Abstract
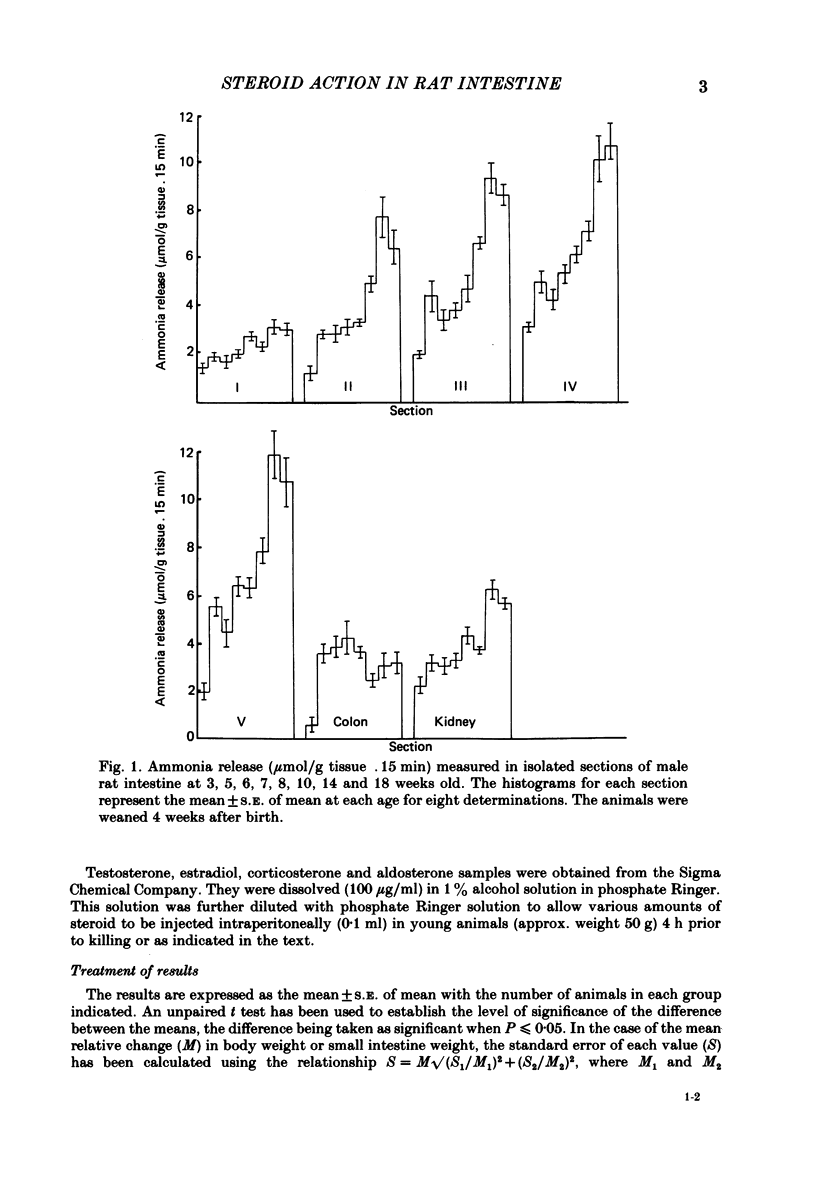
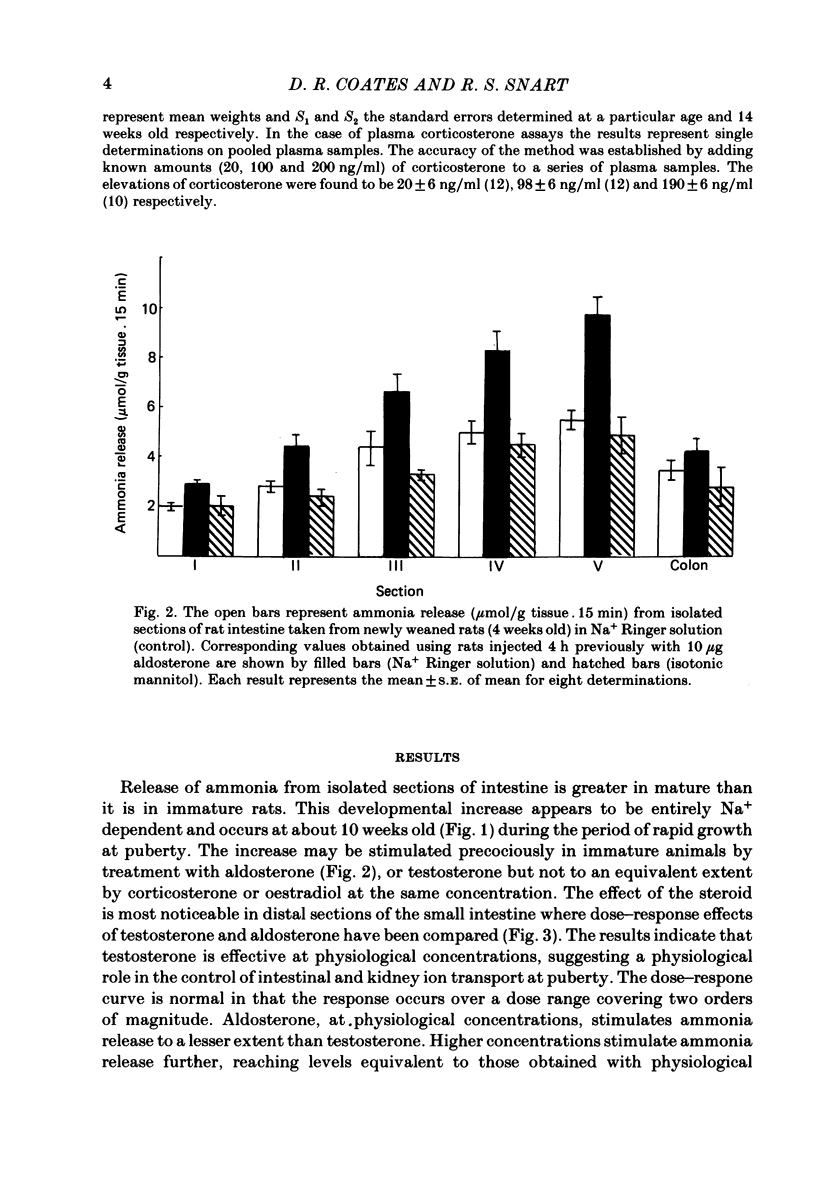
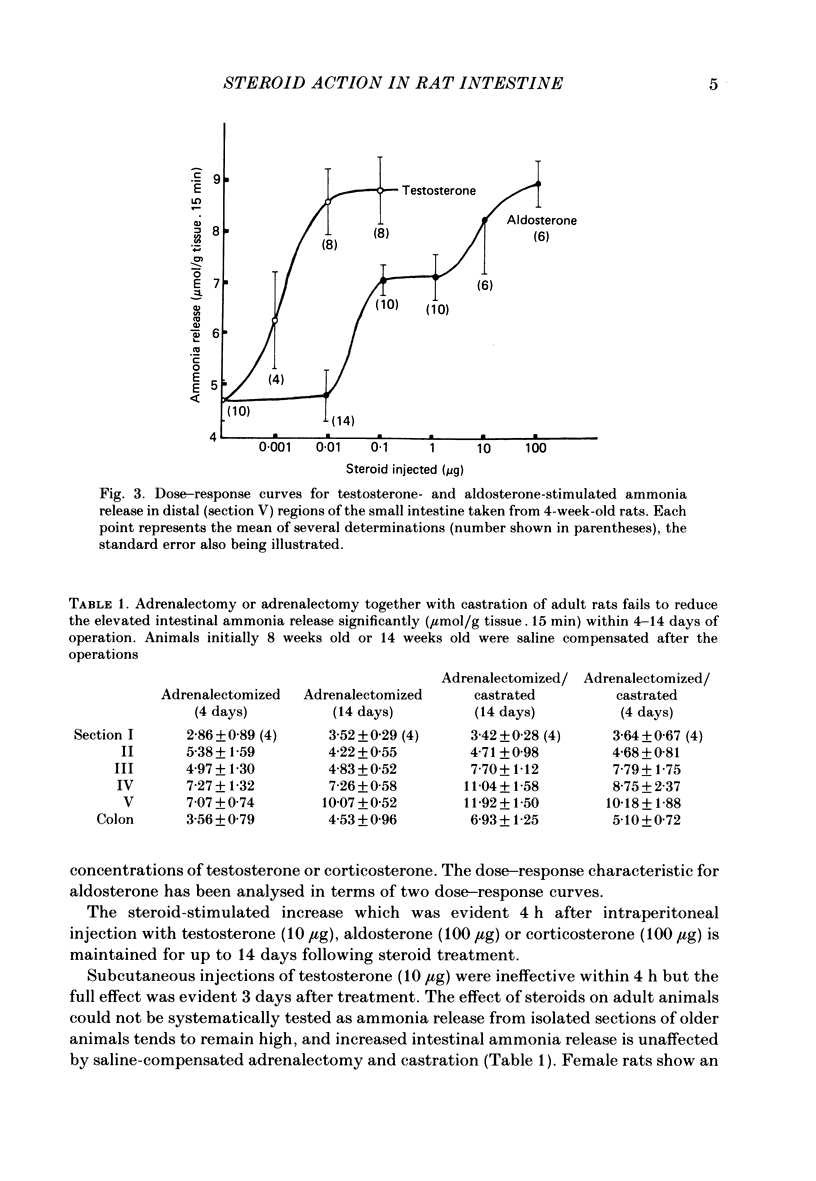
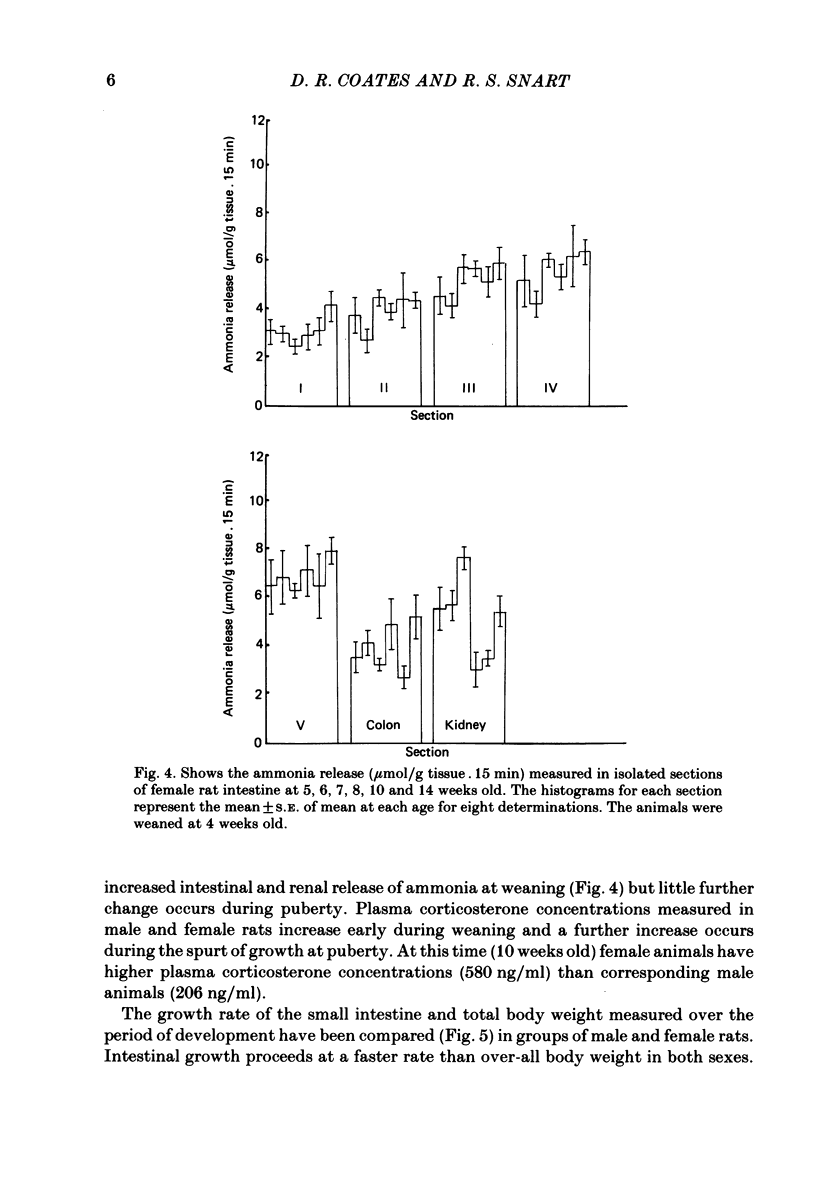
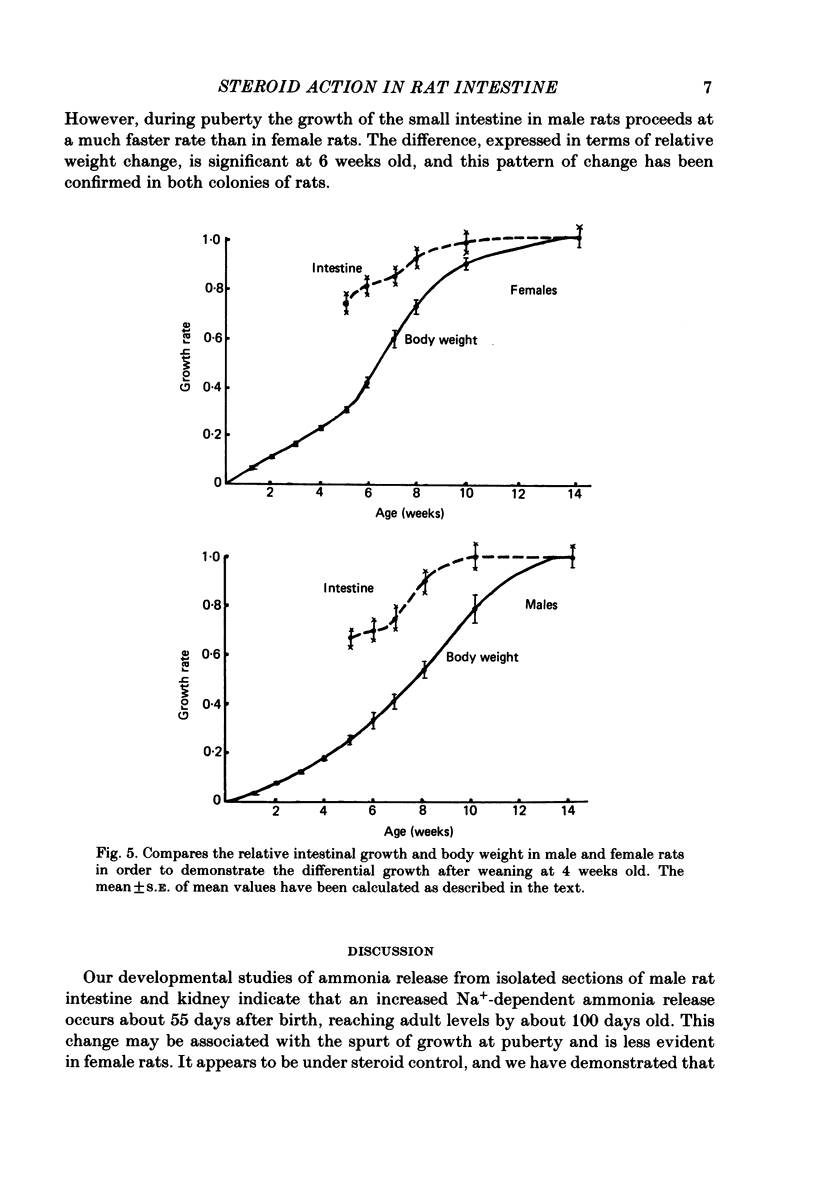
Release of ammonia from isolated intestinal sections of adult male rats is higher than that measured using immature animals. The increase appears to be Na+ dependent and develops during the spurt of growth at puberty. Developmental changes in Na+-dependent ammonia release from isolated sections of the intestine and growth of the small intestine in male and female rats have been compared. Intestinal growth increases far more rapidly than body weight and in the males critical developmental changes occur early during weaning and during puberty. In females the major change is at weaning and little further change occurs during puberty. Treatment of young animals with aldosterone or testosterone increases the Na+-dependent ammonia release precociously. Dose-response effects of testosterone and aldosterone in distal sections of the small intestine have been compared.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke R. M., Hardy R. N. An analysis of the mechanism of cessation of uptake of macromolecular substances by the intestine of the young rat ('closure'). J Physiol. 