Abstract
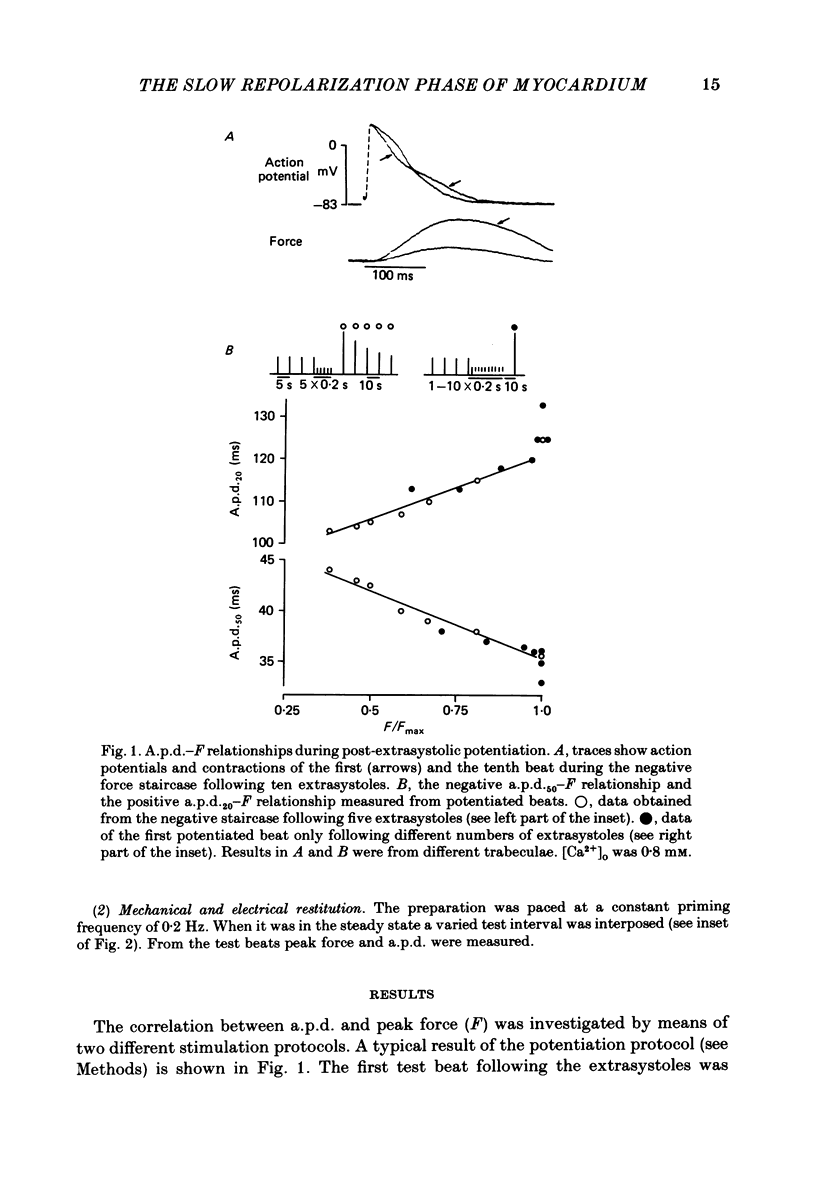
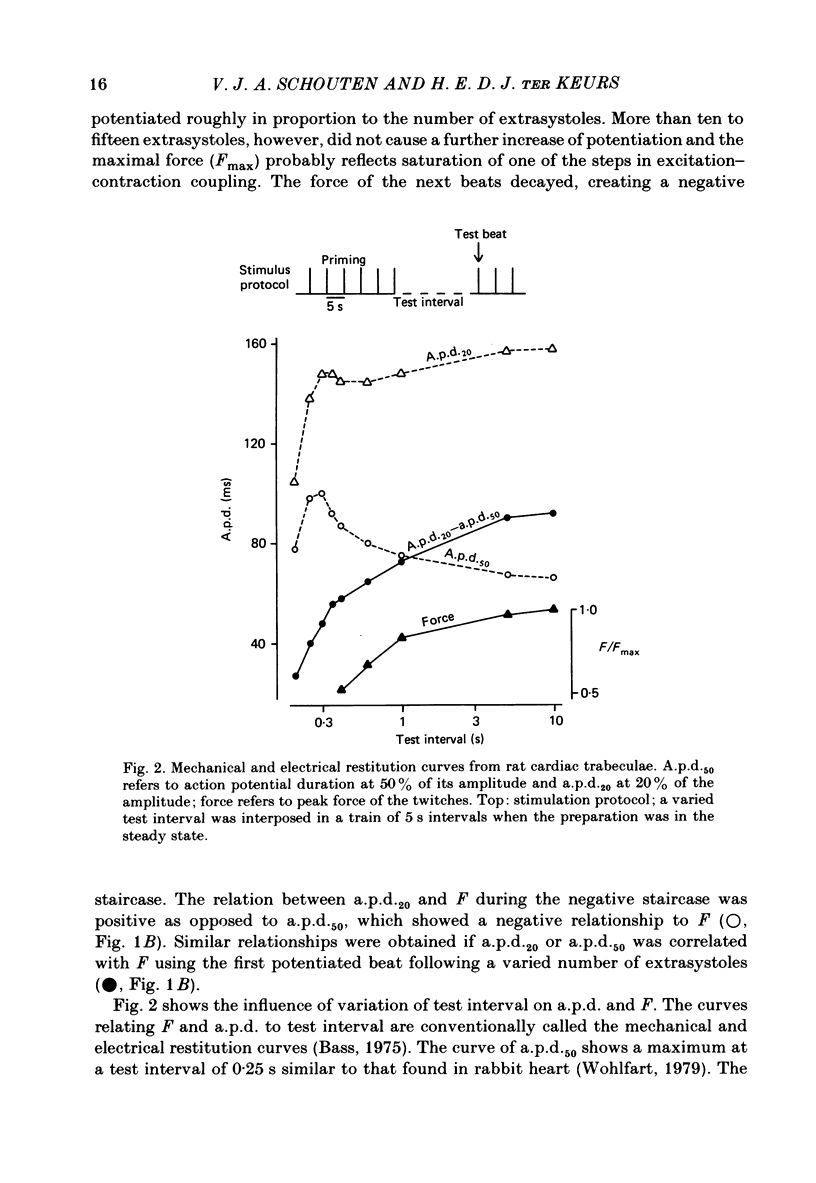
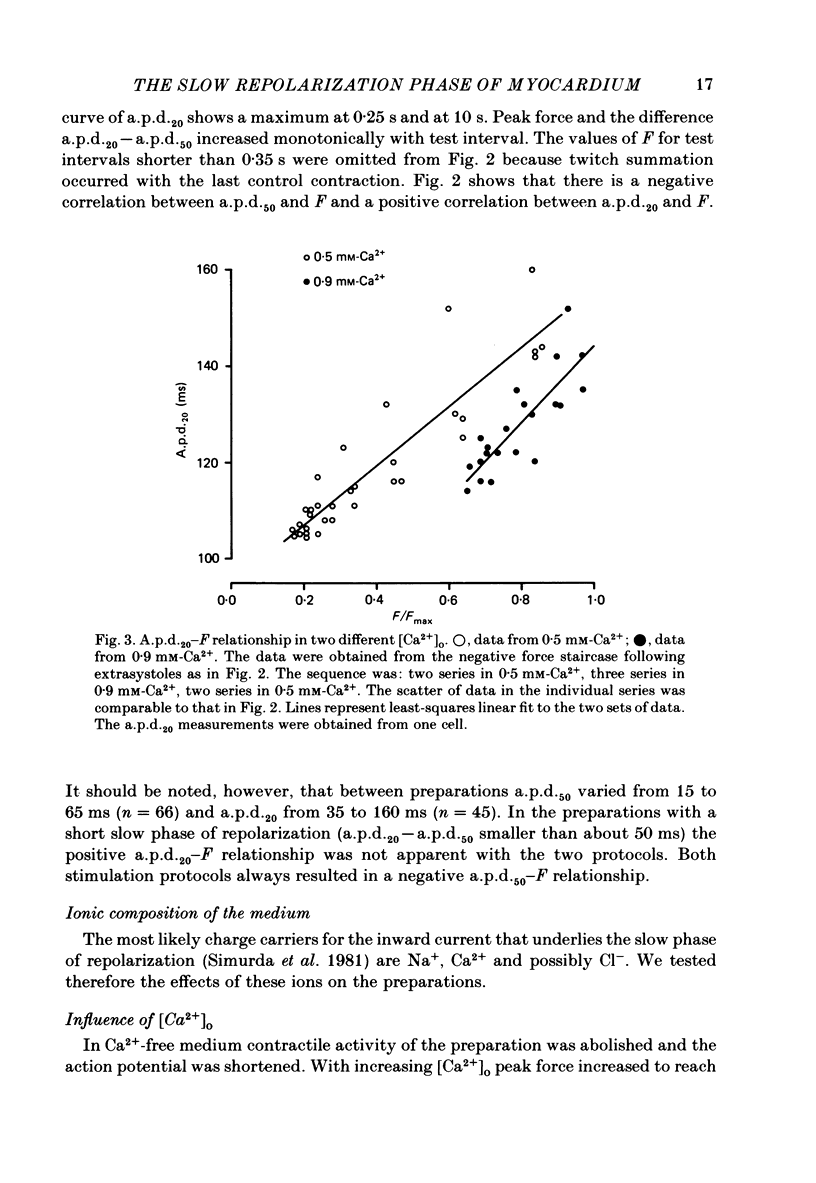
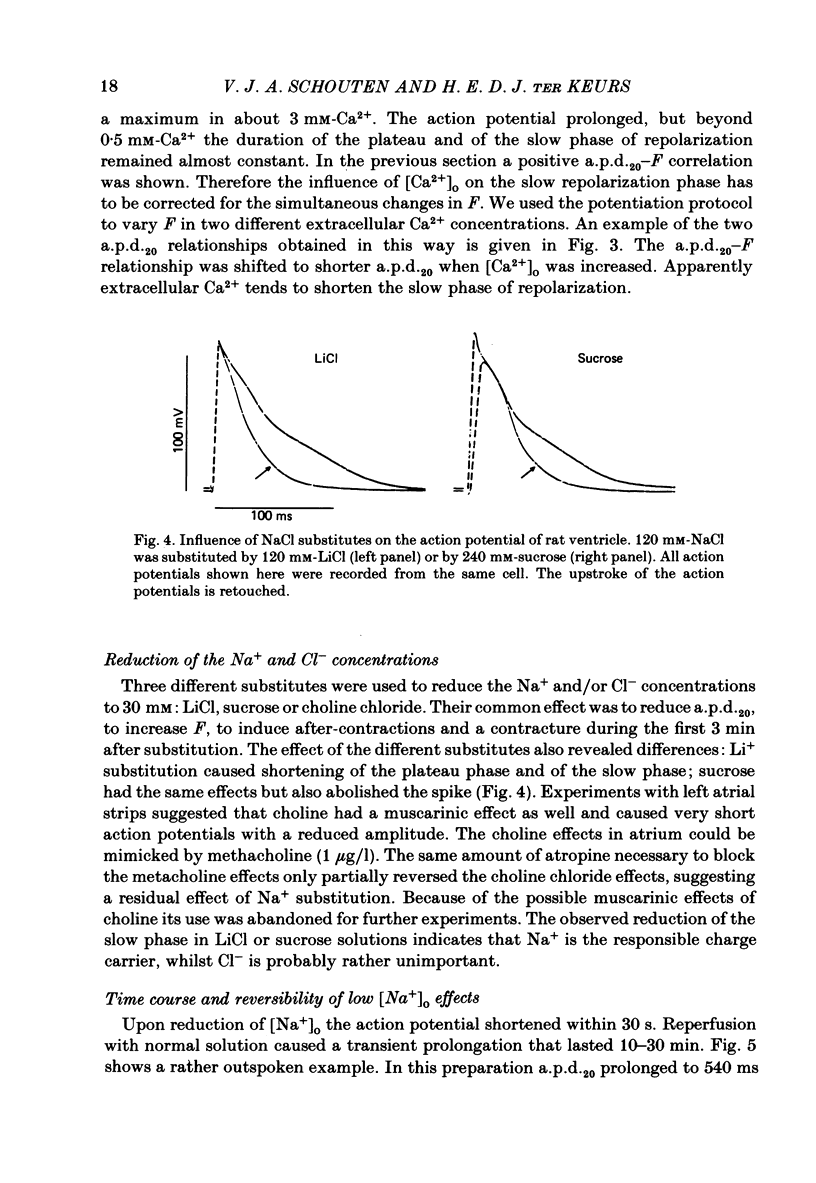
Intracellular action potentials and isometric force were measured from thin trabeculae of the right ventricle of rat heart. Characteristic for the action potential of rat myocardium is a short plateau and a slow final repolarization phase. We have studied the influence of ionic composition of the medium and of stimulation frequency on the slow phase of repolarization and its relation to peak force. The results confirmed a positive correlation between peak force and the duration of the slow phase of repolarization, as has been reported for other species. An increase of [Ca2+]o caused a shortening of the slow phase of repolarization when peak force was kept constant. In low [Na+]o peak force was increased and the slow phase of repolarization was shortened. Reperfusion with normal medium after a period in low [Na+]o induced a transient prolongation of the slow phase of repolarization and reduction of peak force. The transient lasted about 20 min. In the presence of the Ca2+ entry blocker nifedipine the action potential duration and peak force were reduced. Low [Na+]o caused less shortening of the slow phase of repolarization and a greater increase of peak force. The slow phase of repolarization was prolonged transiently following