Abstract
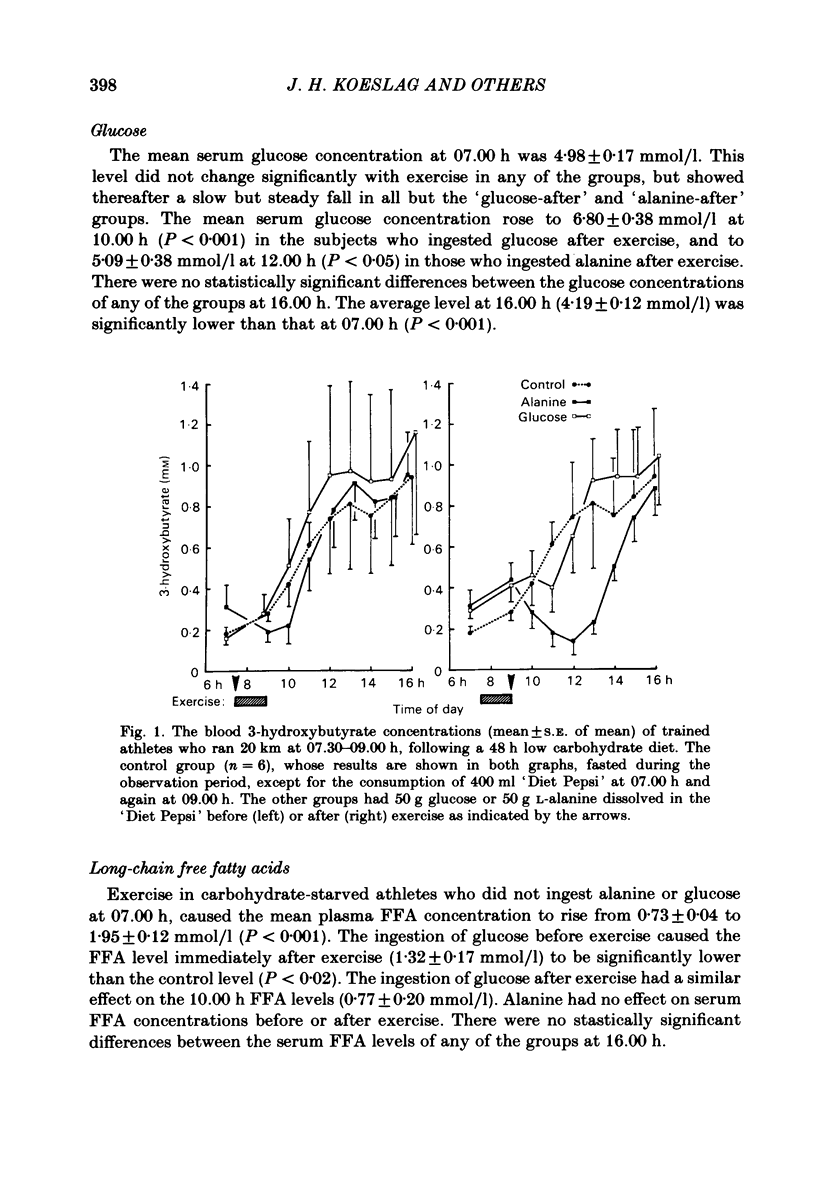
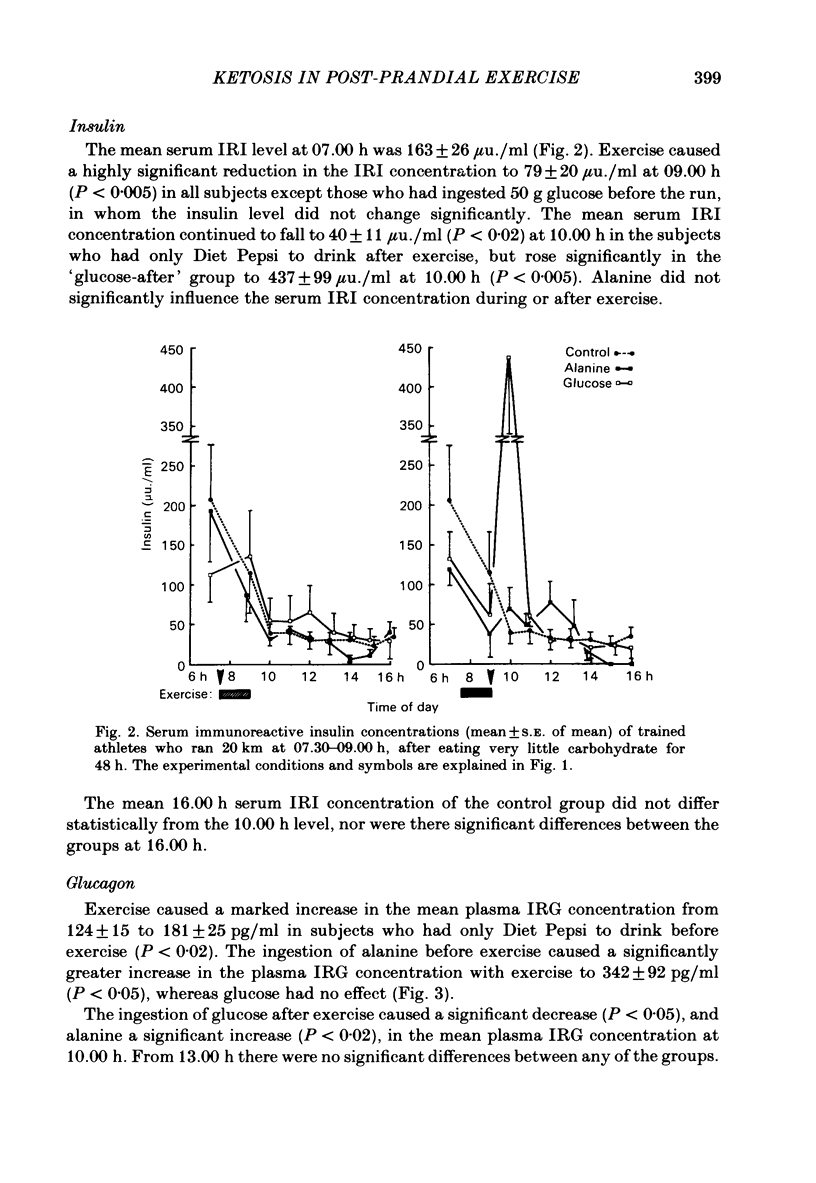
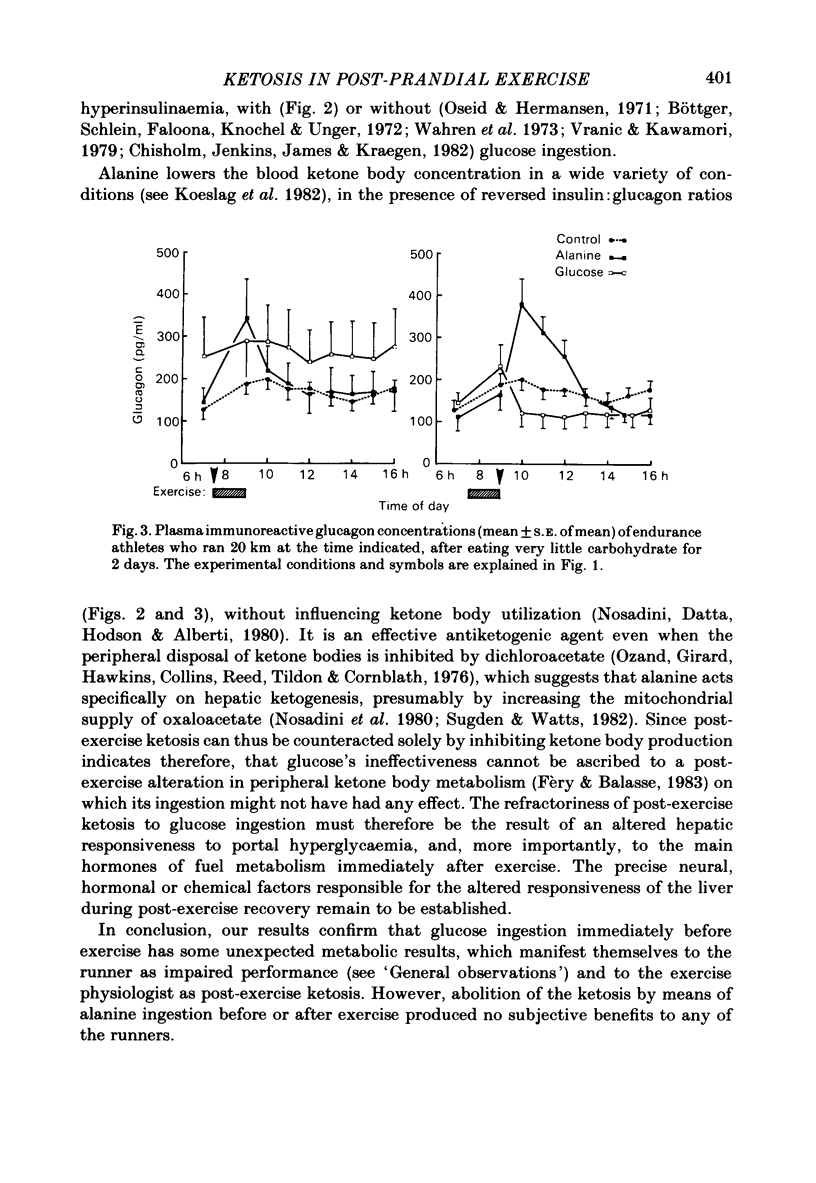
This study examined ketosis in response to 90 min of running before and after the ingestion of 50 g glucose or 50 g L-alanine in thirty-three athletes. Everyone ran 20 km at 07.30 h and then rested, while fasting, till 16.00 h. There were four test groups: 'glucose-before', 'glucose-after', 'alanine-before' and 'alanine-after' according to whether glucose or alanine was ingested at 07.00 h, or 09.00 h. Controls did not ingest either test substance. The control 3-hydroxybutyrate concentration rose from 0.23 +/- 0.03 mmol/l (S.E. of mean) at 07.00 h to 0.74 +/- 0.27 mmol/l at 12.00 h, and 0.94 +/- 0.33 mmol/l at 16.00 h. Glucose ingestion before or after exercise did not influence post-exercise ketosis significantly, despite high insulin: glucagon ratios, low free fatty acid concentrations and hyperglycaemia. Alanine significantly lowered the 3-hydroxybutyrate levels, especially after exercise (to 0.14 +/- 0.07 mmol/l at 12.00 h; P less than 0.05) despite reversed insulin: glucagon ratios. This suggests that hepatic responsiveness to portal hyperglycaemia and the main hormones of metabolism is altered immediately after exercise, presumably to promote muscle glycogen synthesis in preference to liver glycogen synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlborg G., Felig P. Influence of glucose ingestion on fuel-hormone response during prolonged exercise. J Appl Physiol. 1976 Nov;41(5 Pt 1):683–688. doi: 10.1152/jappl.1976.41.5.683. [DOI] [PubMed] [Google Scholar]
- Ahlborg G., Felig P. Substrate utilization during prolonged exercise preceded by ingestion of glucose. Am J Physiol. 1977 Sep;233(3):E188–E194. doi: 10.1152/ajpendo.1977.233.3.E188. [DOI] [PubMed] [Google Scholar]
