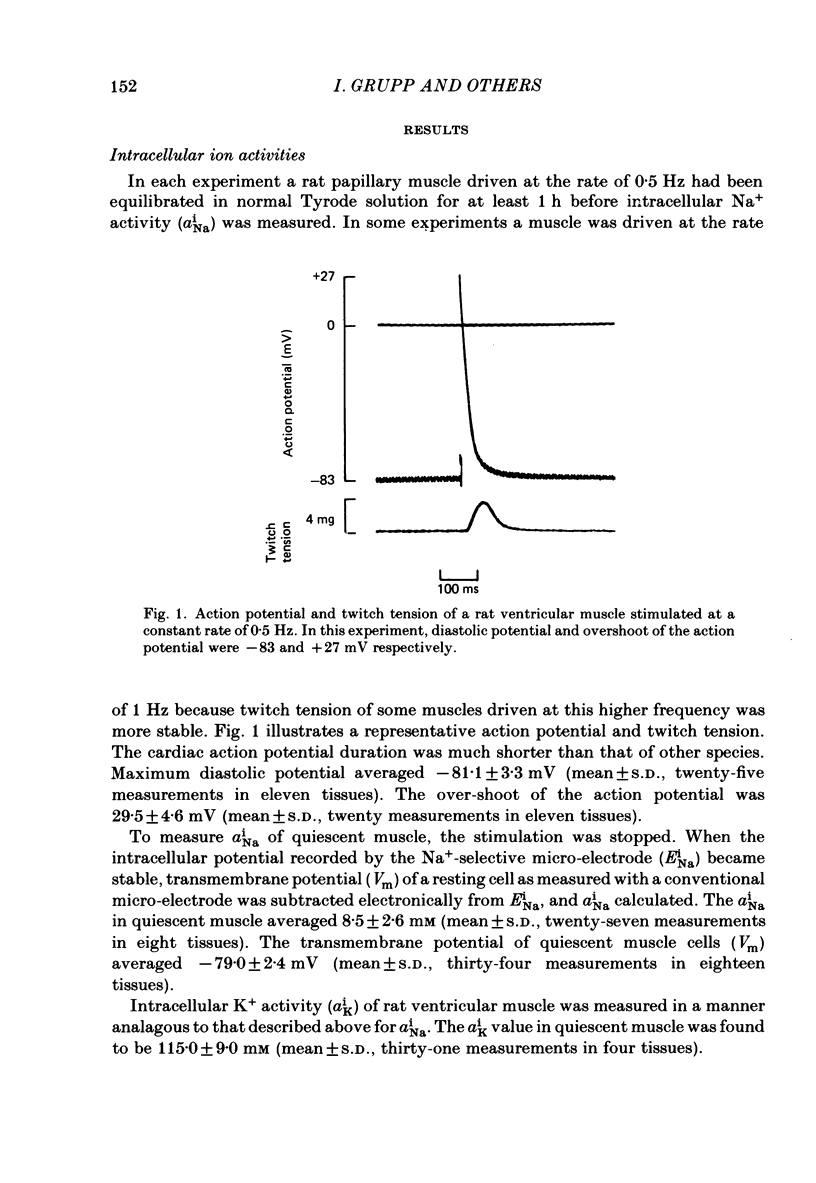
Abstract
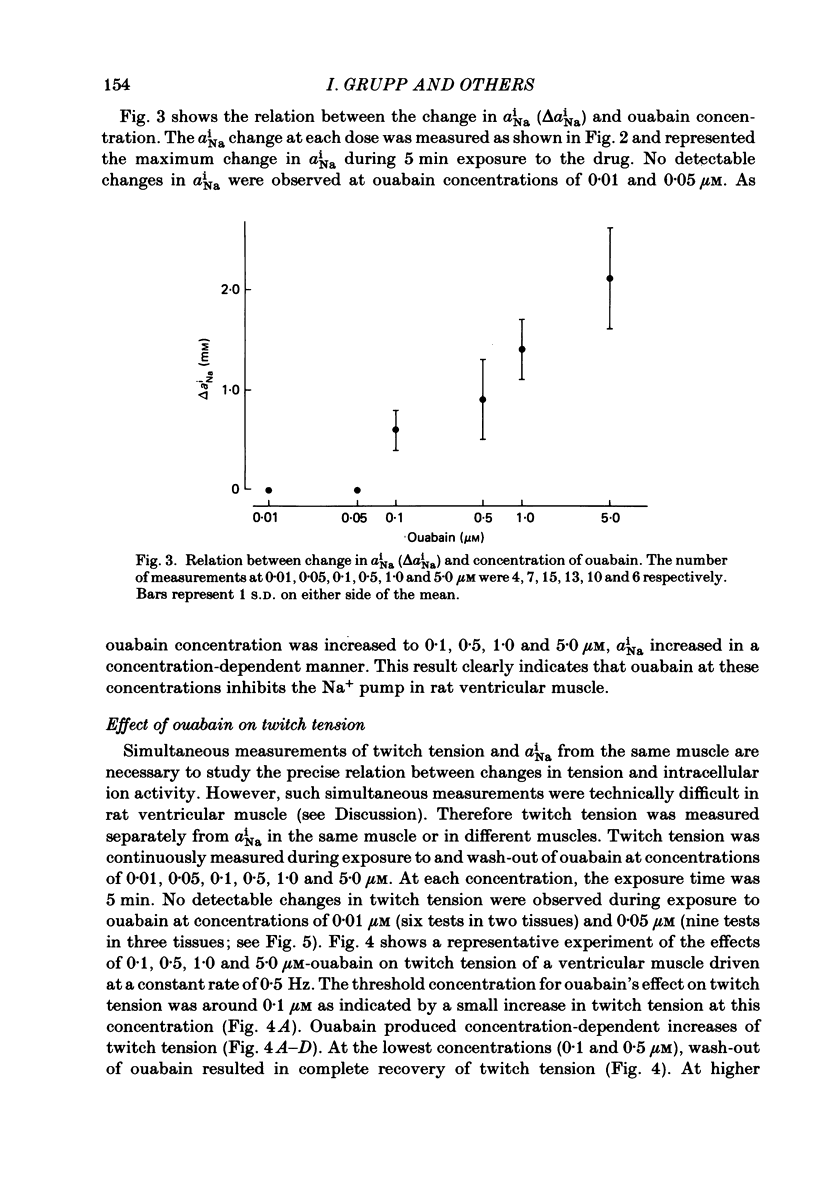
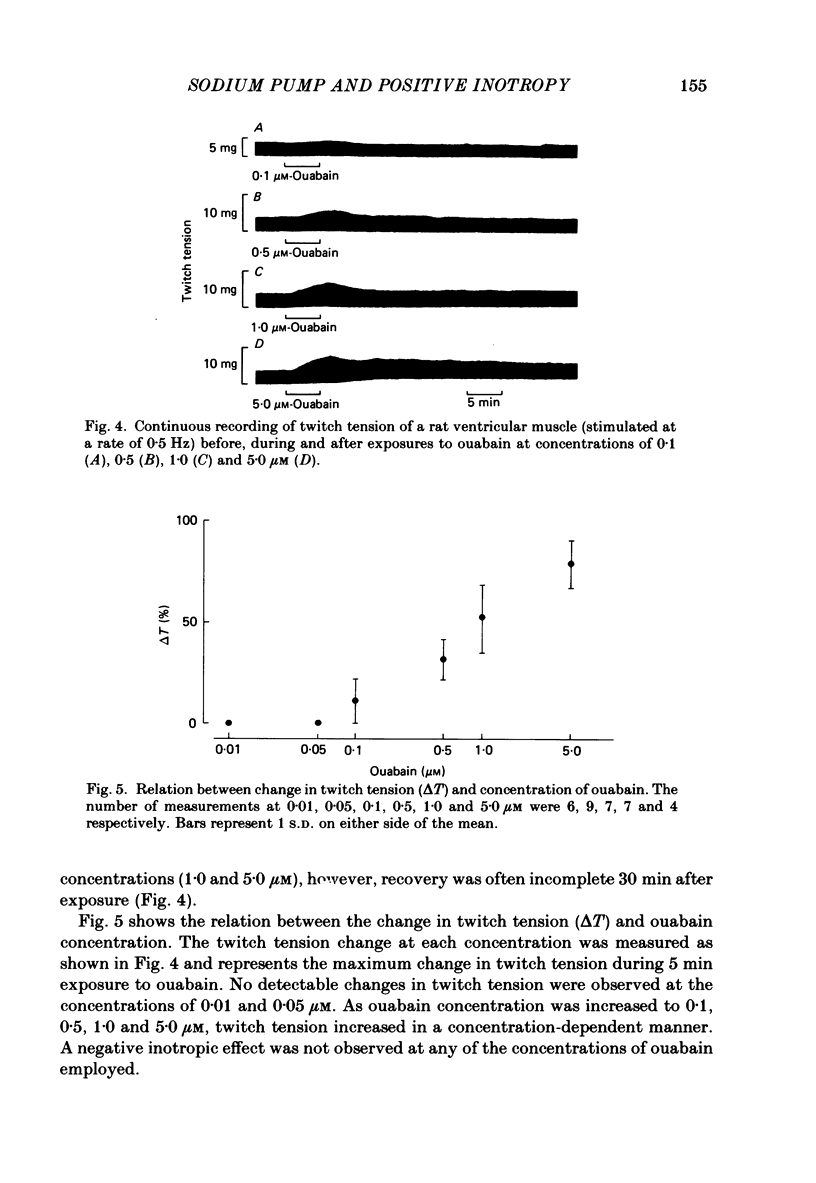
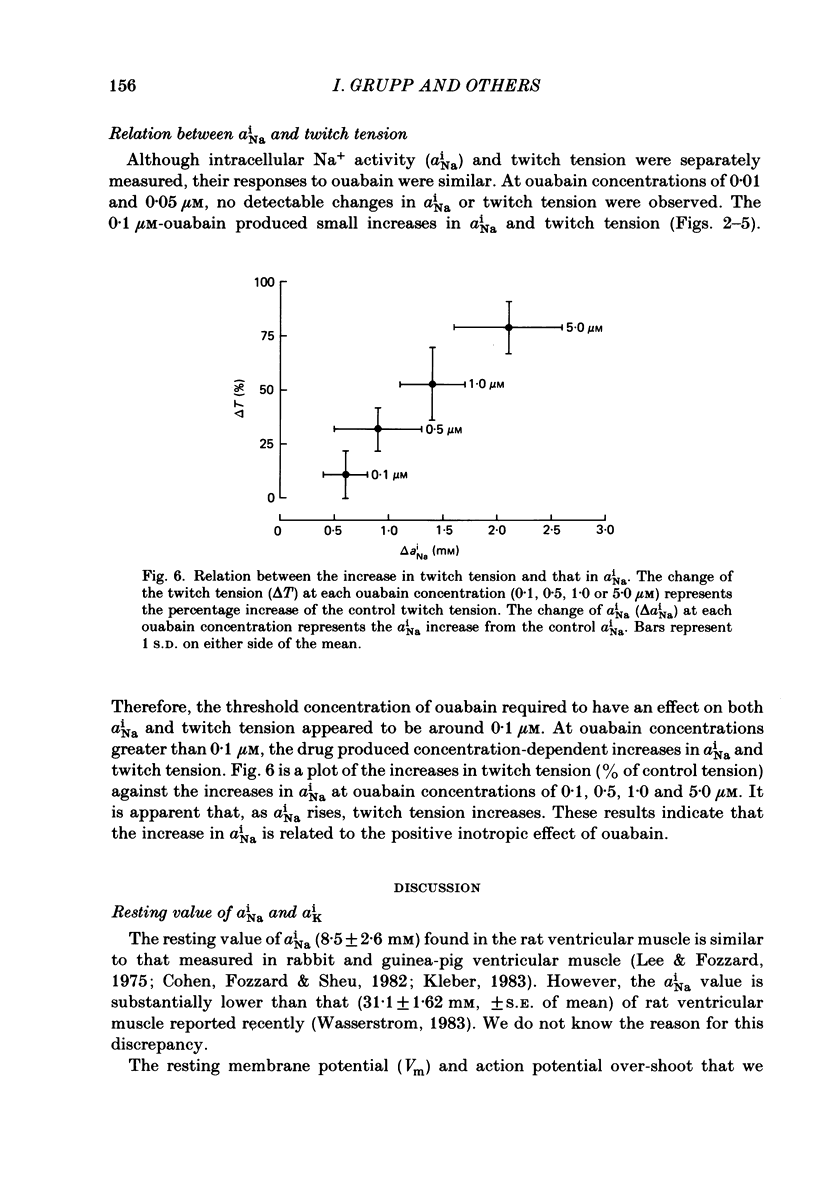
Low concentrations of ouabain which produce a positive inotropic effect on rat ventricular muscle do not inhibit the isolated Na+-K+-ATPase enzyme from this tissue, suggesting that these low-concentration inotropic effects are not related to sodium pump inhibition (Erdmann, Philipp & Scholz, 1980; Adams, Schwartz, Grupp, Grupp, Lee, Wallick, Powell, Twist & Gathiram, 1982). We tested this hypothesis by continuously measuring intracellular Na+ activity with Na+-selective micro-electrodes and, separately, twitch tension of rat ventricular muscle during exposure to and wash-out of ouabain. Intracellular Na+ activity (aiNa) and transmembrane potential of quiescent muscle cells averaged 8.5 +/- 2.6 mM (mean +/- S.D., n = 27) and -79.2 +/- 2.4 mV (n = 34) respectively. Low concentrations of ouabain (0.1, 0.5 and 1.0 microM) produced concentration-dependent increases in both aiNa and twitch tension. At lower concentrations of ouabain (0.01 and 0.05 microM), no detectable changes in aiNa and twitch tension were observed. The data strongly indicate that in rat ventricular muscle sodium pump inhibition is present at low concentrations of ouabain which produce positive inotropy. This is consistent with previous results in canine and sheep cardiac Purkinje fibres.