reperfusion at normal [Na+]o, but only during a few beats. These results are in agreement with the hypothesis that the slow phase of repolarization is due to an inward current generated by Na+-Ca2+ exchange, as latter mechanism is known to be sensitive to the intracellular and extracellular concentrations of both Na+ and Ca2+.
Full text
PDF

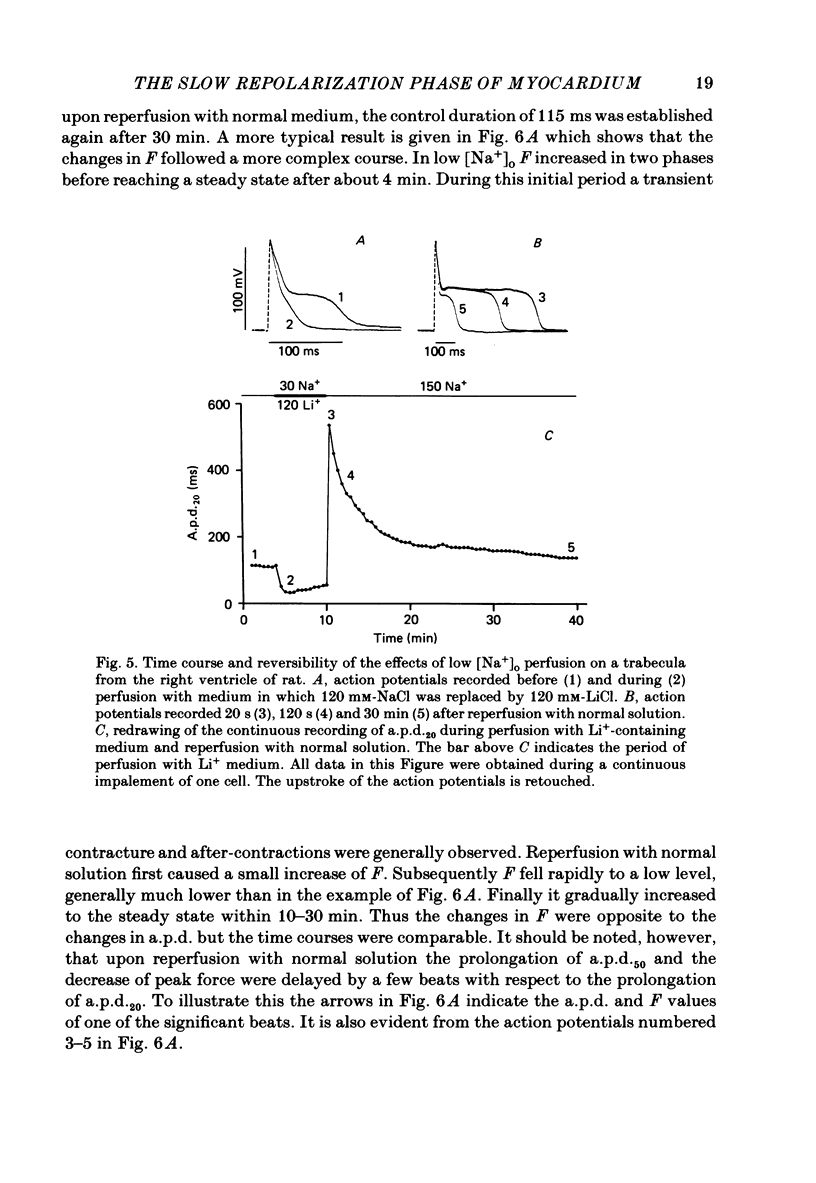





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Eisner D. A., Lab M. J., Orchard C. H. The effects of low sodium solutions on intracellular calcium concentration and tension in ferret ventricular muscle. J Physiol. 1983 Dec;345:391–407. doi: 10.1113/jphysiol.1983.sp014984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. Calcium transients in mammalian ventricular muscle. Eur Heart J. 1980;Suppl A:5–15. doi: 10.1093/eurheartj/1.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- Arlock P., Katzung B. G. Effects of sodium substitutes on ouabain induced transient inward current. Proc West Pharmacol Soc. 1982;25:57–60. [PubMed] [Google Scholar]
- Bass B. G. Restitution of the action potential in cat papillary muscle. Am J Physiol. 1975 Jun;228(6):1717–1724. doi: 10.1152/ajplegacy.1975.228.6.1717. [DOI] [PubMed] [Google Scholar]
- Boyett M. R., Jewell B. R. Analysis of the effects of changes in rate and rhythm upon electrical activity in the heart. Prog Biophys Mol Biol. 1980;36(1):1–52. doi: 10.1016/0079-6107(81)90003-1. [DOI] [PubMed] [Google Scholar]