- Balasse E. O., Fery F., Neef M. A. Changes induced by exercise in rates of turnover and oxidation of ketone bodies in fasting man. J Appl Physiol Respir Environ Exerc Physiol. 1978 Jan;44(1):5–11. doi: 10.1152/jappl.1978.44.1.5. [DOI] [PubMed] [Google Scholar]
- Böttger I., Schlein E. M., Faloona G. R., Knochel J. P., Unger R. H. The effect of exercise on glucagon secretion. J Clin Endocrinol Metab. 1972 Jul;35(1):117–125. doi: 10.1210/jcem-35-1-117. [DOI] [PubMed] [Google Scholar]
- Chisholm D. J., Jenkins A. B., James D. E., Kraegen E. W. The effect of hyperinsulinemia on glucose homeostasis during moderate exercise in man. Diabetes. 1982 Jul;31(7):603–608. doi: 10.2337/diab.31.7.603. [DOI] [PubMed] [Google Scholar]
- Cohen P. The hormonal control of glycogen metabolism in mammalian muscle by multivalent phosphorylation. Biochem Soc Trans. 1979 Jun;7(3):459–480. doi: 10.1042/bst0070459. [DOI] [PubMed] [Google Scholar]
- Costill D. L., Coyle E., Dalsky G., Evans W., Fink W., Hoopes D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1977 Oct;43(4):695–699. doi: 10.1152/jappl.1977.43.4.695. [DOI] [PubMed] [Google Scholar]
- DOLE V. P., MEINERTZ H. Microdetermination of long-chain fatty acids in plasma and tissues. J Biol Chem. 1960 Sep;235:2595–2599. [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man. Diabetes. 1975 May;24(5):468–475. doi: 10.2337/diab.24.5.468. [DOI] [PubMed] [Google Scholar]
- Fell R. D., McLane J. A., Winder W. W., Holloszy J. O. Preferential resynthesis of muscle glycogen in fasting rats after exhausting exercise. Am J Physiol. 1980 May;238(5):R328–R332. doi: 10.1152/ajpregu.1980.238.5.R328. [DOI] [PubMed] [Google Scholar]
- Fell R. D., Terblanche S. E., Ivy J. L., Young J. C., Holloszy J. O. Effect of muscle glycogen content on glucose uptake following exercise. J Appl Physiol Respir Environ Exerc Physiol. 1982 Feb;52(2):434–437. doi: 10.1152/jappl.1982.52.2.434. [DOI] [PubMed] [Google Scholar]