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. J., Schwartz A., Grupp G., Grupp I., Lee S. W., Wallick E. T., Powell T., Twist V. W., Gathiram P. High-affinity ouabain binding site and low-dose positive inotropic effect in rat myocardium. Nature. 1982 Mar 11;296(5853):167–169. doi: 10.1038/296167a0. [DOI] [PubMed] [Google Scholar]
- Baumgarten C. M., Cohen C. J., McDonald T. F. Heterogeneity of intracellular potassium activity and membrane potential in hypoxic guinea pig ventricle. Circ Res. 1981 Nov;49(5):1181–1189. doi: 10.1161/01.res.49.5.1181. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Fozzard H. A., Sheu S. S. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982 May;50(5):651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Dagostino M., Lee C. O. Neutral carrier Na+- and Ca2+-selective microelectrodes for intracellular application. Biophys J. 1982 Dec;40(3):199–207. doi: 10.1016/S0006-3495(82)84475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Characterization of the electrogenic sodium pump in cardiac Purkinje fibres. J Physiol. 1980 Jun;303:441–474. doi: 10.1113/jphysiol.1980.sp013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The control of tonic tension by membrane potential and intracellular sodium activity in the sheep cardiac Purkinje fibre. J Physiol. 1983 Feb;335:723–743. doi: 10.1113/jphysiol.1983.sp014560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J Physiol. 1981 Aug;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Vaughan-Jones R. D., Lederer W. J. Active transport and inotropic state in guinea pig left atrium. Circ Res. 1983 Dec;53(6):834–836. doi: 10.1161/01.res.53.6.834. [DOI] [PubMed] [Google Scholar]
