Abstract
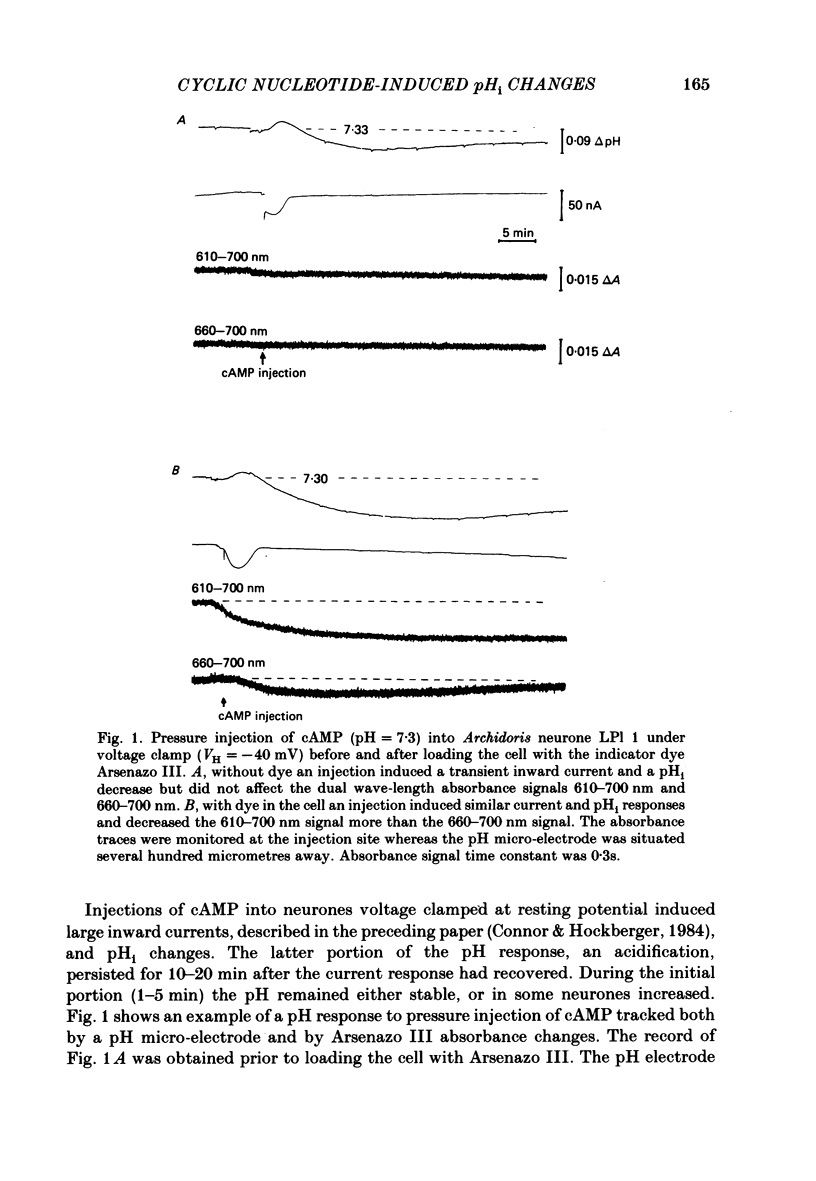
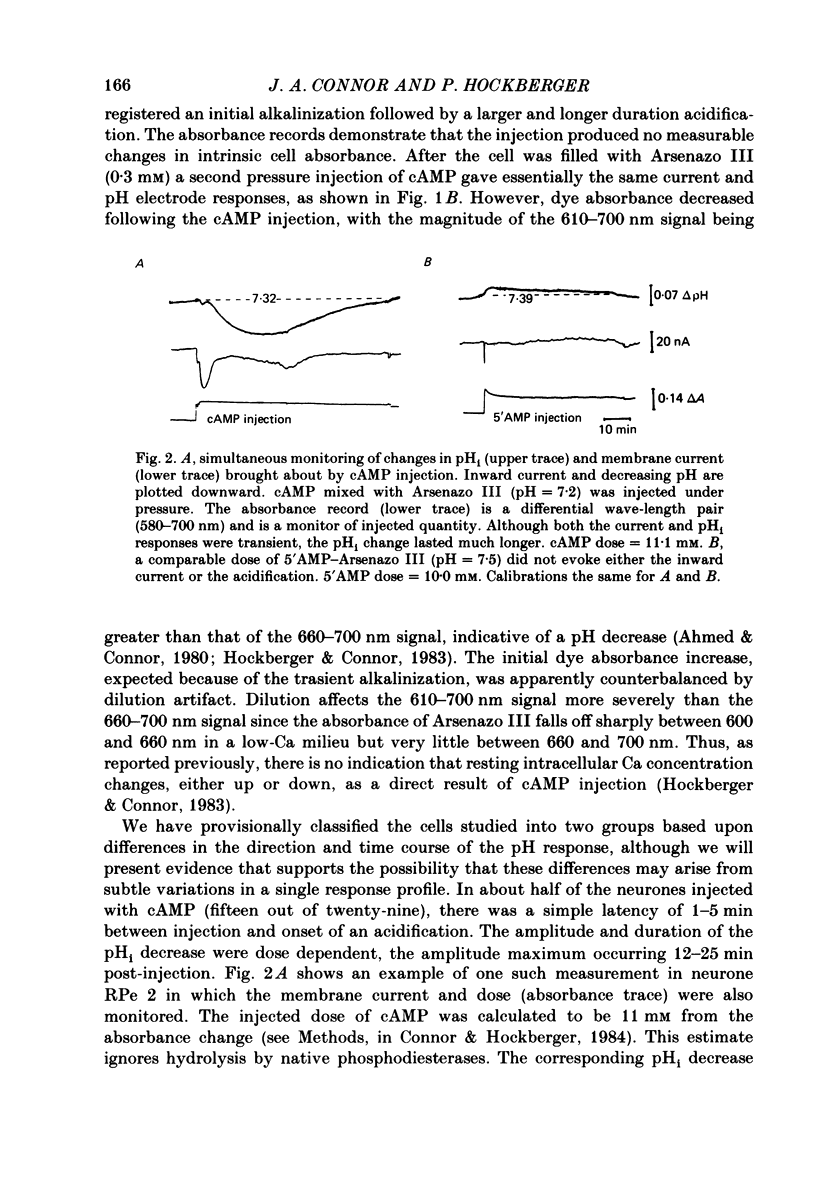
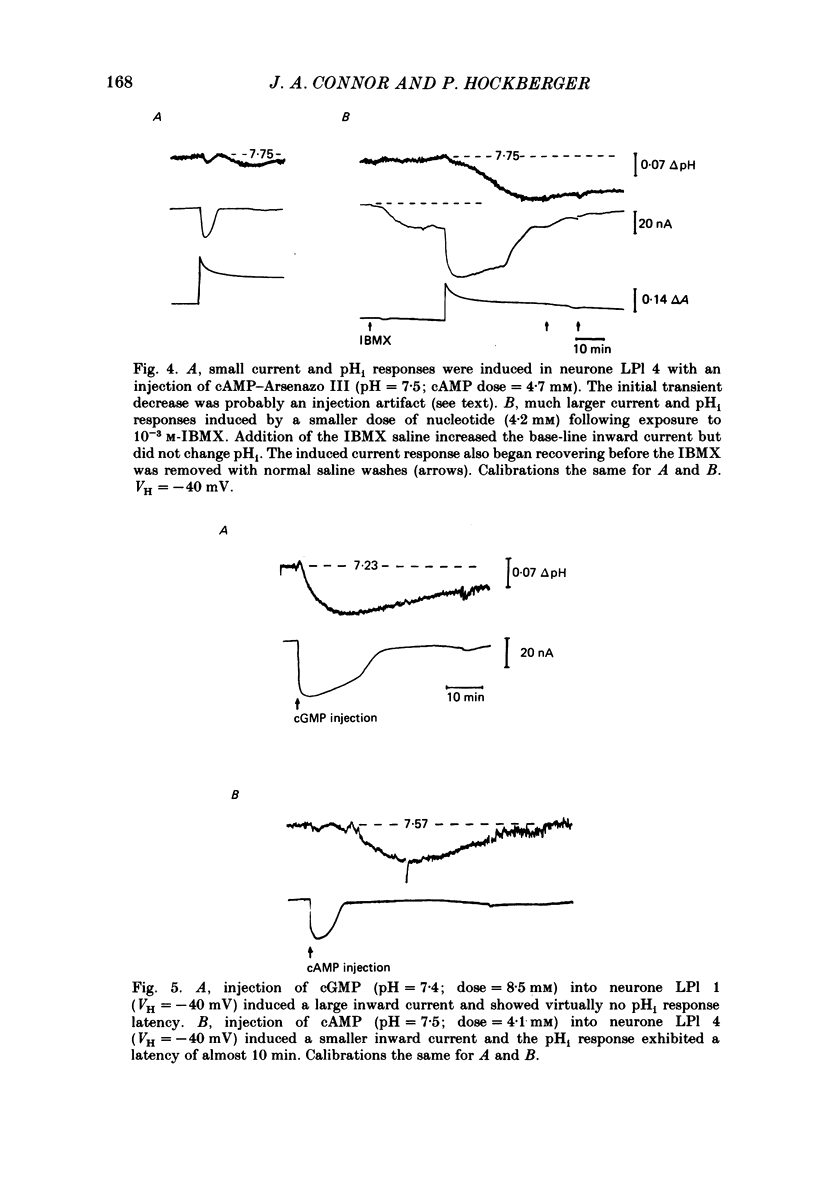
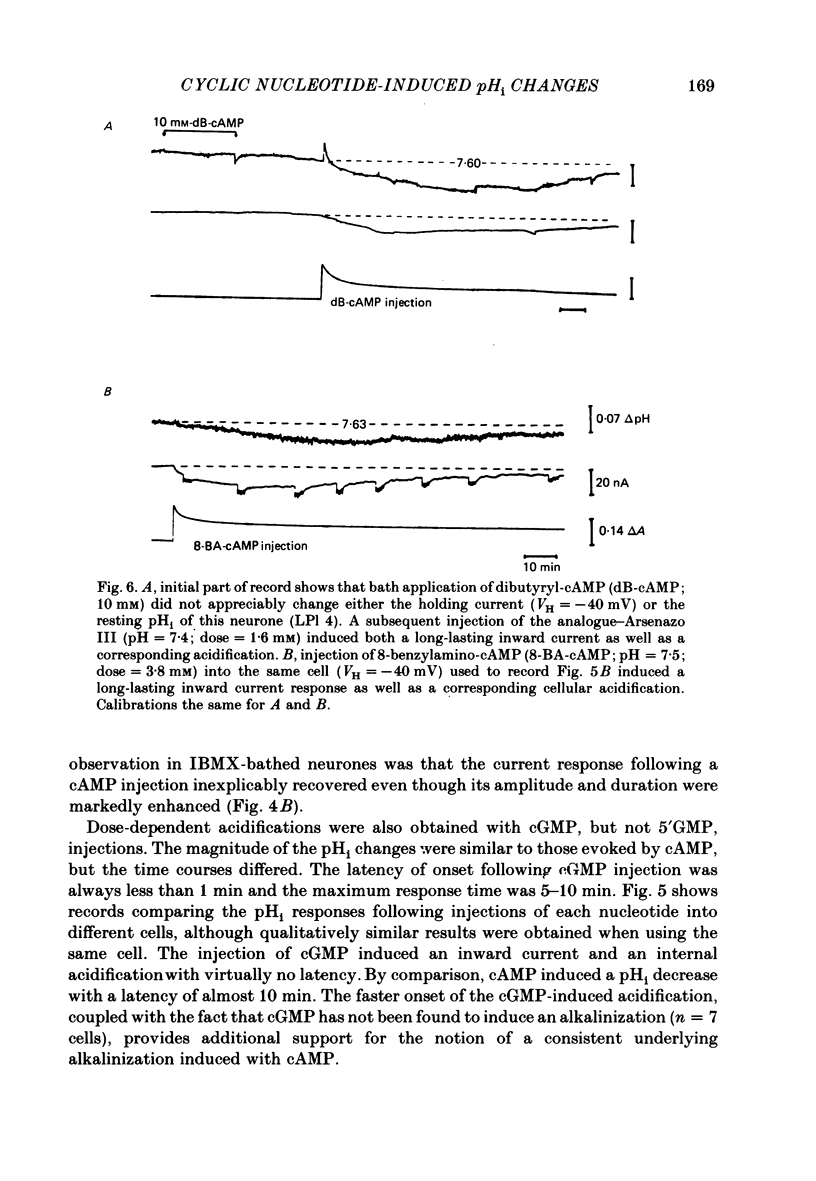
Injections of cyclic AMP (cAMP) or cyclic GMP (cGMP) into identifiable gastropod neurones under voltage clamp induced reversible cytoplasmic pH changes which were measured using the indicator dye Arsenazo III and pH micro-electrodes. Similar injections of 5' AMP or 5' GMP did not induce such effects. In about one-half of the population of neurones examined, cAMP injections (0.1-10 mM) induced pH decreases with latencies of 1-5 min and maximum response times 12-25 min post-injection. Comparable injections of cGMP into these cells resulted in decreases with latencies less than 1 min and maximum response times 5-10 min. The amplitude and duration of these pHi decreases were dose dependent. In the other half of the population of neurones tested, cAMP injection induced an immediate alkalinization