Abstract
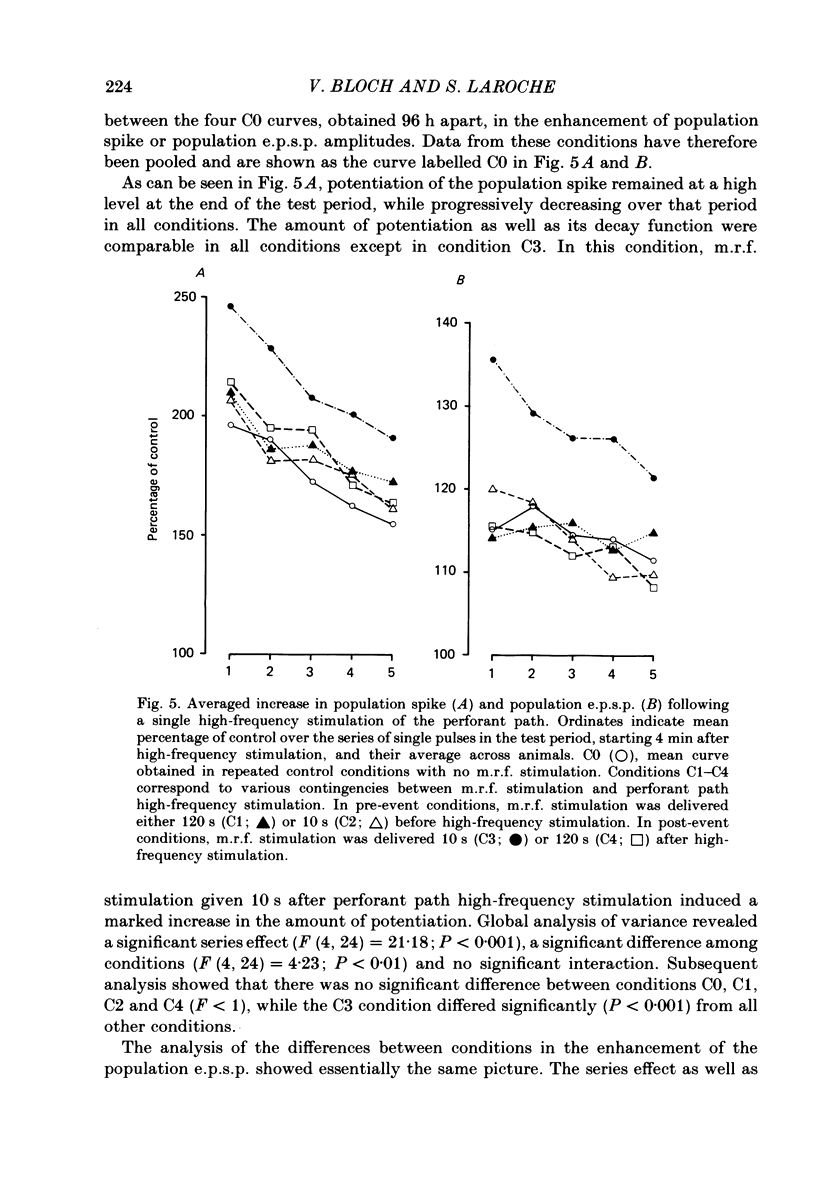
The possibility that post-trial stimulation of the mesencephalic reticular formation (m.r.f.) may modulate long-term potentiation (l.t.p.) at the perforant path to dentate granule cell synapses was studied in freely moving rats. Extracellular potentials evoked in the dentate gyrus by test pulses to the perforant path were recorded before and at various delays after a series of high-frequency stimulus trains to the perforant path (ten trains of eight pulses at 400 Hz, delivered at 5 min intervals). We have compared the magnitude and duration of l.t.p. of the population spike in this control condition with that observed when a low-intensity m.r.f. stimulation was delivered 10 s after each train to the perforant path. Post-event m.r.f. stimulation enhanced the amount of l.t.p. induced by the series of high-frequency stimulus trains and prolonged its duration for several days. The size of the population spike was unaffected by repeated m.r.f. stimulation in the absence of perforant path high-frequency stimulation, or when this failed to induce significant l.t.p. The temporal gradient of efficacy of m.r.f. stimulation was investigated. M.r.f. stimulation delivered 10 s after a single high-frequency stimulation of perforant path fibres resulted in an enhanced l.t.p. of both the population excitatory post-synaptic potential (e.p.s.p.) and population spike. L.t.p. was unaffected by m.r.f. stimulation given either before, or 120 s after perforant path high-frequency stimulation. These results show that low-intensity m.r.f. stimulation enhances lasting changes in synaptic function in the dentate gyrus when delivered during a critical period following high-frequency activation of perforant path fibres. These results are discussed in the light of our previous findings on the effects of post-event m.r.f. stimulation on memory and on the development of associative changes in hippocampal multiunit activity during conditioning. It is hypothesized that l.t.p.-like mechanisms may be involved in the stabilization of neural networks by experience and that this process might be reinforced by diffuse m.r.f. activation.
