Abstract
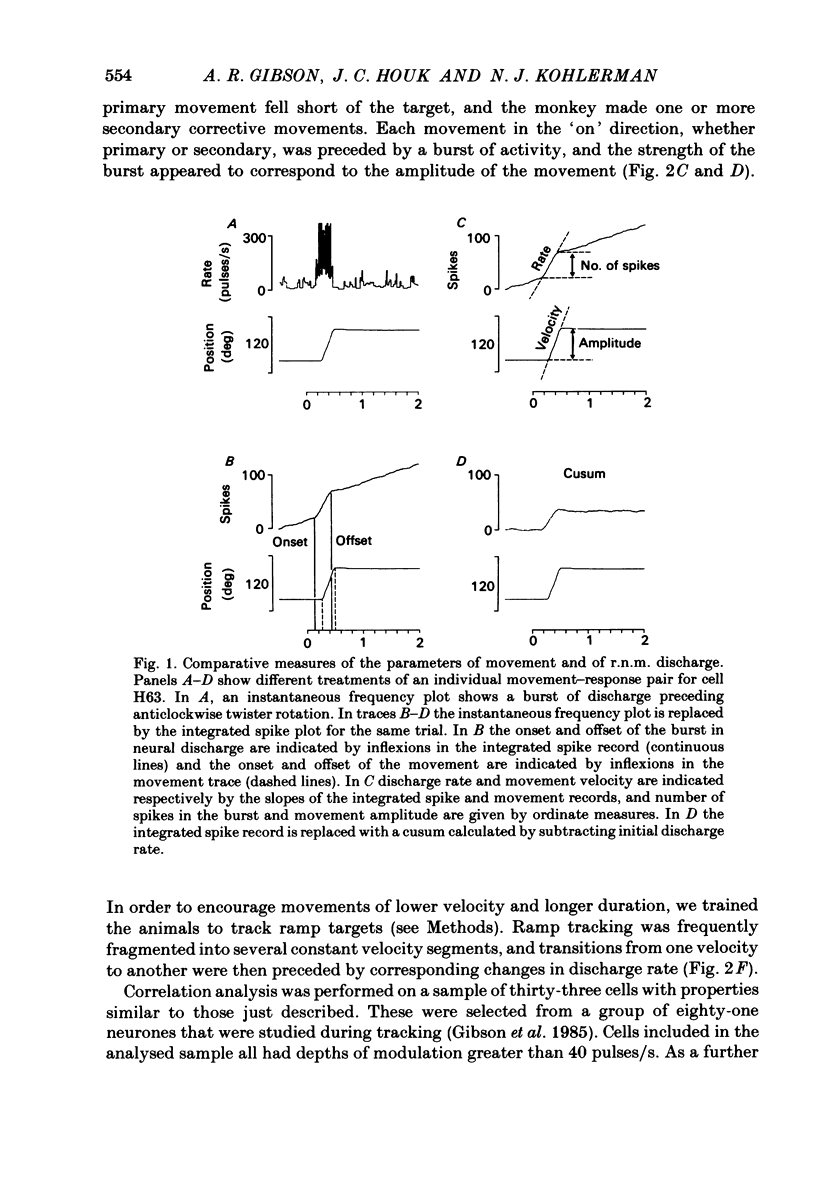
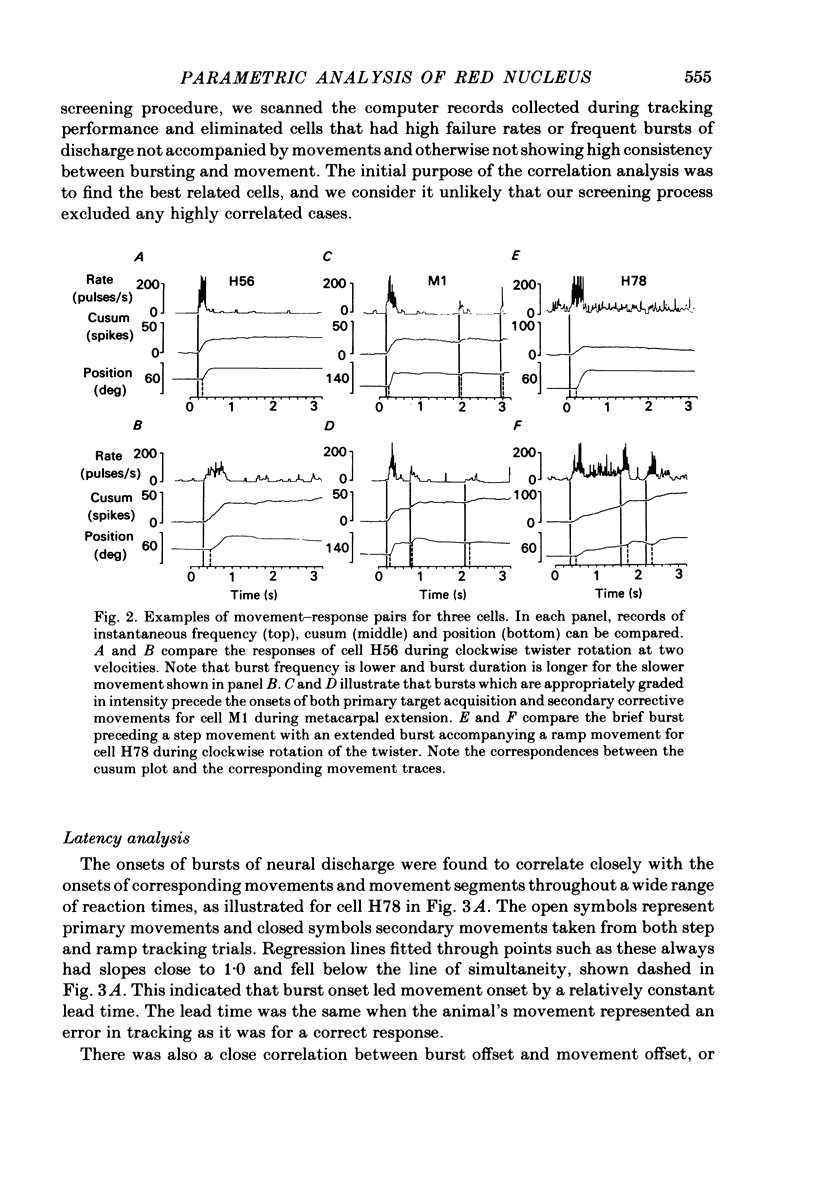
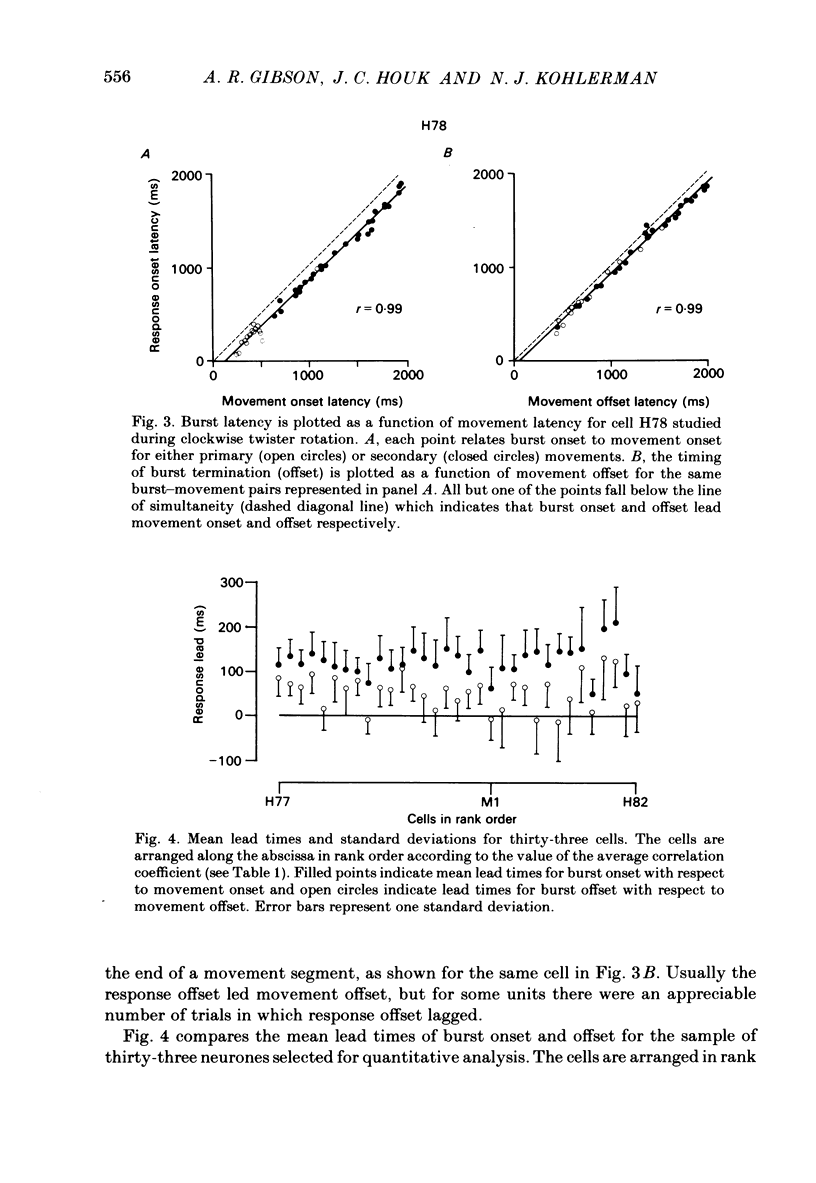
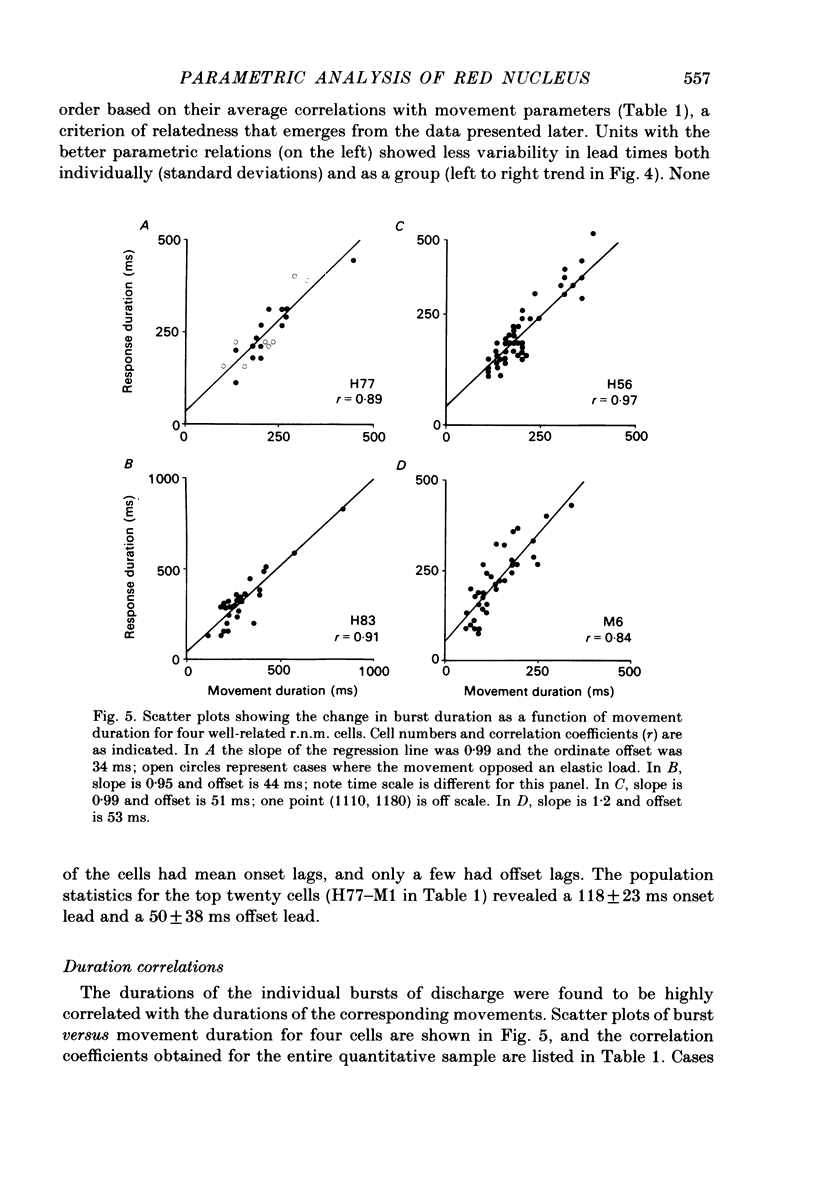
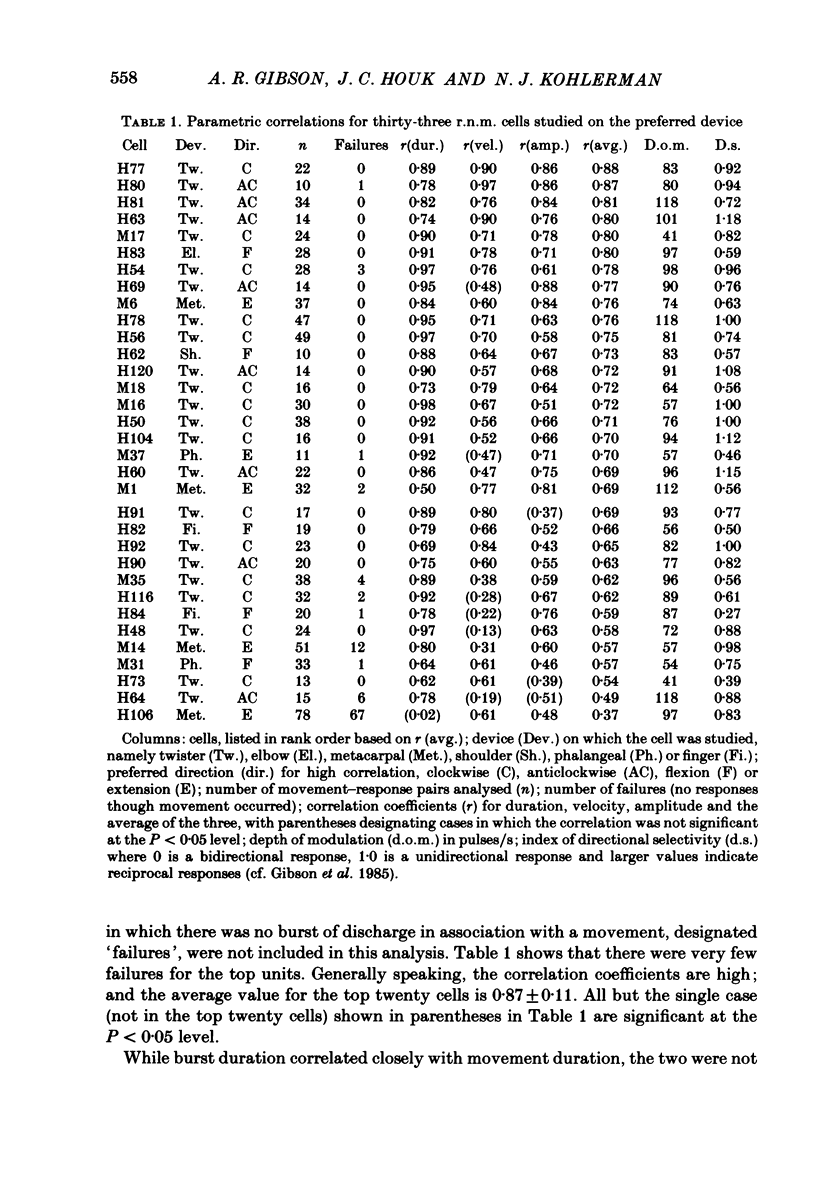
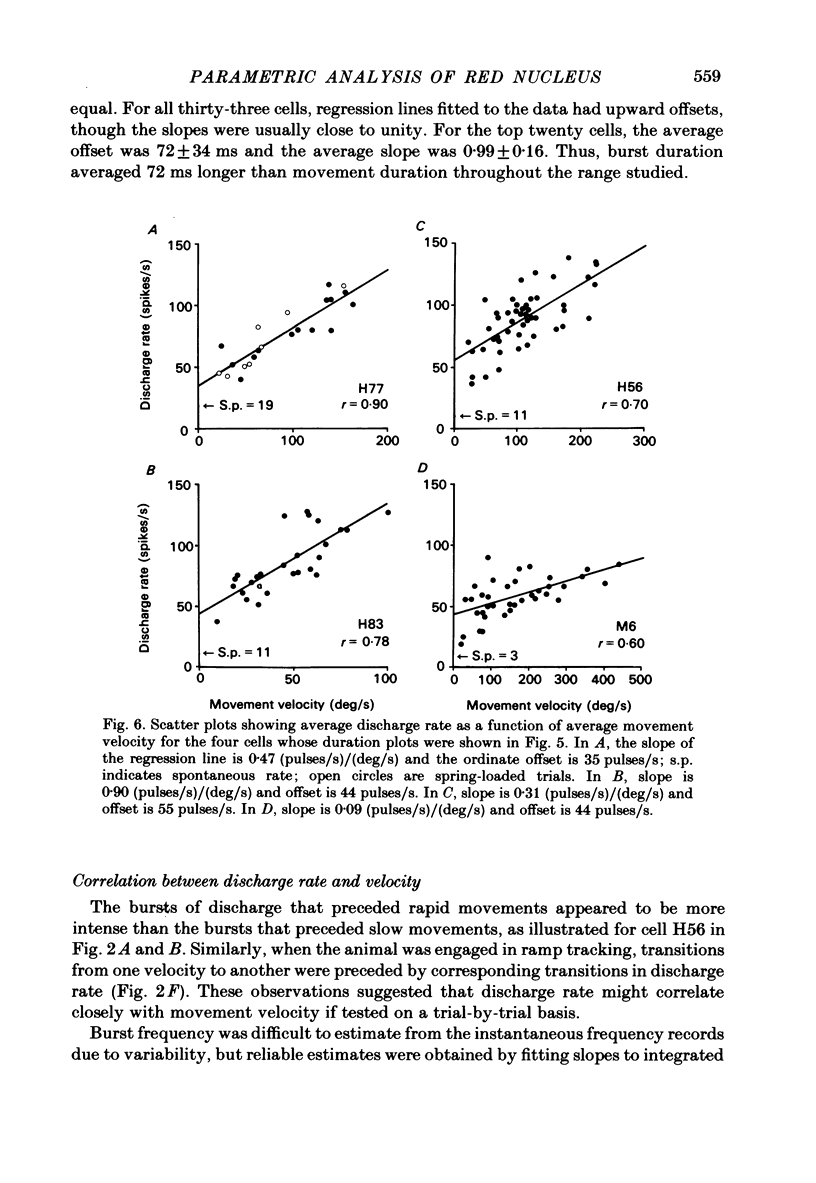
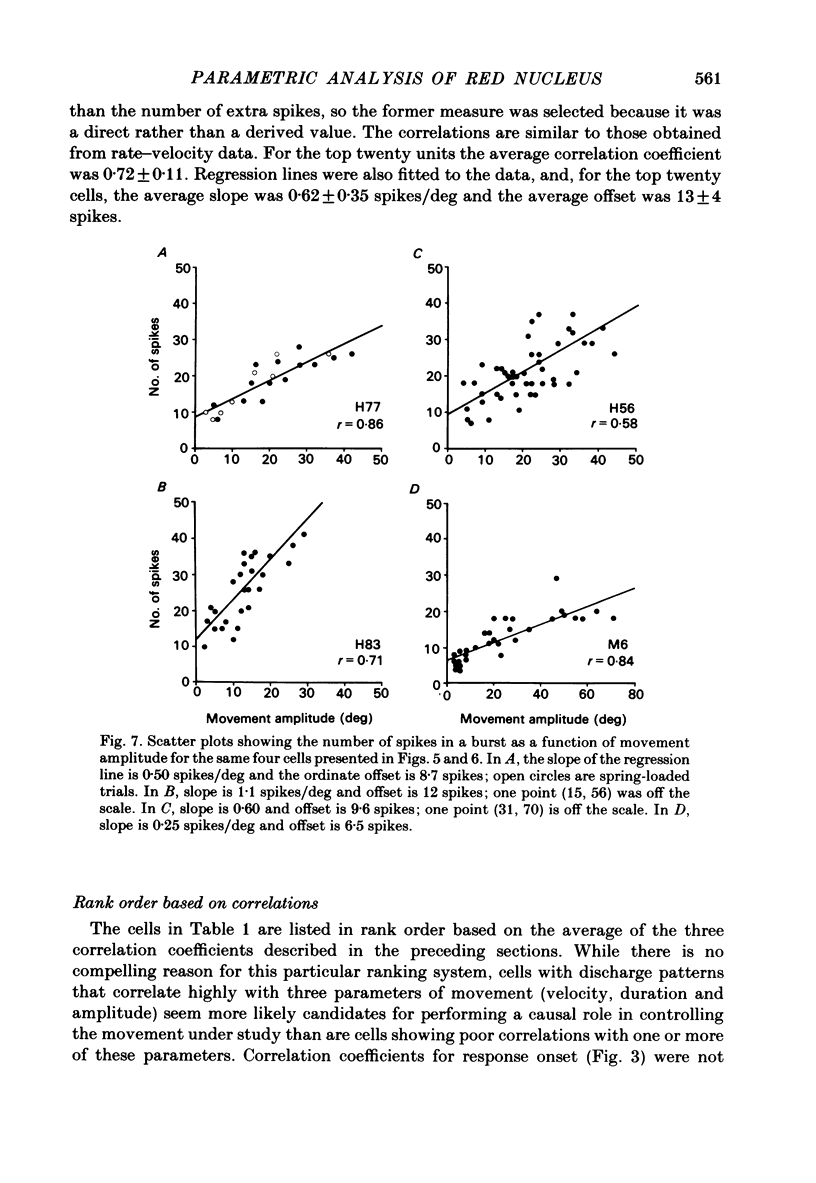
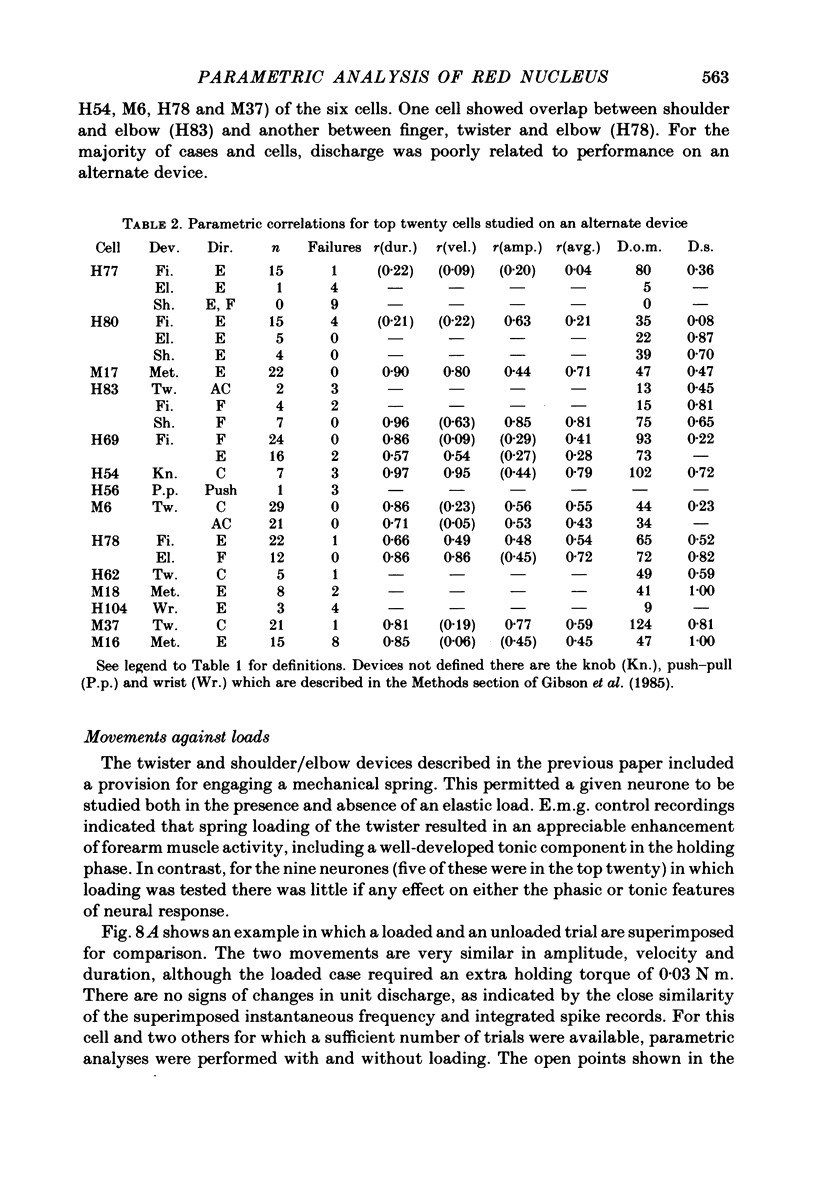
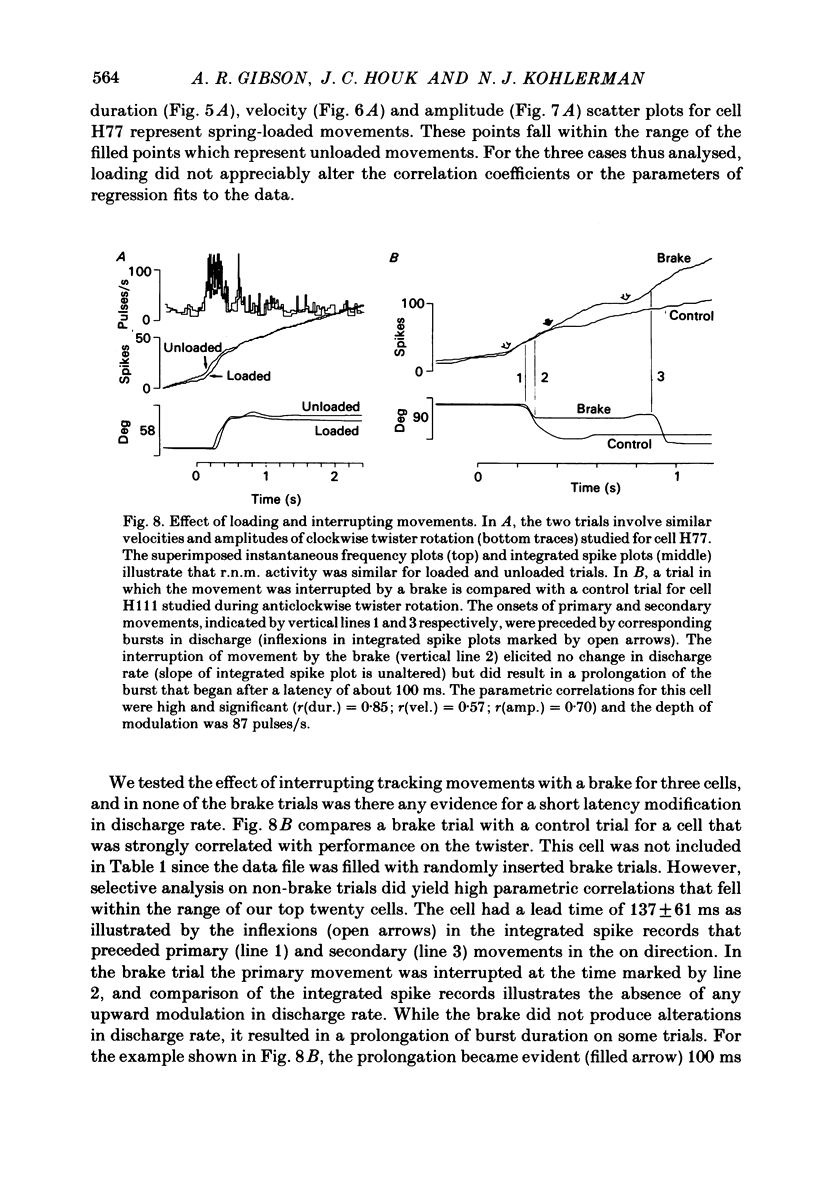
Correlation and regression analyses were performed on thirty-three of the magnocellular red nucleus cells described in the previous paper. We sought to test for reliable relations between the parameters of individual tracking movements and corresponding bursts of neural discharge. High correlations were found between the following burst and movement parameters: (i) burst latency versus movement latency; (ii) burst