- Erdmann E., Philipp G., Scholz H. Cardiac glycoside receptor, (Na+ + K+)-ATPase activity and force of contraction in rat heart. Biochem Pharmacol. 1980 Dec;29(24):3219–3229. doi: 10.1016/0006-2952(80)90295-6. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C., Cranefield P. F. Direct measurement of changes in sodium pump current in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1783–1787. doi: 10.1073/pnas.76.4.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghysel-Burton J., Godfraind T. Importance of the lactone ring for the action of therapeutic doses of ouabain in guinea-pig atria [proceedings]. J Physiol. 1977 Mar;266(1):75P–76P. [PubMed] [Google Scholar]
- Glitsch H. G., Reuter H., Scholz H. The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol. 1970 Jul;209(1):25–43. doi: 10.1113/jphysiol.1970.sp009153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T., Ghysel-Burton J. Independence of the positive inotropic effect of ouabain from the inhibition of the heart Na+/K+ pump. Proc Natl Acad Sci U S A. 1980 May;77(5):3067–3069. doi: 10.1073/pnas.77.5.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougen T. J., Spicer N., Smith T. W. Stimulation of monovalent cation active transport by low concentrations of cardiac glycosides. Role of catecholamines. J Clin Invest. 1981 Nov;68(5):1207–1214. doi: 10.1172/JCI110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kléber A. G. Resting membrane potential, extracellular potassium activity, and intracellular sodium activity during acute global ischemia in isolated perfused guinea pig hearts. Circ Res. 1983 Apr;52(4):442–450. doi: 10.1161/01.res.52.4.442. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Serena S. D. Effects of strophanthidin upon contraction and ionic exchange in rabbit ventricular myocardium: relation to control of active state. J Mol Cell Cardiol. 1970 Mar;1(1):65–90. doi: 10.1016/0022-2828(70)90029-5. [DOI] [PubMed] [Google Scholar]
- Lechat P., Malloy C. R., Smith T. W. Active transport and inotropic state in guinea pig left atrium. Circ Res. 1983 Apr;52(4):411–422. doi: 10.1161/01.res.52.4.411. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Dagostino M. Effect of strophanthidin on intracellular Na ion activity and twitch tension of constantly driven canine cardiac Purkinje fibers. Biophys J. 1982 Dec;40(3):185–198. doi: 10.1016/S0006-3495(82)84474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O., Fozzard H. A. Activities of potassium and sodium ions in rabbit heart muscle. J Gen Physiol. 1975 Jun;65(6):695–708. doi: 10.1085/jgp.65.6.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. O. Ionic activities in cardiac muscle cells and application of ion-selective microelectrodes. Am J Physiol. 1981 Oct;241(4):H459–H478. doi: 10.1152/ajpheart.1981.241.4.H459. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Kang D. H., Sokol J. H., Lee K. S. Relation between intracellular Na ion activity and tension of sheep cardiac Purkinje fibers exposed to dihydro-ouabain. Biophys J. 1980 Feb;29(2):315–330. doi: 