- Chapman R. A. Control of cardiac contractility at the cellular level. Am J Physiol. 1983 Oct;245(4):H535–H552. doi: 10.1152/ajpheart.1983.245.4.H535. [DOI] [PubMed] [Google Scholar]
- Chapman R. A., Coray A., McGuigan J. A. Sodium/calcium exchange in mammalian ventricular muscle: a study with sodium-sensitive micro-electrodes. J Physiol. 1983 Oct;343:253–276. doi: 10.1113/jphysiol.1983.sp014891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clusin W. T., Fischmeister R., DeHaan R. L. Caffeine-induced current in embryonic heart cells: time course and voltage dependence. Am J Physiol. 1983 Sep;245(3):H528–H532. doi: 10.1152/ajpheart.1983.245.3.H528. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Coraboeuf E., Vassort G. Effects of some inhibitors of ionic permeabilities on ventricular action potential and contraction of rat and guinea-pig hearts. J Electrocardiol. 1968;1(1):19–29. doi: 10.1016/s0022-0736(68)80005-6. [DOI] [PubMed] [Google Scholar]
- Croaboeuf E., Gautier P., Giuraudou P. Potential and tension changes induced by sodium removal in dog Purkinje fibres: role of an electrogenic sodium-calcium exchange. J Physiol. 1981 Feb;311:605–622. doi: 10.1113/jphysiol.1981.sp013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Vassort G. The electrogenic Na-Ca exchange and the cardiac electrical activity. I--Simulation on Purkinje fibre action potential. J Physiol (Paris) 1981 Sep;77(6-7):705–709. [PubMed] [Google Scholar]