- Foster C., Costill D. L., Fink W. J. Effects of preexercise feedings on endurance performance. Med Sci Sports. 1979 Spring;11(1):1–5. [PubMed] [Google Scholar]
- Foster D. W., McGarry J. D. The regulation of ketogenesis. Ciba Found Symp. 1982;87:120–131. doi: 10.1002/9780470720691.ch7. [DOI] [PubMed] [Google Scholar]
- Féry F., Balasse E. O. Ketone body turnover during and after exercise in overnight-fasted and starved humans. Am J Physiol. 1983 Oct;245(4):E318–E325. doi: 10.1152/ajpendo.1983.245.4.E318. [DOI] [PubMed] [Google Scholar]
- Houghton C. R., Hawkins R. A., Williamson D. H., Krebs H. A. An explanation for the lowering of blood ketone-body concentrations in starved rats during short-term exercise. Biochem J. 1971 Oct;124(5):56P–57P. doi: 10.1042/bj1240056pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy J. L., Holloszy J. O. Persistent increase in glucose uptake by rat skeletal muscle following exercise. Am J Physiol. 1981 Nov;241(5):C200–C203. doi: 10.1152/ajpcell.1981.241.5.C200. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. E., PASSMORE R. Interrelations among post-exercise ketosis (Courtice-Douglas effect), hydration and metabolic state. Metabolism. 1960 May;9:443–451. [PubMed] [Google Scholar]
- Jansson E., Kaijser L. Effect of diet on muscle glycogen and blood glucose utilization during a short-term exercise in man. Acta Physiol Scand. 1982 Jul;115(3):341–347. doi: 10.1111/j.1748-1716.1982.tb07087.x. [DOI] [PubMed] [Google Scholar]
