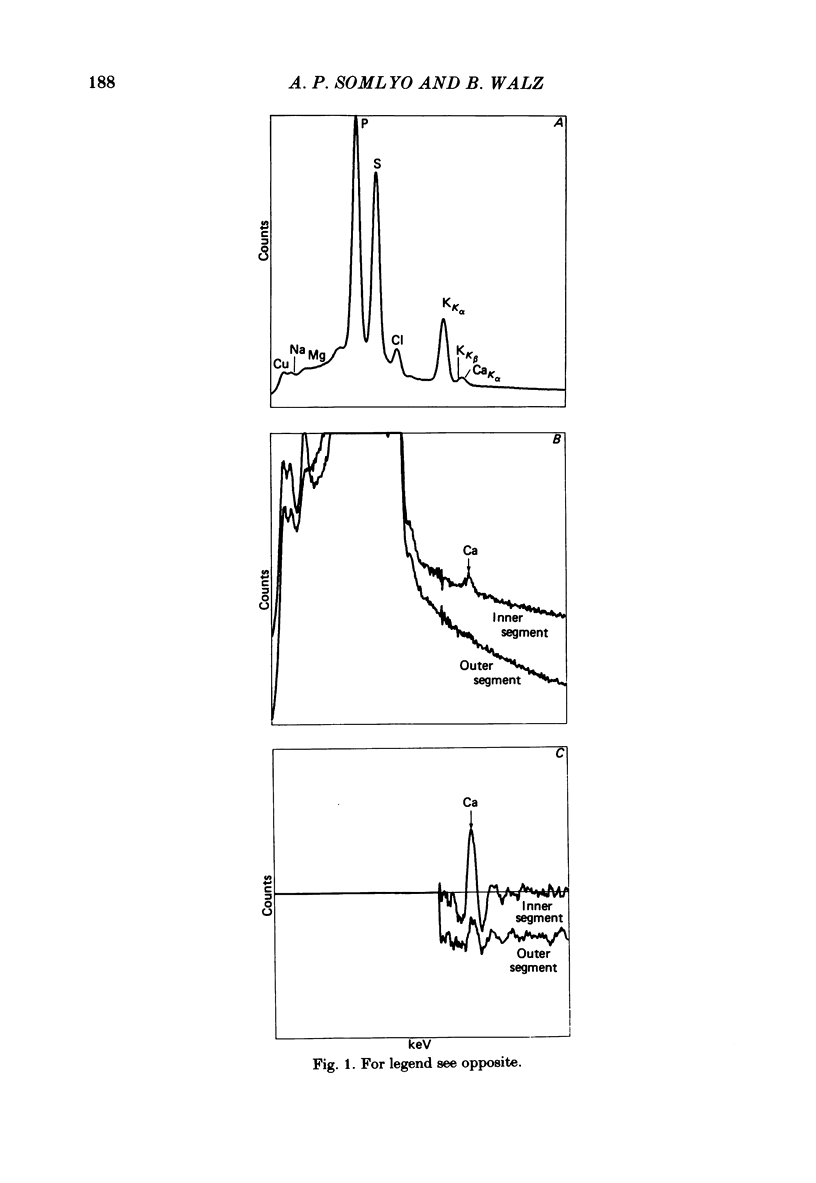
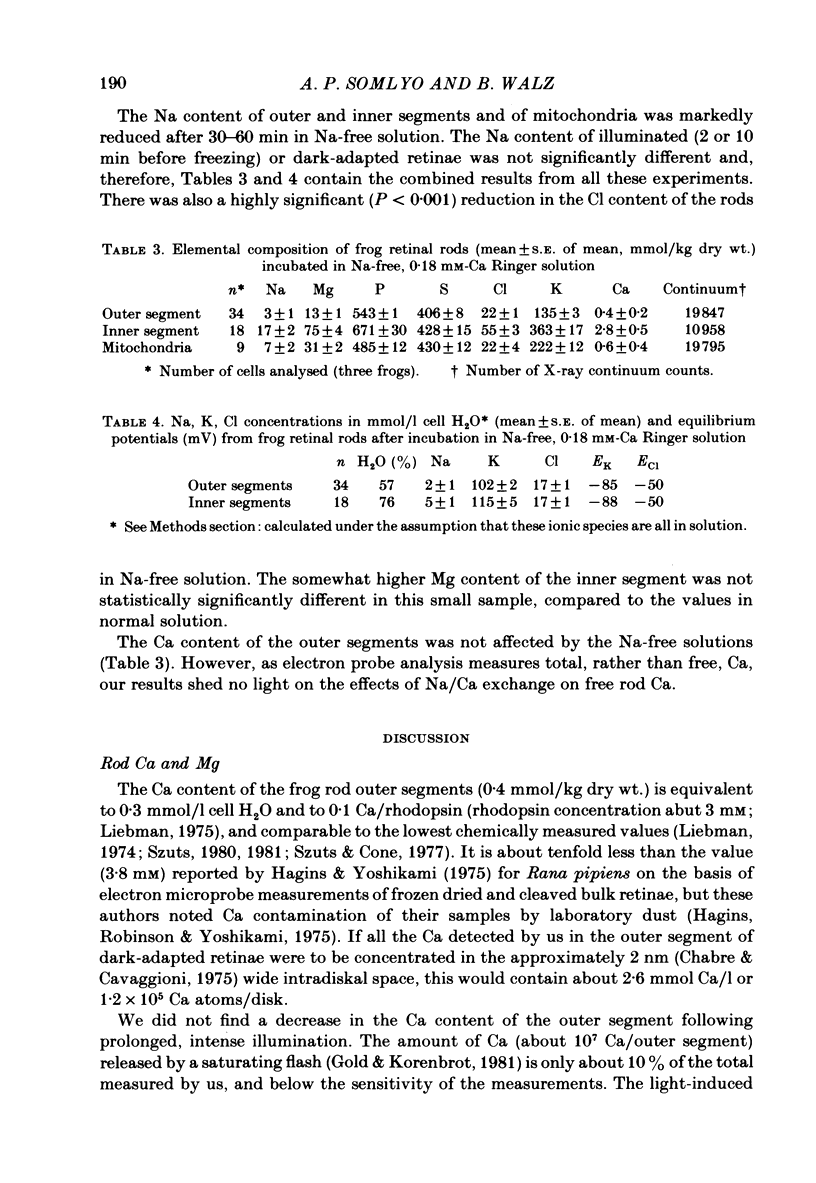
The composition of dark-adapted and illuminated retinal rod outer and inner segments and mitochondria was determined with electron probe X-ray micro-analysis of cryosections. The concentration of Ca in the outer segment was 0.4 mmol/kg dry wt. (0.1 Ca/rhodopsin) and did not measurably change upon illumination with saturating light for 5 min. The non-mitochondrial regions of the inner segment contained the highest concentrations (up to 13 mmol/kg dry wt.) of Ca in rods; these regions probably represent the endoplasmic reticulum. The equilibrium potentials estimated from the measured elemental concentrations and the known water content of dark-adapted outer segments were (mV): ENa = +17, EK = -83, ECl = -27. The respective values in the inner segment were: ENa = +20, EK = -89, ECl = -26. The above values were obtained in frog rods bathed in 0.18 mM-Ca Ringer solution. In the outer segment of toad rods bathed in 1.8 mM-Ca Ringer, ENa = +33 mV. The Mg content of the rods was high. The (computed) concentration in the dark-adapted retinae was 11 mM in the outer segment and 24 mM in the inner segment. Illumination caused a reduction in Mg to 9 mM (outer segment) and 16 mM (inner segment). Illumination caused a highly significant reduction in Na and Cl concentrations, and an increase in K concentration in both outer and inner segments. Exposure to Na-free (choline Ringer) solution resulted in reduction in Na to just-detectable levels (3 +/- 1 mmol/kg dry wt.) in the outer segment and to 5 +/- 1 mM in the inner segment. This was associated with a significant loss of Cl and decrease in ECl to -50 mV. The low Na content of the outer segment in the Na-depleted rods is not compatible with an extracellular concentration (105 mM) of inexchangeable Na in the intradiskal space. Mitochondrial Na and Mg paralleled the changes in the cytoplasmic concentrations: both mitochondrial Na and Mg were significantly decreased in illuminated, compared to dark-adapted rods. There was no detectable Ca (0 +/- 0.2 mmol/kg dry wt.) in mitochondria of dark-adapted rods containing high concentrations of Na; mitochondrial Ca was slightly higher (0.5 +/- 0.2 mmol/kg dry wt.) in the mitochondria that contained low Na following illumination.