Full text
PDF



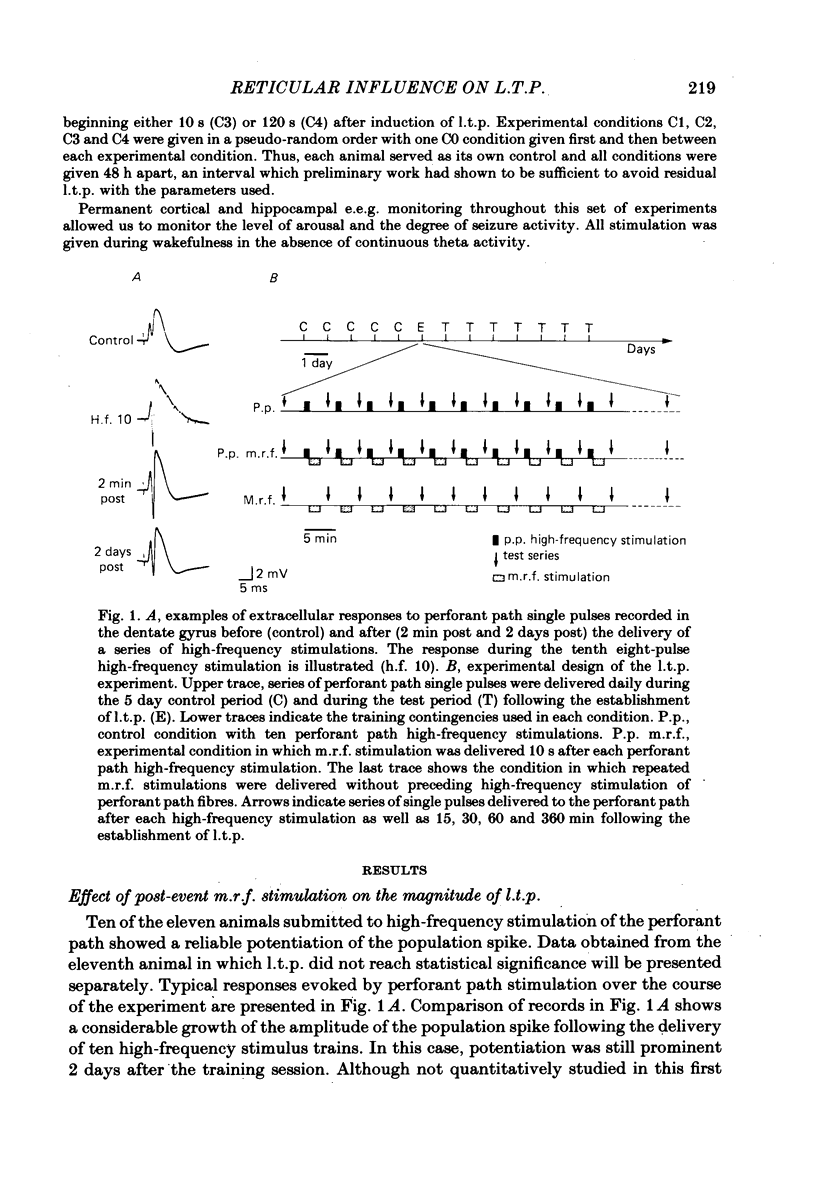
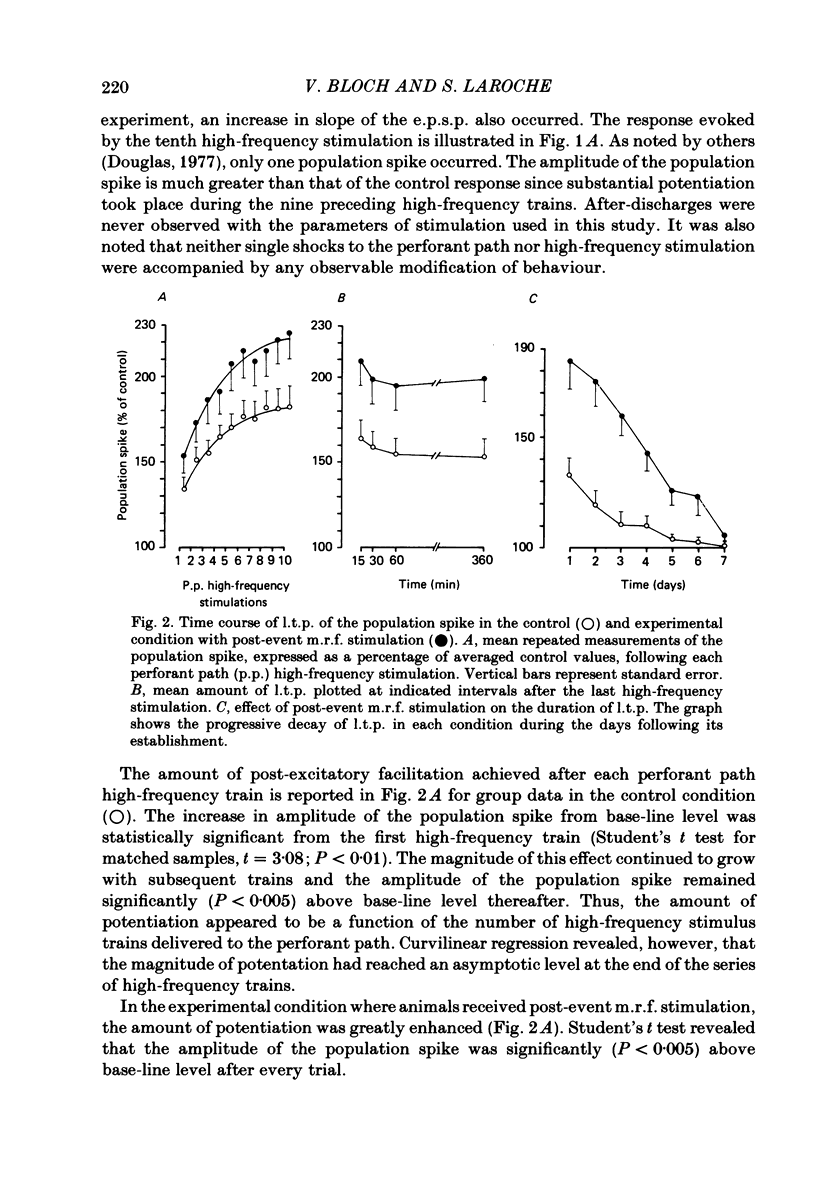

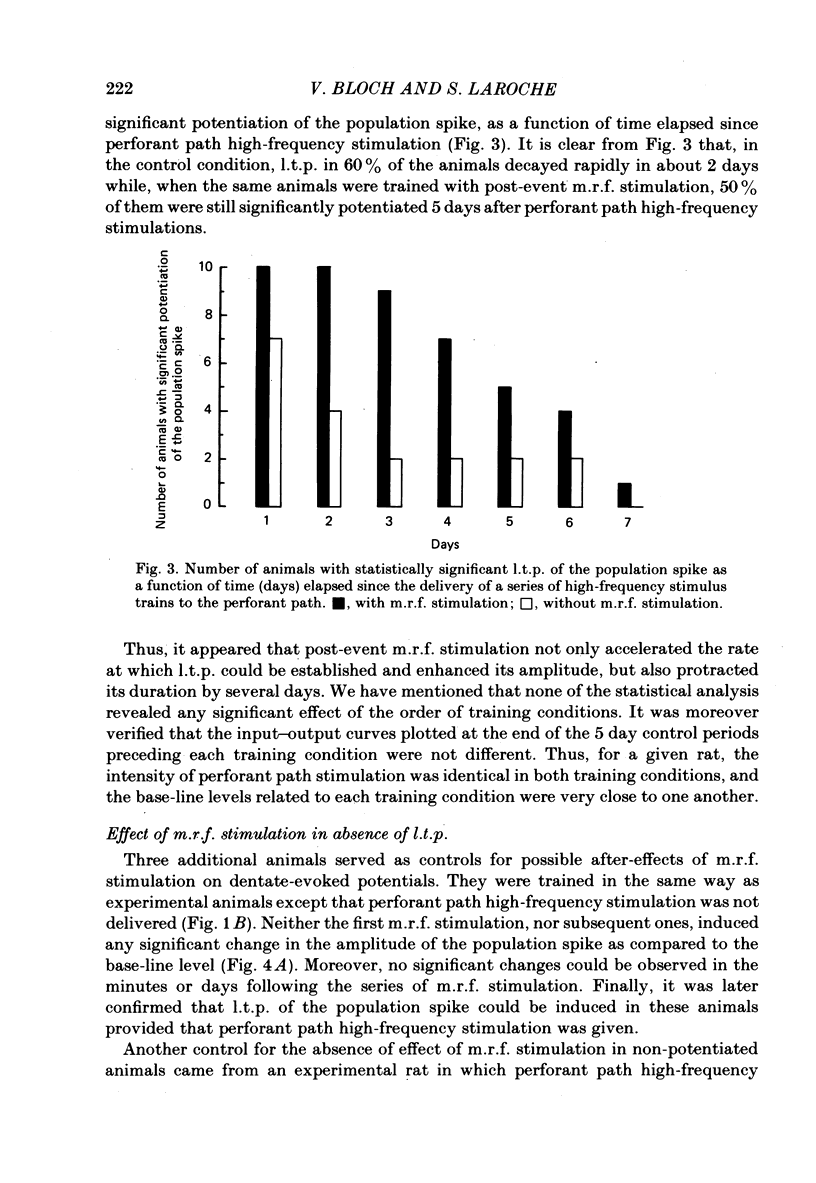
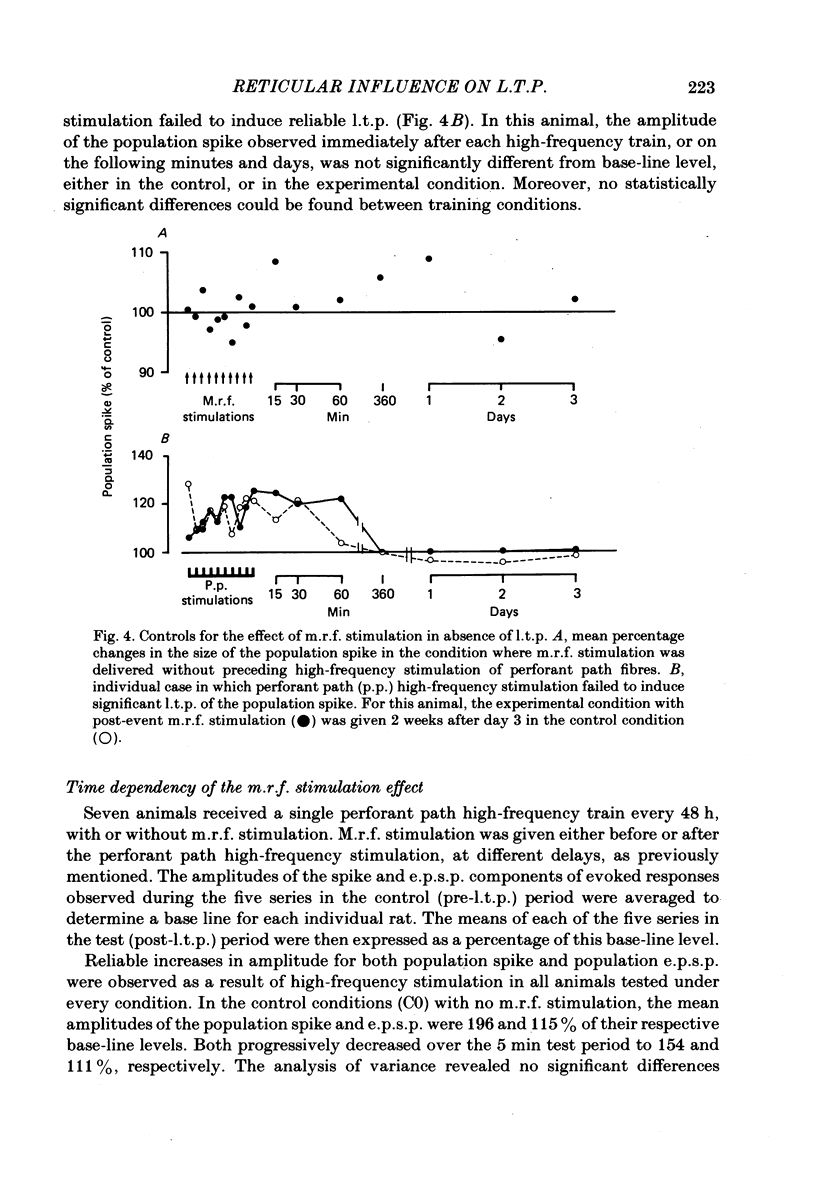







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Bliss T. V., Skrede K. K. Unit analysis of hippocampal polulation spikes. Exp Brain Res. 1971;13(2):208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- Andersen P., Sundberg S. H., Sveen O., Swann J. W., Wigström H. Possible mechanisms for long-lasting potentiation of synaptic transmission in hippocampal slices from guinea-pigs. J Physiol. 1980 May;302:463–482. doi: 10.1113/jphysiol.1980.sp013256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf S. Y., Mason S. T., Miller J. J. Noradrenergic modulation transmission between the entorhinal cortex and the dentate gyrus of the rat [proceedings]. J Physiol. 1979 Jul;292:52P–52P. [PubMed] [Google Scholar]
- Assaf S. Y., Miller J. J. Neuronal transmission in the dentate gyrus: role of inhibitory mechanisms. Brain Res. 1978 Aug 11;151(3):587–592. doi: 10.1016/0006-8993(78)91091-0. [DOI] [PubMed] [Google Scholar]
- Avarez-Leefmans F. J., Gardner-Medwin A. R. Proceedings: Influences of the septum on the hippocampal dentate area which are unaccompanied by field potentials. J Physiol. 1975 Jul;249(1):14P–16P. [PubMed] [Google Scholar]