duration versus movement duration; (iii) burst frequency versus movement velocity and (iv) number of spikes in the burst versus movement amplitude. Cells were ranked according to the average of the duration, velocity and amplitude correlation coefficients. The top twenty cells had average correlation coefficients ranging from 0.69 to 0.88 for their preferred movement. These cases were judged most likely to reveal the control functions of the red nucleus, and the following points refer to this sample. Burst onset led movement onset by 118 +/- 23 ms, and burst offset led movement offset by 50 +/- 38 ms. Burst duration increased as the duration of the movement increased (r = 0.87 +/- 0.11). The duration of the burst was approximately equal to movement duration (slope of 0.99 +/- 0.16) plus a constant (72 +/- 34 ms) throughout a broad range. Average discharge rate during the burst increased with average movement velocity (r = 0.69 +/- 0.15). The slope of the relation was 0.36 +/- 0.21 (pulses/s)/(deg/s) of joint rotation. The regression lines had consistent upward offsets (56 +/- 15 pulses/s) that exceeded the spontaneous discharge rate (17 +/- 10 pulses/s). The number of spikes in the burst increased with movement amplitude independent of velocity (r = 0.72 +/- 0.11). The slope of the relation was 0.62 spikes/deg and the offset was 13 +/- 4 spikes. The preferred movement was co-ordinated hand in fifteen cases, digit in three, elbow in one and shoulder in one. When these cells were tested with an alternate movement, the failure rate (cases in which a burst did not