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastian B. L., Fain G. L. The effects of low calcium and background light on the sensitivity of toad rods. J Physiol. 1982 Sep;330:307–329. doi: 10.1113/jphysiol.1982.sp014343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Blaurock A. E., Wilkins M. H. Structure of frog photoreceptor membranes. Nature. 1969 Aug 30;223(5209):906–909. doi: 10.1038/223906a0. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Pinto L. H. Ionic mechanism for the photoreceptor potential of the retina of Bufo marinus. J Physiol. 1974 Feb;236(3):575–591. doi: 10.1113/jphysiol.1974.sp010453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabre M., Cavaggioni A. X-ray diffraction studies of retinal rods. II. Light effect on the osmotic properties. Biochim Biophys Acta. 1975 Mar 25;382(3):336–343. doi: 10.1016/0005-2736(75)90275-8. [DOI] [PubMed] [Google Scholar]
- George J. S., Hagins W. A. Control of Ca2+ in rod outer segment disks by light and cyclic GMP. Nature. 1983 May 26;303(5915):344–348. doi: 10.1038/303344a0. [DOI] [PubMed] [Google Scholar]
- Gold G. H., Korenbrot J. I. Light-induced calcium release by intact retinal rods. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5557–5561. doi: 10.1073/pnas.77.9.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Robinson W. E., Yoshikami S. Ionic aspects of excitation in rod outer segments. Ciba Found Symp. 1975;(31):169–189. doi: 10.1002/9780470720134.ch10. [DOI] [PubMed] [Google Scholar]
- Hagins W. A. The visual process: Excitatory mechanisms in the primary receptor cells. Annu Rev Biophys Bioeng. 1972;1:131–158. doi: 10.1146/annurev.bb.01.060172.001023. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Ionic mechanisms in excitation of photoreceptors. Ann N Y Acad Sci. 1975 Dec 30;264:314–325. doi: 10.1111/j.1749-6632.1975.tb31492.x. [DOI] [PubMed] [Google Scholar]
- Hall T. A., Gupta B. L. The localization and assay of chemical elements by microprobe methods. Q Rev Biophys. 1983 Aug;16(3):279–339. doi: 10.1017/s0033583500005114. [DOI] [PubMed] [Google Scholar]