- Fozzard H. A. Heart: excitation-contraction coupling. Annu Rev Physiol. 1977;39:201–220. doi: 10.1146/annurev.ph.39.030177.001221. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Glycocalyx is not required for show inward calcium current in isolated rat heart myocytes. Nature. 1980 Mar 27;284(5754):358–360. doi: 10.1038/284358a0. [DOI] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ Res. 1984 Feb;54(2):144–156. doi: 10.1161/01.res.54.2.144. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W., Weingart R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:209–226. doi: 10.1113/jphysiol.1978.sp012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan M. J., Niedergerke R. Intracellular sodium concentration and resting sodium fluxes of the frog heart ventricle. J Physiol. 1967 Jan;188(2):235–260. doi: 10.1113/jphysiol.1967.sp008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Mechanism of calcium channel blockade by verapamil, D600, diltiazem and nitrendipine in single dialysed heart cells. Nature. 1983 Apr 28;302(5911):790–794. doi: 10.1038/302790a0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Weeks T. A., Kao R. L., Akaike N., Brown A. M. Sodium current in single heart muscle cells. Nature. 1979 Mar 15;278(5701):269–271. doi: 10.1038/278269a0. [DOI] [PubMed] [Google Scholar]
- Lipsius S. L., Gibbons W. R. Membrane currents, contractions, and aftercontractions in cardiac Purkinje fibers. Am J Physiol. 1982 Jul;243(1):H77–H86. doi: 10.1152/ajpheart.1982.243.1.H77. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. Characteristics of the second inward current in cells isolated from rat ventricular muscle. Proc R Soc Lond B Biol Sci. 1983 Oct 22;219(1217):447–469. doi: 10.1098/rspb.1983.0084. [DOI] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. The effects of ryanodine, EGTA and low-sodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during the plateau. Br J Pharmacol. 1984 Mar;81(3):543–550. doi: 10.1111/j.1476-5381.1984.tb10107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. The generation of electric currents in cardiac fibers by Na/Ca exchange. Am J Physiol. 1979 Mar;236(3):C103–C110. doi: 10.1152/ajpcell.1979.236.3.C103. [DOI] [PubMed] [Google Scholar]
- Payet M. D., Schanne O. F., Ruiz-Ceretti E. Frequency dependence of the ionic currents determining the action potential repolarization in rat ventricular muscle. J Mol Cell Cardiol. 1981 Feb;13(2):207–215. doi: 10.1016/0022-2828(81)90217-0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Exchange of calcium ions in the mammalian myocardium. Mechanisms and physiological significance. Circ Res. 1974 May;34(5):599–605. doi: 10.1161/01.res.34.5.599. [DOI] [PubMed] [Google Scholar]