10.1016/S0006-3495(80)85135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui H., Schwartz A. Mechanism of cardiac glycoside inhibition of the (Na+-K+)-dependent ATPase from cardiac tissue. Biochim Biophys Acta. 1968 Mar 25;151(3):655–663. doi: 10.1016/0005-2744(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Noble D. Mechanism of action of therapeutic levels of cardiac glycosides. Cardiovasc Res. 1980 Sep;14(9):495–514. doi: 10.1093/cvr/14.9.495. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- Sheu S. S., Fozzard H. A. Transmembrane Na+ and Ca2+ electrochemical gradients in cardiac muscle and their relationship to force development. J Gen Physiol. 1982 Sep;80(3):325–351. doi: 10.1085/jgp.80.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalle M., Lee C. O. The relationship among intracellular sodium activity, calcium, and strophanthidin inotropy in canine cardiac Purkinje fibers. J Gen Physiol. 1984 Feb;83(2):287–307. doi: 10.1085/jgp.83.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstrom J. A., Schwartz D. J., Fozzard H. A. Relation between intracellular sodium and twitch tension in sheep cardiac Purkinje strands exposed to cardiac glycosides. Circ Res. 1983 Jun;52(6):697–705. doi: 10.1161/01.res.52.6.697. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Delbridge L. M., Bustamante J. O., McDonald T. F. Heterogeneity of the action potential in isolated rat ventricular myocytes and tissue. Circ Res. 1983 Mar;52(3):280–290. doi: 10.1161/01.res.52.3.280. [DOI] [PubMed] [Google Scholar]