- Hodgkin A. L., McNaughton P. A., Nunn B. J., Yau K. W. Effect of ions on retinal rods from Bufo marinus. J Physiol. 1984 May;350:649–680. doi: 10.1113/jphysiol.1984.sp015223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp R. D., Silcox J. C., Somlyo A. V. Cryoultramicrotomy: evidence against melting and the use of a low temperature cement for specimen orientation. J Microsc. 1982 Feb;125(Pt 2):157–165. doi: 10.1111/j.1365-2818.1982.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Schnetkamp P. P. Calcium metabolism in vertebrate photoreceptors. Cell Calcium. 1982 May;3(2):83–112. doi: 10.1016/0143-4160(82)90008-2. [DOI] [PubMed] [Google Scholar]
- Kitazawa T., Somlyo A. P., Somlyo A. V. The effects of valinomycin on ion movements across the sarcoplasmic reticulum in frog muscle. J Physiol. 1984 May;350:253–268. doi: 10.1113/jphysiol.1984.sp015199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A. Light-dependent Ca++ content of rod outer segment disc membranes. Invest Ophthalmol. 1974 Sep;13(9):700–701. [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969 Jul 12;223(5202):201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Schröder W. H., Fain G. L. Light-dependent calcium release from photoreceptors measured by laser micro-mass analysis. Nature. 1984 May 17;309(5965):268–270. doi: 10.1038/309268a0. [DOI] [PubMed] [Google Scholar]
- Shuman H., Somlyo A. V., Somlyo A. P. Quantitative electron probe microanalysis of biological thin sections: methods and validity. Ultramicroscopy. 1976 Sep-Oct;1(4):317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- Sloane B. F., Scarpa A., Somlyo A. P. Vascular smooth muscle mitochondria: magnesium content and transport. Arch Biochem Biophys. 1978 Aug;189(2):409–416. doi: 10.1016/0003-9861(78)90228-x. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P. Cell physiology: cellular site of calcium regulation. Nature. 1984 Jun 7;309(5968):516–517. doi: 10.1038/309516b0. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Shuman H. Electron probe and electron energy loss analysis in biology. Ultramicroscopy. 1982;8(1-2):219–233. doi: 10.1016/0304-3991(82)90290-x. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H. Electron probe analysis of vascular smooth muscle. Composition of mitochondria, nuclei, and cytoplasm. J Cell Biol. 1979 May;81(2):316–335. doi: 10.1083/jcb.81.