accompany a movement) increased from 1.4 to 20%, and the correlation coefficients generally were low and lacked significance. Cells in the top twenty had directionally specific responses, low variance in lead time, large depths of modulation (41-118 pulses/s) and low failure rates. Cells that failed to show strong parametric correlations often had one or more of the former attributes. It appears that high parametric correlations with individual movements are particularly restrictive criteria of relatedness.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton J. E., Onoda N. Dependence of the activity of interpositus and red nucleus neurons on sensory input data generated by movement. Brain Res. 1978 Aug 18;152(1):41–63. doi: 10.1016/0006-8993(78)90133-6. [DOI] [PubMed] [Google Scholar]
- Cheney P. D. Response of rubromotoneuronal cells identified by spike-triggered averaging of EMG activity in awake monkeys. Neurosci Lett. 1980 Apr;17(1-2):137–142. doi: 10.1016/0304-3940(80)90075-0. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H. An application of cumulative sum technique (cusums) to neurophysiology [proceedings]. J Physiol. 1977 Feb;265(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- Ghez C., Vicario D. Discharge of red nucleus neurons during voluntary muscle contraction: activity patterns and correlations with isometric force. J Physiol (Paris) 1978;74(3):283–285. [PubMed] [Google Scholar]
- Gibson A. R., Houk J. C., Kohlerman N. J. Magnocellular red nucleus activity during different types of limb movement in the macaque monkey. J Physiol. 