- Barnes C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979 Feb;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Baudry M., Oliver M., Creager R., Wieraszko A., Lynch G. Increase in glutamate receptors following repetitive electrical stimulation in hippocampal slices. Life Sci. 1980 Jul 28;27(4):325–330. doi: 10.1016/0024-3205(80)90200-3. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Gardner-Medwin A. R. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Goddard G. V., Riives M. Reduction of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J Physiol. 1983 Jan;334:475–491. doi: 10.1113/jphysiol.1983.sp014507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch V. Facts and hypotheses concerning memory consolidation processes. Brain Res. 1970 Dec 18;24(3):561–575. doi: 10.1016/0006-8993(70)90228-3. [DOI] [PubMed] [Google Scholar]
- Bloch V., Laroche S. Conditioning of hippocampal cells: its acceleration and long-term facilitation by post-trial reticular stimulation. Behav Brain Res. 1981 Jul;3(1):23–42. doi: 10.1016/0166-4328(81)90026-7. [DOI] [PubMed] [Google Scholar]
- Browning M., Dunwiddie T., Bennett W., Gispen W., Lynch G. Synaptic phosphoproteins: specific changes after repetitive stimulation of the hippocampal slice. Science. 1979 Jan 5;203(4375):60–62. doi: 10.1126/science.214855. [DOI] [PubMed] [Google Scholar]
- Bär P. R., Schotman P., Gispen W. H., Tielen A. M., Lopes Da Silva F. H. Changes in synaptic membrane phosphorylation after tetanic stimulation in the dentate area of the rat hippocampal slice. Brain Res. 1980 Oct 6;198(2):478–484. doi: 10.1016/0006-8993(80)90764-7. [DOI] [PubMed] [Google Scholar]