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo A. V., Shuman H., Somlyo A. P. Elemental distribution in striated muscle and the effects of hypertonicity. Electron probe analysis of cryo sections. J Cell Biol. 1977 Sep;74(3):828–857. doi: 10.1083/jcb.74.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuts E. Z. Calcium flux across disk membranes. Studies with intact rod photoreceptors and purified disks. J Gen Physiol. 1980 Sep;76(3):253–286. doi: 10.1085/jgp.76.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuts E. Z., Cone R. A. Calcium content of frog rod outer segments and discs. Biochim Biophys Acta. 1977 Jul 14;468(2):194–208. doi: 10.1016/0005-2736(77)90114-6. [DOI] [PubMed] [Google Scholar]
- Torre V. The contribution of the electrogenic sodium-potassium pump to the electrical activity of toad rods. J Physiol. 1982 Dec;333:315–341. doi: 10.1113/jphysiol.1982.sp014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl R., Kuras P. V., Anderson K., Abrahamson E. W. A light scattering study on the ion permeabilities of dark-adapted bovine rod outer segment disk membranes. Biochim Biophys Acta. 1980 Oct 2;601(3):462–477. doi: 10.1016/0005-2736(80)90550-7. [DOI] [PubMed] [Google Scholar]
- Ungar F., Piscopo I., Holtzman E. Calcium accumulation in intracellular compartments of frog retinal rod photoreceptors. Brain Res. 1981 Jan 26;205(1):200–206. doi: 10.1016/0006-8993(81)90733-2. [DOI] [PubMed] [Google Scholar]
- Walz B. Ca2+-sequestering smooth endoplasmic reticulum in an invertebrate photoreceptor. II. Its properties as revealed by microphotometric measurements. J Cell Biol. 1982 Jun;93(3):849–859. doi: 10.1083/jcb.93.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz B. Calcium-sequestering smooth endoplasmic reticulum in retinula cells of the blowfly. J Ultrastruct Res. 1982 Nov;81(2):240–248. doi: 10.1016/s0022-5320(82)90079-x. [DOI] [PubMed] [Google Scholar]
- Yoshikami S., George J. S., Hagins W. A. Light-induced calcium fluxes from outer segment layer of vertebrate retinas. Nature. 1980 Jul 24;286(5771):395–398. doi: 10.1038/286395a0. [DOI] [PubMed] [Google Scholar]



