Abstract
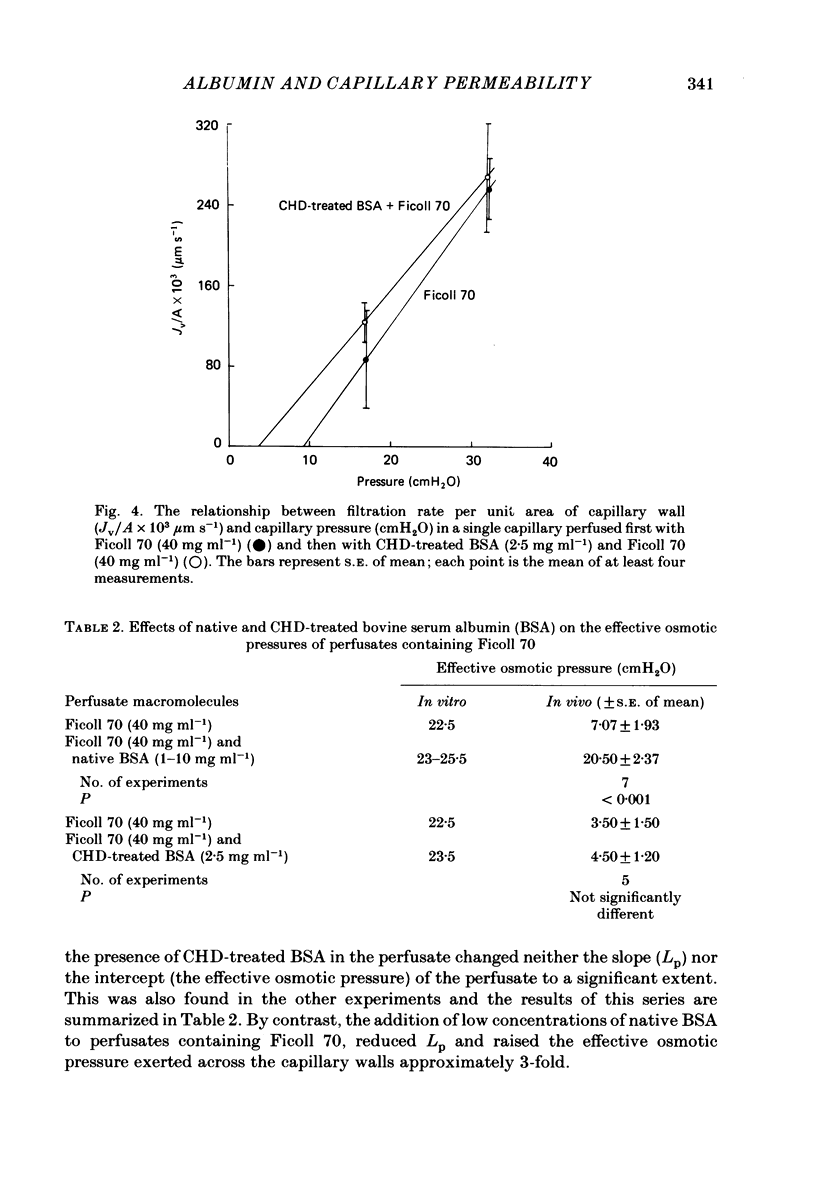
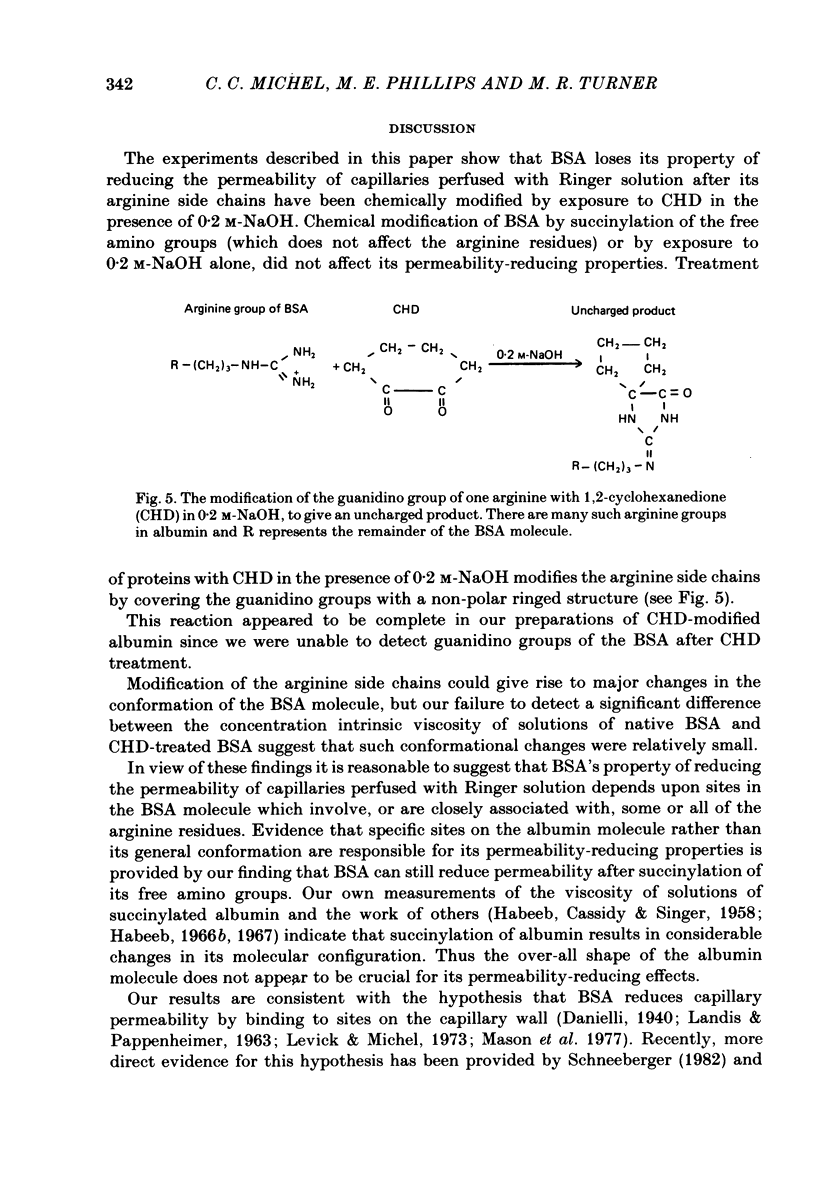
Single capillaries in the mesenteries of pithed frogs were perfused sequentially with two frog Ringer solutions. The first solution contained no protein; the second solution contained either native or chemically modified bovine serum albumin (BSA) at a concentration of 3-5 mg ml-1. During each perfusion capillary permeability was assessed from the hydraulic conductivity of the capillary wall (Lp) which was determined from measurements of fluid filtration rate at two or more different capillary pressures (Michel, Mason, Curry, Tooke & Hunter, 1974). Lp measured during perfusion with protein-free Ringer solution was on average three times greater than its value for the same vessel perfused with Ringer solution containing native BSA. This confirms the findings of Mason, Curry & Michel (1977). BSA, which had been succinylated to modify the free amino groups of its lysine residues, appeared to be as effective as native BSA in reducing Lp. After modification of its arginine side chains by exposure to 1,2-cyclohexanedione (CHD) in the presence of 0.2 M-NaOH, BSA lost its property of reducing Lp in capillaries perfused with Ringer solution. Exposure of BSA to 0.2 M-NaOH followed by dialysis against normal Ringer solution did not affect its property of reducing Lp. CHD-treated BSA at a concentration of 2.5 mg ml-1 had no effect upon the effective osmotic pressure exerted across capillary walls by Ringer perfusates containing the neutral polymer Ficoll 70 at a concentration of 40 mg ml-1. Native BSA raised the effective osmotic pressure from 7.07 +/- 1.93 cmH2O to 20.50 +/- 2.37 cmH2O (n = 7; P less than 0.001). It is concluded that the effects of BSA on permeability depend upon specific sites in the BSA molecule. It is suggested that these sites involve positively charged arginine side chains of the albumin molecule. The results are discussed in terms of the fibre-matrix hypothesis of capillary permeability and in terms of Brown's (1976) theory for the structure of albumin.