- SLEATOR W., Jr, FURCHGOTT R. F., DE GUBAREFF T., KRESPI V. ACTION POTENTIALS OF GUINEA PIG ATRIA UNDER CONDITIONS WHICH ALTER CONTRACTION. Am J Physiol. 1964 Feb;206:270–282. doi: 10.1152/ajplegacy.1964.206.2.270. [DOI] [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simurda J., Simurdová M., Bravený P., Sumbera J. Activity-dependent changes of slow inward current in ventricular heart muscle. Pflugers Arch. 1981 Oct;391(4):277–283. doi: 10.1007/BF00581507. [DOI] [PubMed] [Google Scholar]
- VAUGHAN WILLIAMS E. M. A study of intracellular potentials and contractions in atria, including evidence for an after-potential. J Physiol. 1959 Dec;149:78–92. doi: 10.1113/jphysiol.1959.sp006326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstrom J. A., Schwartz D. J., Fozzard H. A. Relation between intracellular sodium and twitch tension in sheep cardiac Purkinje strands exposed to cardiac glycosides. Circ Res. 1983 Jun;52(6):697–705. doi: 10.1161/01.res.52.6.697. [DOI] [PubMed] [Google Scholar]
- Wohlfart B., Noble M. I. The cardiac excitation-contraction cycle. Pharmacol Ther. 1982;16(1):1–43. doi: 10.1016/0163-7258(82)90030-4. [DOI] [PubMed] [Google Scholar]
- Wohlfart B. Relationships between peak force, action potential duration and stimulus interval in rabbit myocardium. Acta Physiol Scand. 1979 Aug;106(4):395–409. doi: 10.1111/j.1748-1716.1979.tb06419.x. [DOI] [PubMed] [Google Scholar]