1985 Jan;358:527–549. doi: 10.1113/jphysiol.1985.sp015565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. Discharges of intracerebellar nuclear cells in monkeys. J Physiol. 1979 Dec;297(0):559–580. doi: 10.1113/jphysiol.1979.sp013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk J. C. Regulation of stiffness by skeletomotor reflexes. Annu Rev Physiol. 1979;41:99–114. doi: 10.1146/annurev.ph.41.030179.000531. [DOI] [PubMed] [Google Scholar]
- Humphrey D. R., Rietz R. R. Cells of origin of corticorubral projections from the arm area of primate motor cortex and their synaptic actions in the red nucleus. Brain Res. 1976 Jun 25;110(1):162–169. doi: 10.1016/0006-8993(76)90217-1. [DOI] [PubMed] [Google Scholar]
- KUYPERS H. G., FLEMING W. R., FARINHOLT J. W. Subcorticospinal projections in the rhesus monkey. J Comp Neurol. 1962 Feb;118:107–137. doi: 10.1002/cne.901180109. [DOI] [PubMed] [Google Scholar]
- Larsen K. D., Yumiya H. The red nucleus of the monkey. Topographic localization of somatosensory input and motor output. Exp Brain Res. 1980;40(4):393–404. doi: 10.1007/BF00236148. [DOI] [PubMed] [Google Scholar]
- Lawrence D. G., Kuypers H. G. The functional organization of the motor system in the monkey. II. The effects of lesions of the descending brain-stem pathways. Brain. 1968 Mar;91(1):15–36. doi: 10.1093/brain/91.1.15. [DOI] [PubMed] [Google Scholar]
- Otero J. B. Comparison between red nucleus and precentral neurons during learned movements in the monkey. Brain Res. 1976 Jan 9;101(1):37–46. doi: 10.1016/0006-8993(76)90986-0. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Karamjan O. A., Kurchavyi G. G., Repina Z. A. Synaptic actions evoked from the red nucleus on the spinal alpha-motorneurons in the Rhesus monkey. Brain Res. 1971 Sep 24;32(2):325–348. doi: 10.1016/0006-8993(71)90328-3. [DOI] [PubMed] [Google Scholar]
- Soechting J. F., Burton J. E., Onoda N. Relationships between sensory input, motor output and unit activity in interpositus and red nuclei during intentional movement. Brain Res. 1978 Aug 18;152(1):65–79. doi: 10.1016/0006-8993(78)90134-8. [DOI] [PubMed] [Google Scholar]
- Strick P. L. The influence of motor preparation on the response of cerebellar neurons to limb displacements. J Neurosci. 1983 Oct;3(10):2007–2020. doi: 10.1523/JNEUROSCI.03-10-02007.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