- Delanoy R. L., Tucci D. L., Gold P. E. Amphetamine effects on long term potentiation in dentate granule cells. Pharmacol Biochem Behav. 1983 Jan;18(1):137–139. doi: 10.1016/0091-3057(83)90263-0. [DOI] [PubMed] [Google Scholar]
- Dolphin A. C., Errington M. L., Bliss T. V. Long-term potentiation of the perforant path in vivo is associated with increased glutamate release. Nature. 1982 Jun 10;297(5866):496–498. doi: 10.1038/297496a0. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975 Mar 21;86(2):205–215. doi: 10.1016/0006-8993(75)90697-6. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Goddard G. V., Riives M. Inhibitory modulation of long-term potentiation: evidence for a postsynaptic locus of control. Brain Res. 1982 May 27;240(2):259–272. doi: 10.1016/0006-8993(82)90221-9. [DOI] [PubMed] [Google Scholar]
- Douglas R. M. Long lasting synaptic potentiation in the rat dentate gyrus following brief high frequency stimulation. Brain Res. 1977 May 6;126(2):361–365. doi: 10.1016/0006-8993(77)90733-8. [DOI] [PubMed] [Google Scholar]
- Edwards S. B., de Olmos J. S. Autoradiographic studies of the projections of the midbrain reticular formation: ascending projections of nucleus cuneiformis. J Comp Neurol. 1976 Feb 15;165(4):417–431. doi: 10.1002/cne.901650403. [DOI] [PubMed] [Google Scholar]
- Fantie B. D., Goddard G. V. Septal modulation of the population spike in the fascia dentata produced by perforant path stimulation in the rat. Brain Res. 1982 Dec 9;252(2):227–237. doi: 10.1016/0006-8993(82)90390-0. [DOI] [PubMed] [Google Scholar]
