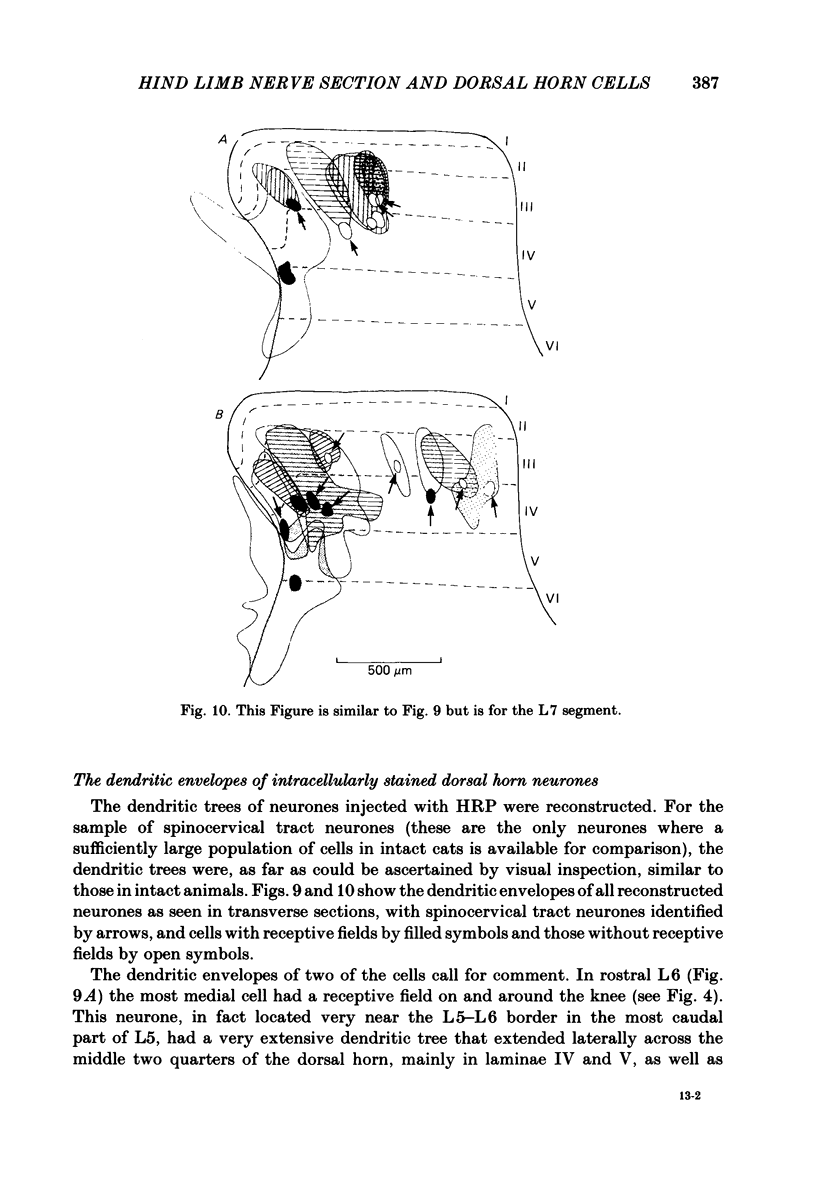
Abstract
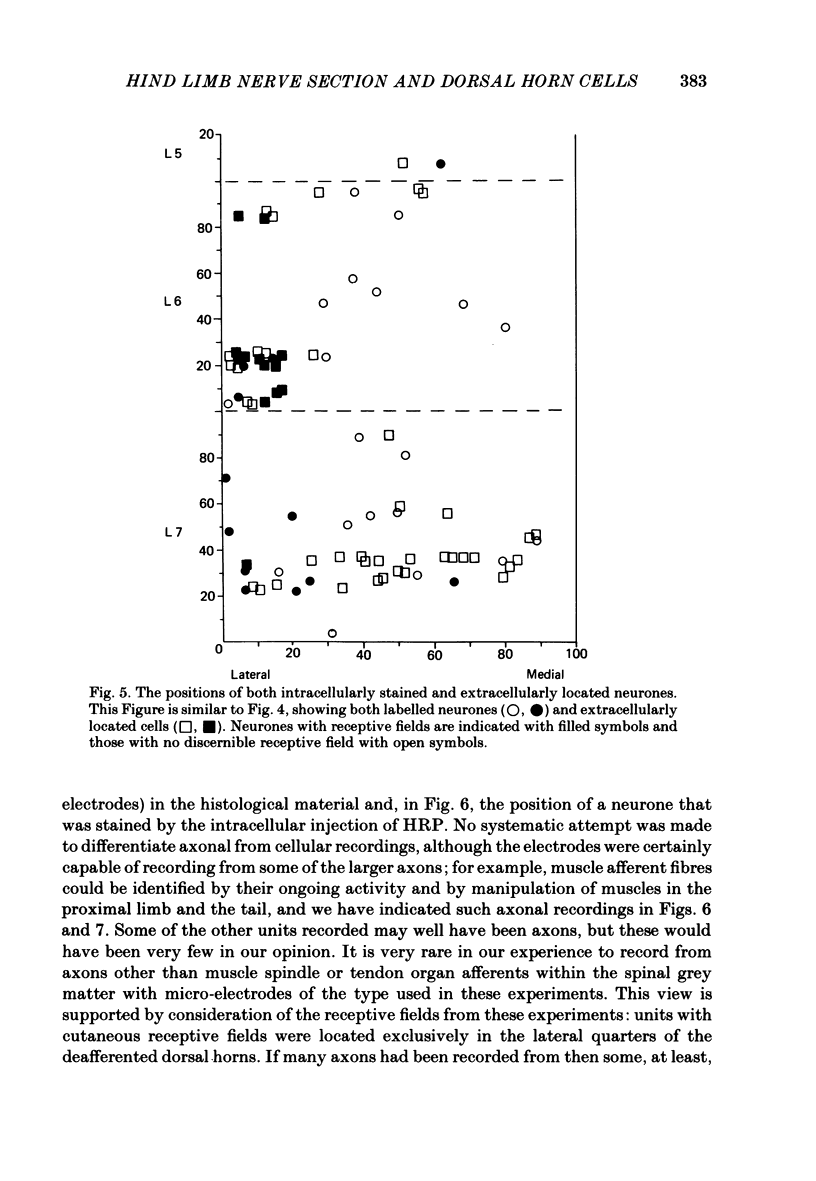
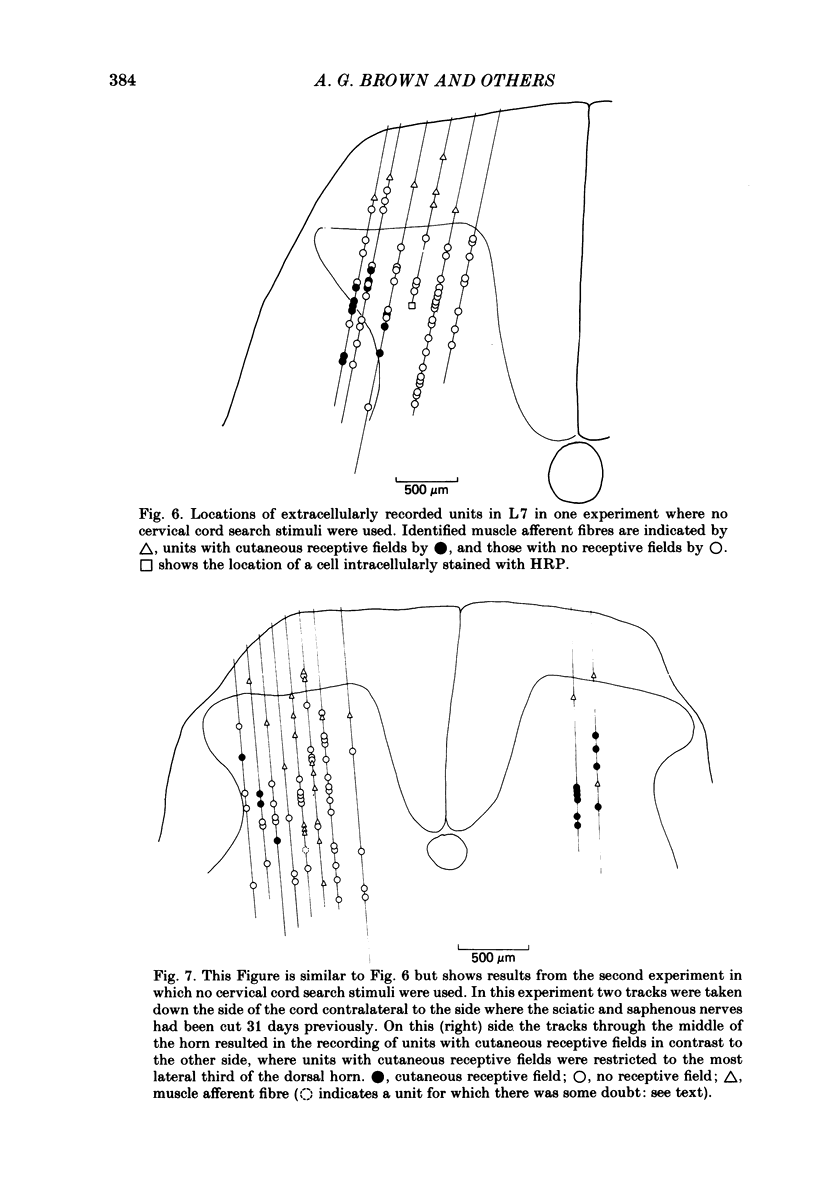
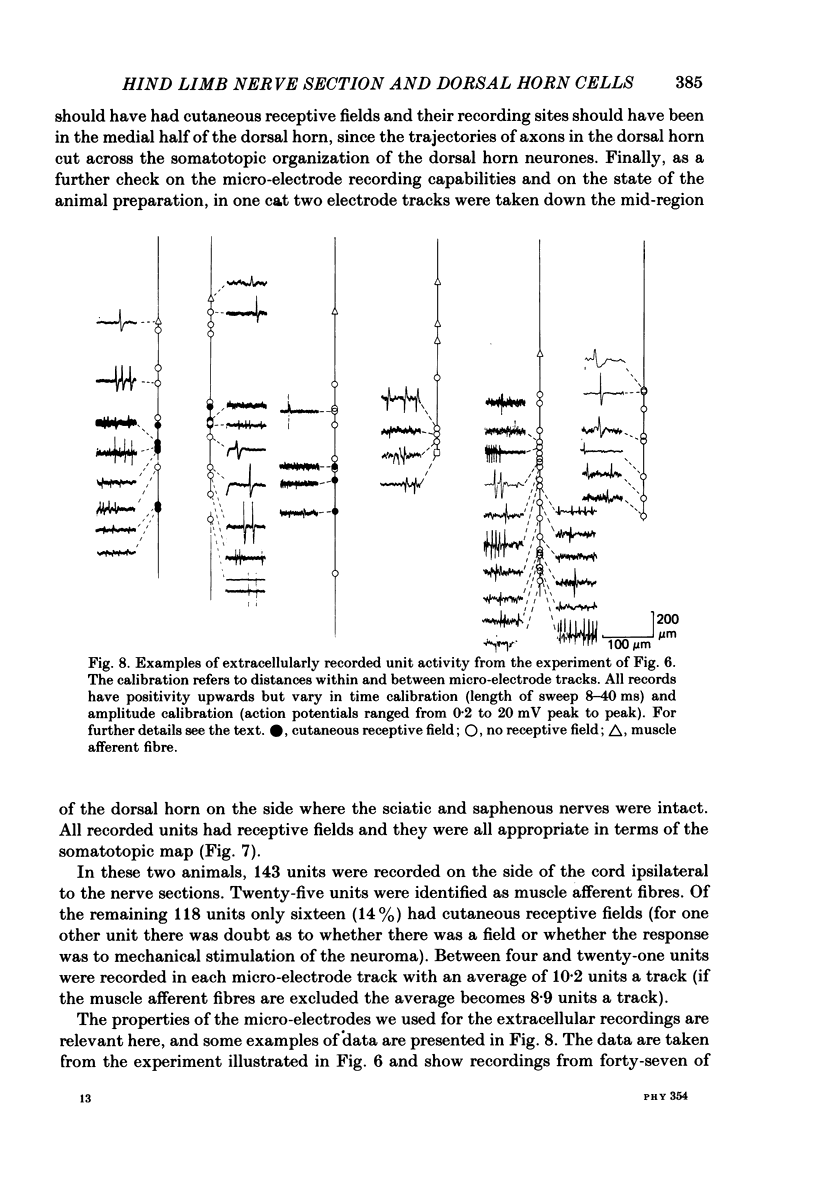
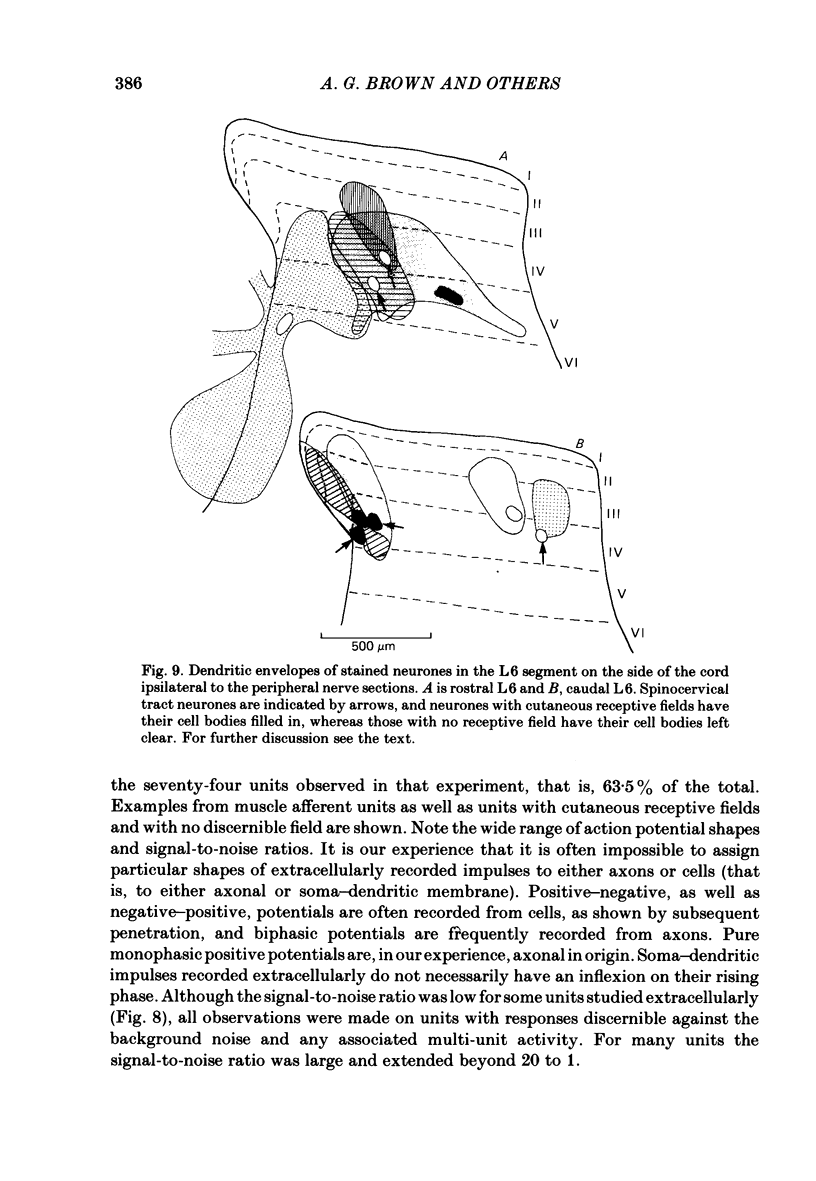
The sciatic and saphenous nerves of one hind limb were sectioned in young adult cats anaesthetized with halothane. Between 19 and 55 days later, under chloralose anaesthesia, dorsal horn neurones in the L6 and L7 segments were recorded and their receptive field properties examined. In seven animals recordings were made from identified spinocervical