- Koeslag J. H., Noakes T. D., Sloan A. W. Post-exercise ketosis. J Physiol. 1980 Apr;301:79–90. doi: 10.1113/jphysiol.1980.sp013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeslag J. H., Noakes T. D., Sloan A. W. The effects of alanine, glucose and starch ingestion on the ketosis produced by exercise and by starvation. J Physiol. 1982 Apr;325:363–376. doi: 10.1113/jphysiol.1982.sp014155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeslag J. H. Post-exercise ketosis and the hormone response to exercise: a review. Med Sci Sports Exerc. 1982;14(5):327–334. [PubMed] [Google Scholar]
- Koivisto V. A., Karonen S. L., Nikkilä E. A. Carbohydrate ingestion before exercise: comparison of glucose, fructose, and sweet placebo. J Appl Physiol Respir Environ Exerc Physiol. 1981 Oct;51(4):783–787. doi: 10.1152/jappl.1981.51.4.783. [DOI] [PubMed] [Google Scholar]
- Madsen N. B., KasvinskyPJ, Fletterick R. J. Allosteric transitions of phosphorylase a and the regulation of glycogen metabolism. J Biol Chem. 1978 Dec 25;253(24):9097–9101. [PubMed] [Google Scholar]
- Maehlum S., Hermansen L. Muscle glycogen concentration during recovery after prolonged severe exercise in fasting subjects. Scand J Clin Lab Invest. 1978 Oct;38(6):557–560. doi: 10.1080/00365517809108819. [DOI] [PubMed] [Google Scholar]
- Maehlum S., Jervell J., Pruett E. D. Arterial-hepatic vein glucose differences in normal and diabetic man after a glucose infusion at rest and after exercise. Scand J Clin Lab Invest. 1976 Sep;36(5):415–422. doi: 10.3109/00365517609054458. [DOI] [PubMed] [Google Scholar]
- Nelson J. D., Poussier P., Marliss E. B., Albisser A. M., Zinman B. Metabolic response of normal man and insulin-infused diabetics to postprandial exercise. Am J Physiol. 1982 May;242(5):E309–E316. doi: 10.1152/ajpendo.1982.242.5.E309. [DOI] [PubMed] [Google Scholar]