- Fifková E., Anderson C. L., Young S. J., Van Harreveld A. Effect of anisomycin on stimulation-induced changes in dendritic spines of the dentate granule cells. J Neurocytol. 1982 Apr;11(2):183–210. doi: 10.1007/BF01258243. [DOI] [PubMed] [Google Scholar]
- Grantyn A., Grantyn R. Postsynaptic responses of hippocampal neurons to subcortical stimulation: differentiation of ascending pathways. Acta Physiol Acad Sci Hung. 1973;43(4):329–345. [PubMed] [Google Scholar]
- HUGHES J. R. Post-tetanic potentiation. Physiol Rev. 1958 Jan;38(1):91–113. doi: 10.1152/physrev.1958.38.1.91. [DOI] [PubMed] [Google Scholar]
- Hesse G. W., Teyler T. J. Reversible loss of hippocampal long term potentiation following electronconvulsive seizures. Nature. 1976 Dec 9;264(5586):562–564. doi: 10.1038/264562a0. [DOI] [PubMed] [Google Scholar]
- Laroche S., Falcou R., Bloch V. Post-trial reticular facilitation of associative changes in multiunit activity; comparison between dentate gyrus and entorhinal cortex. Behav Brain Res. 1983 Sep;9(3):381–387. doi: 10.1016/0166-4328(83)90139-0. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Schottler F., Oliver M., Lynch G. Brief bursts of high-frequency stimulation produce two types of structural change in rat hippocampus. J Neurophysiol. 1980 Aug;44(2):247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979 Oct 19;175(2):233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Barnes C. A. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol. 1977 Oct 15;175(4):439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L., Douglas R. M., Goddard G. V. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978 Nov 24;157(2):277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- McNaughton B. L. Long-term synaptic enhancement and short-term potentiation in rat fascia dentata act through different mechanisms. J Physiol. 1982 Mar;324:249–262. doi: 10.1113/jphysiol.1982.sp014110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine R. J., Milgram N. W., Hafner S. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983 Feb 7;260(2):217–231. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- Racine R. J., Milgram N. W. Short-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983 Feb 7;260(2):201–216. doi: 10.1016/0006-8993(83)90675-3. [DOI] [PubMed] [Google Scholar]
- Robinson G. B., Racine R. J. Heterosynaptic interactions between septal and entorhinal inputs to the dentate gyrus: long-term potentiation effects. Brain Res. 1982 Oct 7;249(1):162–166. doi: 10.1016/0006-8993(82)90182-2. [DOI] [PubMed] [Google Scholar]
- Robinson T. E., Vanderwolf C. H. Electrical stimulation of the brain stem in freely moving rats: II. Effects on hippocampal and neocortical electrical activity, and relations to behavior. Exp Neurol. 1978 Sep 15;61(3):485–515. doi: 10.1016/0014-4886(78)90018-3. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A., Fifkova E. Swelling of dendritic spines in the fascia dentata after stimulation of the perforant fibers as a mechanism of post-tetanic potentiation. Exp Neurol. 1975 Dec;49(3):736–749. doi: 10.1016/0014-4886(75)90055-2. [DOI] [PubMed] [Google Scholar]