lasting 5-10 min followed by an acidification which displayed a maximum response time within the same range as those in the first group. cGMP injection into these cells induced only acidifications with faster peak responses than with cAMP. Since cGMP did not elicit alkalinizing responses, the slower response time of the first group of cells with cAMP may reflect an always present underlying alkalinization. The nucleotide-induced acidification was potentiated in neurones bathed in the phosphodiesterase inhibitor IBMX. Injections of non-hydrolysable cAMP analogues also induced acidifications which were longer lasting than comparable doses of cyclic AMP. These results indicate that the elevated [H+]i was not simply due to hydrolysis of the injected cyclic nucleotide. The pHi changes were invariably of much longer duration than a nucleotide-induced inward current (Connor & Hockberger, 1984) and persisted in Na-free salines which eliminated the current response. These results support the notion that cyclic nucleotide elevation can affect cellular metabolic processes distinct from effects on ion transport mechanisms.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Intracellular pH changes induced by calcium influx during electrical activity in molluscan neurons. J Gen Physiol. 1980 Apr;75(4):403–426. doi: 10.1085/jgp.75.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Russell J. M., Brodwick M. S., Keifer D. W., Roos A. Influence of cyclic AMP on intracellular pH regulation and chloride fluxes in barnacle muscle fibers. Nature. 1978 Nov 30;276(5687):511–513. doi: 10.1038/276511a0. [DOI] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Scarpa A. Ionized magnesium concentration in axoplasm of dialyzed squid axons. FEBS Lett. 1975 Jan 15;50(1):82–85. doi: 10.1016/0014-5793(75)81046-5. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Hockberger P. A novel membrane sodium current induced by injection of cyclic nucleotides into gastropod neurones. J Physiol. 1984 Sep;354:139–162. doi: 10.1113/jphysiol.1984.sp015368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Hockberger P., Connor J. A. Intracellular calcium measurements with arsenazo III during cyclic AMP injections into molluscan neurons. Science. 1983 Feb 18;219(4586):869–871. doi: 10.1126/science.6297009. [DOI] [PubMed] [Google Scholar]
- Illingworth J. A. A common source of error in pH measurements. Biochem J. 1981 Apr 1;195(1):259–262. doi: 10.1042/bj1950259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogan K., Simons E. R. The influence of pH on arsenazo III. Anal Biochem. 1979 Jul 1;96(1):70–76. doi: 10.1016/0003-2697(79)90555-4. [DOI] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D. A. Role of the cAMP-dependent protein kinase as the transducer of cAMP action. Biochem Pharmacol. 1978;27(14):1801–1804. doi: 10.1016/0006-2952(78)90022-9. [DOI] [PubMed] [Google Scholar]