- Nosadini R., Datta H., Hodson A., Alberti K. G. A possible mechanism for the anti-ketogenic action of alanine in the rat. Biochem J. 1980 Aug 15;190(2):323–332. doi: 10.1042/bj1900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oseid S., Hermansen L. Hormonal and metabolic changes during and after prolonged muscular work in pre-pubertal boys. Acta Paediatr Scand Suppl. 1971;217:147–153. doi: 10.1111/j.1651-2227.1971.tb05716.x. [DOI] [PubMed] [Google Scholar]
- Radziuk J., McDonald T. J., Rubenstein D., Dupre J. Initial splanchnic extraction of ingested glucose in normal man. Metabolism. 1978 Jun;27(6):657–669. doi: 10.1016/0026-0495(78)90003-3. [DOI] [PubMed] [Google Scholar]
- Richter E. A., Garetto L. P., Goodman M. N., Ruderman N. B. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982 Apr;69(4):785–793. doi: 10.1172/JCI110517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I. Effects of L-alanine on ketogenesis in vitro. Biochim Biophys Acta. 1982 Aug 6;717(2):385–386. doi: 10.1016/0304-4165(82)90193-3. [DOI] [PubMed] [Google Scholar]
- Vranic M., Kawamori R. Essential roles of insulin and glucagon in regulating glucose fluxes during exercise in dogs. Diabetes. 1979 Jan;28 (Suppl 1):45–52. doi: 10.2337/diab.28.1.s45. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hendler R., Ahlborg G. Glucose and amino acid metabolism during recovery after exercise. J Appl Physiol. 1973 Jun;34(6):838–845. doi: 10.1152/jappl.1973.34.6.838. [DOI] [PubMed] [Google Scholar]
- Young J. C., Garthwaite S. M., Bryan J. E., Cartier L. J., Holloszy J. O. Carbohydrate feeding speeds reversal of enhanced glucose uptake in muscle after exercise. Am J Physiol. 1983 Nov;245(5 Pt 1):R684–R688. doi: 10.1152/ajpregu.1983.245.5.R684. [DOI] [PubMed] [Google Scholar]
- Zinman B., Murray F. T., Vranic M., Albisser A. M., Leibel B. S., Mc Clean P. A., Marliss E. B. Glucoregulation during moderate exercise in insulin treated diabetics. J Clin Endocrinol Metab. 1977 Oct;45(4):641–652. doi: 10.1210/jcem-45-4-641. [DOI] [PubMed] [Google Scholar]