- Vertes R. P. Brain stem generation of the hippocampal EEG. Prog Neurobiol. 1982;19(3):159–186. doi: 10.1016/0301-0082(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Wilson R. C. Changes in translation of synaptic excitation to dentate granule cell discharge accompanying long-term potentiation. I. Differences between normal and reinnervated dentate gyrus. J Neurophysiol. 1981 Aug;46(2):324–338. doi: 10.1152/jn.1981.46.2.324. [DOI] [PubMed] [Google Scholar]
- Wilson R. C., Levy W. B., Steward O. Changes in translation of synaptic excitation to dentate granule cell discharge accompanying long-term potentiation. II. An evaluation of mechanisms utilizing dentate gyrus dually innervated by surviving ipsilateral and sprouted crossed temporodentate inputs. J Neurophysiol. 1981 Aug;46(2):339–355. doi: 10.1152/jn.1981.46.2.339. [DOI] [PubMed] [Google Scholar]
- Winson J. Influence of raphe nuclei on neuronal transmission from perforant pathway through dentate gyrus. J Neurophysiol. 1980 Nov;44(5):937–950. doi: 10.1152/jn.1980.44.5.937. [DOI] [PubMed] [Google Scholar]
- Winson J. Reticular formation influence on neuronal transmission from perforant pathway through dentate gyrus. Brain Res. 1981 Nov 23;225(1):37–49. doi: 10.1016/0006-8993(81)90316-4. [DOI] [PubMed] [Google Scholar]