1969 Sep;204(1):127–134. doi: 10.1113/jphysiol.1969.sp008903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A. D., Munday K. A. Factors affecting mucosal water and sodium transfer in everted sacs of rat jejunum. J Physiol. 1969 Jun;202(2):329–338. doi: 10.1113/jphysiol.1969.sp008814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A. D., Munday K. A. The effect of the renin-angiotensin system on mucosal water and sodium transfer in everted sacs of rat jejunum. J Physiol. 1970 Feb;206(2):323–333. doi: 10.1113/jphysiol.1970.sp009015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn J. S. The fine structure of the absorptive epithelial cells of the developing small intestine of the rat. J Anat. 1967 Jan;101(Pt 1):57–68. [PMC free article] [PubMed] [Google Scholar]
- Feldman D., Funder J. W., Edelman I. S. Evidence for a new class of corticosterone receptors in the rat kidney. Endocrinology. 1973 May;92(5):1429–1441. doi: 10.1210/endo-92-5-1429. [DOI] [PubMed] [Google Scholar]
- Feldman D., Funder J. W. The binding of 18-hydroxydeoxycorticosterone and 18-hydroxycorticosterone to mineralocorticoid and glucocorticoid receptors in the rat kidney. Endocrinology. 1973 May;92(5):1389–1396. doi: 10.1210/endo-92-5-1389. [DOI] [PubMed] [Google Scholar]