tract, post-synaptic dorsal column and dorsolateral funicular neurones as well as from neurones that did not project through these pathways. Thirty-one neurones were intracellularly stained with horseradish peroxidase, and fifty-three were recorded extracellularly and located by reference to stained cells. In two animals (both 31 days after nerve section) no attempt was made to identify axonal projections of the dorsal horn neurones in order to avoid any effects of cervical cord search stimuli on the cells' properties, but all isolated extracellularly recorded units were examined. On the side ipsilateral to the nerve sections 143 units were recorded. In all experiments, neurones in the medial three-quarters of the dorsal horn had no discernible cutaneous, mechanosensitive receptive fields between 19 and 55 days after nerve section. There were only two exceptions to this generalization, one neurone being one of the most rostral cells in the sample (in caudal L5) and the other being one of the most caudal cells (in caudal L7). We present evidence to show that neither of these two neurones had inappropriate receptive fields in terms of the somatotopic organization of the dorsal horn. All other neurones with receptive fields on the skin were appropriately located in the somatotopic map laid out in the dorsal horn. There was no evidence for gross anatomical changes in the dendritic trees of dorsal horn neurones following sciatic and saphenous nerve sections. We have been unable to confirm that, following loss of cutaneous receptive fields by peripheral nerve section, dorsal horn neurones in adult cats acquire 'inappropriate' receptive fields. Possible reasons for this are discussed.