- Ferguson D. R., James P. S., Paterson J. Y., Saunders J. C., Smith M. W. Aldosterone induced changes in colonic sodium transport occurring naturally during development in the neonatal pig. J Physiol. 1979 Jul;292:495–504. doi: 10.1113/jphysiol.1979.sp012867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funder J. W., Feldman D., Edelman I. S. Glucocorticoid receptors in rat kidney: the binding of tritiated-dexamethasone. Endocrinology. 1973 Apr;92(4):1005–1013. doi: 10.1210/endo-92-4-1005. [DOI] [PubMed] [Google Scholar]
- Gross H. A., Ruder H. J., Brown K. S., Lipsett M. B. A radioimmunoassay for plasma corticosterone. Steroids. 1972 Dec;20(6):681–695. doi: 10.1016/0039-128x(72)90051-7. [DOI] [PubMed] [Google Scholar]
- Henning S. J., Ballard P. L., Kretchmer N. A study of the cytoplasmic receptors for glucocorticoids in intestine of pre- and postweanling rats. J Biol Chem. 1975 Mar 25;250(6):2073–2079. [PubMed] [Google Scholar]
- Henning S. J., Kretchmer N. Development of intestinal function in mammals. Enzyme. 1973;15(1):3–23. [PubMed] [Google Scholar]
- Henning S. J., Sims J. M. Delineation of the glucocorticoid-sensitive period of intestinal development in the rat. Endocrinology. 1979 Apr;104(4):1158–1163. doi: 10.1210/endo-104-4-1158. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Averner M. Gene regulation in mammalian cells: a model for the interaction of steroids and 3',5'-cyclic AMP. J Theor Biol. 1975 Feb;49(2):337–344. doi: 10.1016/0022-5193(75)90176-9. [DOI] [PubMed] [Google Scholar]
- Koldovsky O., Sunshine P., Kretchmer N. Cellular migration of intestinal epithelia in suckling and weaned rats. Nature. 1966 Dec 17;212(5068):1389–1390. doi: 10.1038/2121389a0. [DOI] [PubMed] [Google Scholar]
- Levin R. J. The effects of hormones on the absorptive, metabolic and digestive functions of the small intestine. J Endocrinol. 1969 Oct;45(2):315–348. doi: 10.1677/joe.0.0450315. [DOI] [PubMed] [Google Scholar]
- Mainwaring W. I., Mangan F. R. A study of the androgen receptors in a variety of androgen-sensitive tissues. J Endocrinol. 1973 Oct;59(1):121–139. doi: 10.1677/joe.0.0590121. [DOI] [PubMed] [Google Scholar]
- Morris B., Morris R. The effects of cortisone acetate on stomach evacuation and the absorption of 125I-labelled globulins in young rats. J Physiol. 1974 Jul;240(1):79–89. doi: 10.1113/jphysiol.1974.sp010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S., Stenius C., Christian L., Harris C., Ivey C. More about the testosterone induction of kidney alcohol dehydrogenase activity in the mouse. Biochem Genet. 1970 Oct;4(5):565–577. doi: 10.1007/BF00486095. [DOI] [PubMed] [Google Scholar]