Full text
PDF

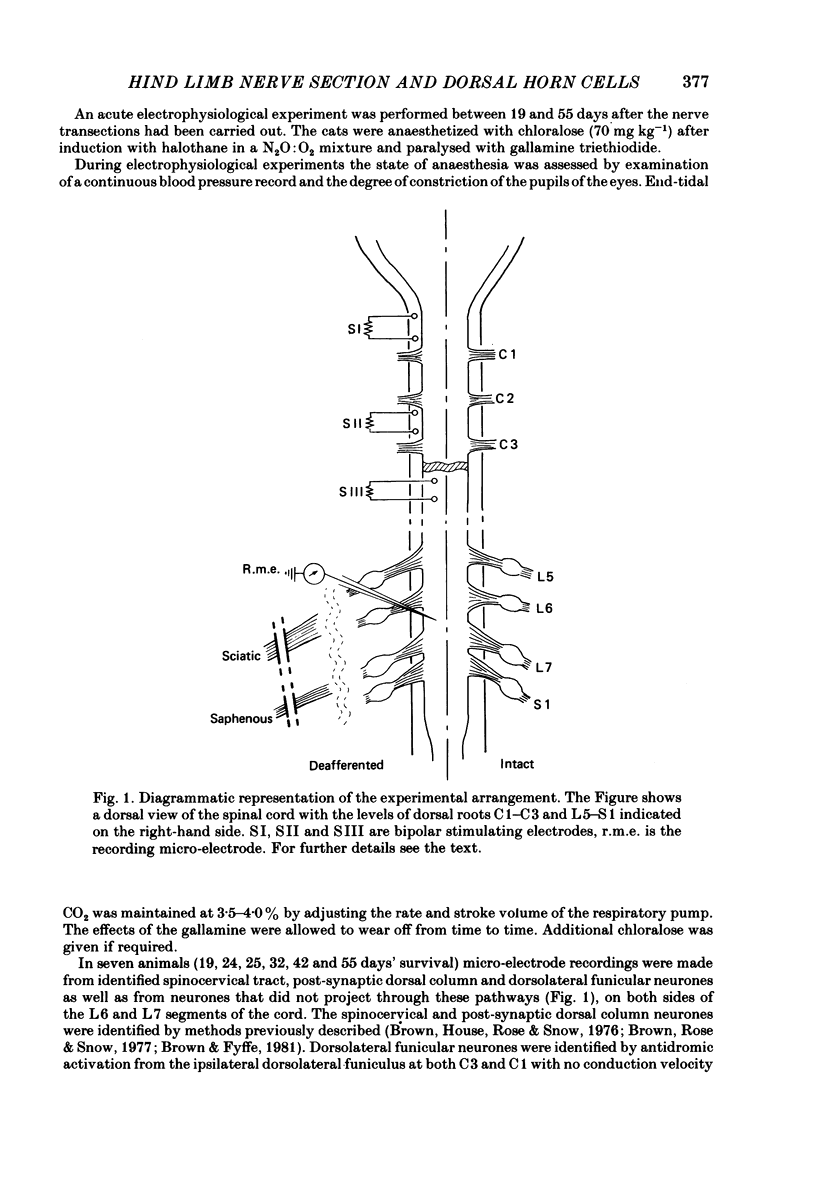

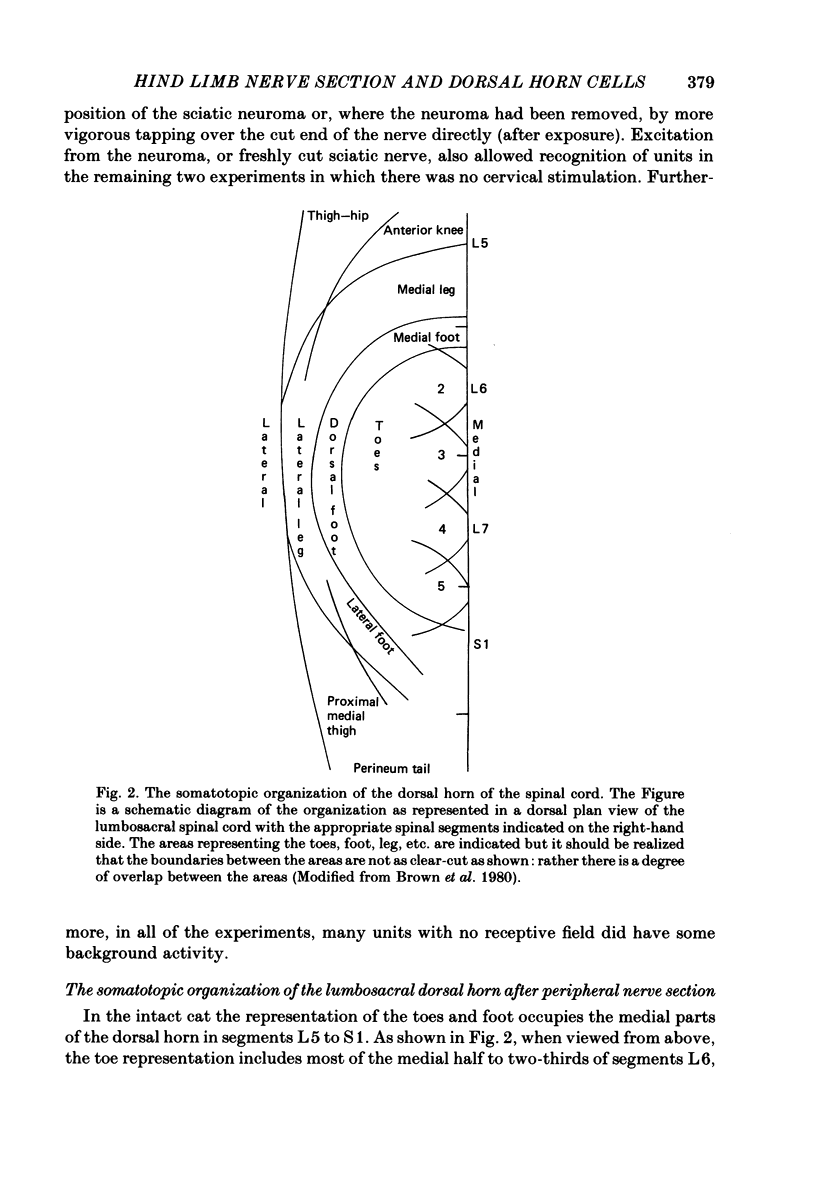
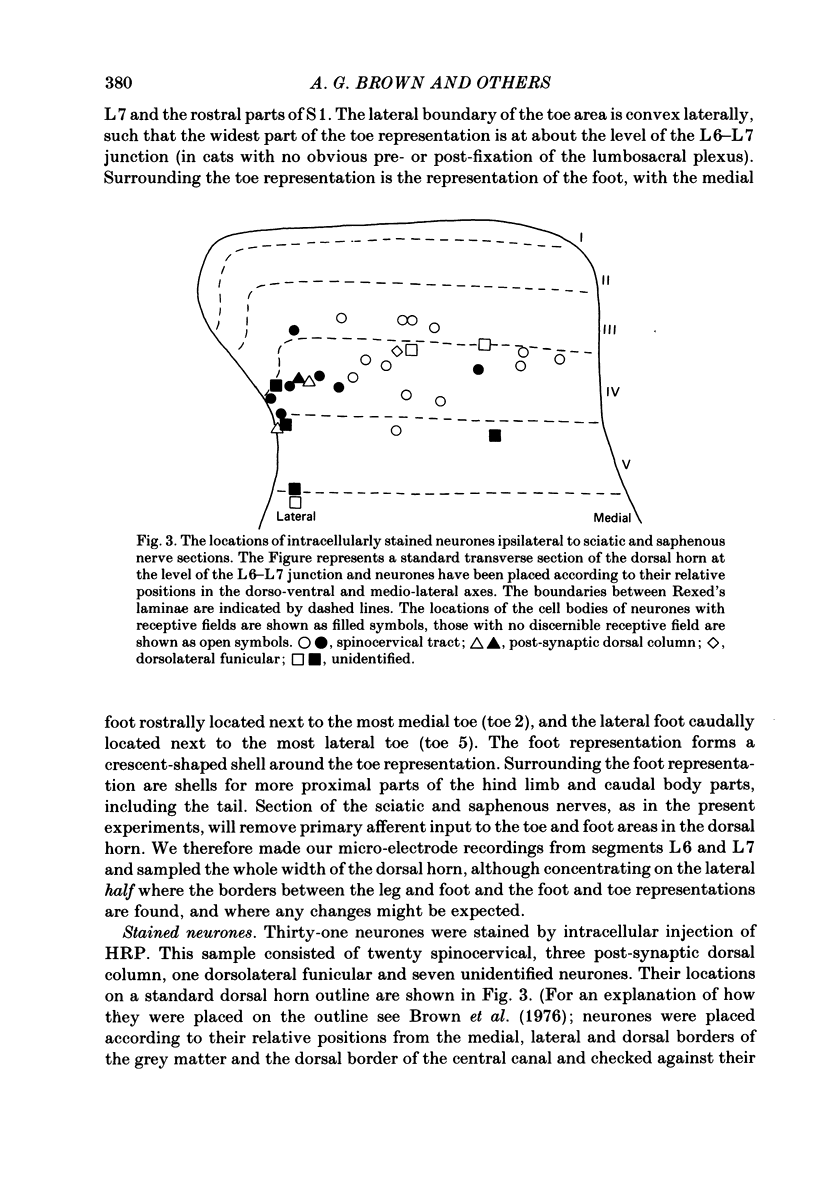

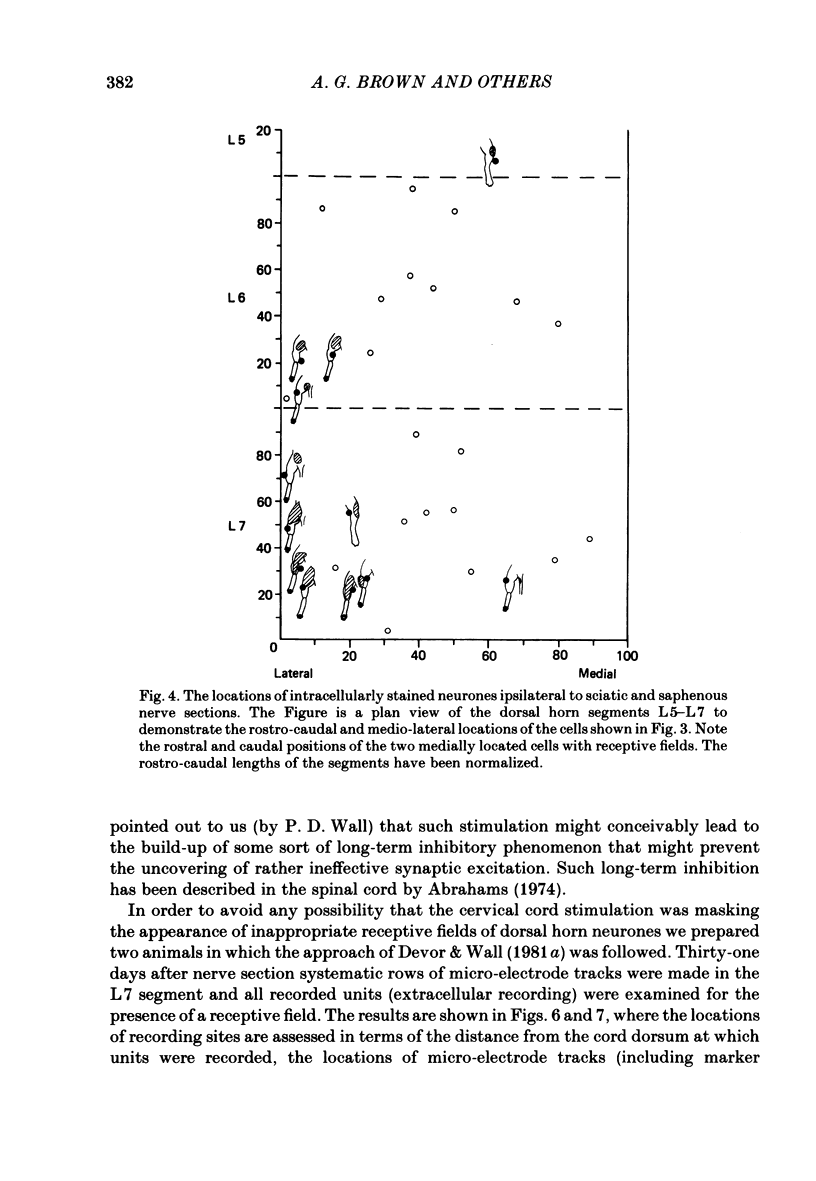







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2(1):141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Basbaum A. I., Wall P. D. Chronic changes in the response of cells in adult cat dorsal horn following partial deafferentation: the appearance of responding cells in a previously non-responsive region. Brain Res. 1976 Nov 5;116(2):181–204. doi: 10.1016/0006-8993(76)90899-4. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Brown P. B., Fyffe R. E., Pubols L. M. Effects of dorsal root section on spinocervical tract neurones in the cat. J Physiol. 1983 Apr;337:589–608. doi: 10.1113/jphysiol.1983.sp014644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Franz D. N. Responses of spinocervical tract neurones to natural stimulation of identified cutaneous receptors. Exp Brain Res. 1969;7(3):231–249. doi: 10.1007/BF00239031. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol. 1981 Dec;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E., Noble R., Rose P. K., Snow P. J. The density, distribution and topographical organization of spinocervical tract neurones in the cat. J Physiol. 1980 Mar;300:409–428. doi: 10.1113/jphysiol.1980.sp013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., House C. R., Rose P. K., Snow P. J. The morphology of spinocervical tract neurones in the cat. J Physiol. 1976 Sep;260(3):719–738. doi: 10.1113/jphysiol.1976.sp011540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Rose P. K., Snow P. J. The morphology of spinocervical tract neurones revealed by intracellular injection of horseradish peroxidase. J Physiol. 1977 Sep;270(3):747–764. doi: 10.1113/jphysiol.1977.sp011980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. B., Fuchs J. L. Somatotopic representation of hindlimb skin in cat dorsal horn. J Neurophysiol. 1975 Jan;38(1):1–9. doi: 10.1152/jn.1975.38.1.1. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Effect of peripheral nerve injury on receptive fields of cells in the cat spinal cord. J Comp Neurol. 1981 Jun 20;199(2):277–291. doi: 10.1002/cne.901990209. [DOI] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Plasticity in the spinal cord sensory map following peripheral nerve injury in rats. J Neurosci. 1981 Jul;1(7):679–684. doi: 10.1523/JNEUROSCI.01-07-00679.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor M., Wall P. D. Reorganisation of spinal cord sensory map after peripheral nerve injury. Nature. 1978 Nov 2;276(5683):75–76. doi: 10.1038/276075a0. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Lisney S. J. Changes in the somatotopic organization of the cat lumbar spinal cord following peripheral nerve transection and regeneration. Brain Res. 1983 Jan 17;259(1):31–39. doi: 10.1016/0006-8993(83)91064-8. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Sassoon E. M., Wall P. D. Properties of synaptic linkage from long ranging afferents onto dorsal horn neurones in normal and deafferented cats. J Physiol. 1978 Dec;285:299–310. doi: 10.1113/jphysiol.1978.sp012572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pubols L. M., Brenowitz G. L. Maintenance of dorsal horn somatotopic organization and increased high-threshold response after single-root or spared-root deafferentiation in cats. J Neurophysiol. 1982 Jan;47(1):103–112. doi: 10.1152/jn.1982.47.1.103. [DOI] [PubMed] [Google Scholar]
- Pubols L. M., Goldberger M. E. Recovery of function in dorsal horn following partial deafferentation. J Neurophysiol. 1980 Jan;43(1):102–117. doi: 10.1152/jn.1980.43.1.102. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- REXED B. The cytoarchitectonic organization of the spinal cord in the cat. J Comp Neurol. 1952 Jun;96(3):414–495. doi: 10.1002/cne.900960303. [DOI] [PubMed] [Google Scholar]