- Pressley L., Funder J. W. Glucocorticoid and mineralocorticoid receptors in gut mucosa. Endocrinology. 1975 Sep;97(3):588–596. doi: 10.1210/endo-97-3-588. [DOI] [PubMed] [Google Scholar]
- RIGGS T. R., WALKER L. M. SEX HORMONE MODIFICATION OF TISSUE LEVELS AND URINARY EXCRETION OF ALPHA-AMINOISOBUTYRIC ACID IN THE RAT. Endocrinology. 1963 Dec;73:781–788. doi: 10.1210/endo-73-6-781. [DOI] [PubMed] [Google Scholar]
- Snart R. S., Taylor E. Aldosterone effects on renal metabolism. J Physiol. 1978 Jan;274:447–454. doi: 10.1113/jphysiol.1978.sp012159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenstad P., Eik-Nes K. B. In vitro metabolism of testosterone to 17 beta-hydroxy-5 alpha-androstane-3-one and 5 alpha-androstane-3 alpha, 17 beta-diol by the rat intestine. J Steroid Biochem. 1982 Oct;17(4):401–406. doi: 10.1016/0022-4731(82)90633-1. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Morley A. R., Apleton D. The effect of testosterone on cell proliferation and differentiation in the small bowel. J Endocrinol. 1972 Jan;52(1):161–175. doi: 10.1677/joe.0.0520161. [DOI] [PubMed] [Google Scholar]