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. R. Structural origins of mammalian albumin. Fed Proc. 1976 Aug;35(10):2141–2144. [PubMed] [Google Scholar]
- Clough G., Michel C. C. The role of vesicles in the transport of ferritin through frog endothelium. J Physiol. 1981 Jun;315:127–142. doi: 10.1113/jphysiol.1981.sp013737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C. A fiber matrix model of capillary permeability. Microvasc Res. 1980 Jul;20(1):96–99. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- Curry F. E., Michel C. C., Mason J. C. Osmotic reflextion coefficients of capillary walls to low molecular weight hydrophilic solutes measured in single perfused capillaries of the frog mesentery. J Physiol. 1976 Oct;261(2):319–336. doi: 10.1113/jphysiol.1976.sp011561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry F. R. Is the transport of hydrophilic substances across the capillary wall determined by a network of fibrous molecules? Physiologist. 1980 Feb;23(1):90–93. [PubMed] [Google Scholar]
- Danielli J. F. Capillary permeability and oedema in the perfused frog. J Physiol. 1940 Mar 14;98(1):109–129. doi: 10.1113/jphysiol.1940.sp003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinker C. K. The permeability and diameter of the capillaries in the web of the brown frog (R. temporaria) when perfused with solutions containing pituitary extract and horse serum. J Physiol. 1927 Aug 8;63(3):249–269. doi: 10.1113/jphysiol.1927.sp002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEEB A. F., CASSIDY H. G., SINGER S. J. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim Biophys Acta. 1958 Sep;29(3):587–593. doi: 10.1016/0006-3002(58)90016-7. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Evaluation of conformational changes in chemically modified bovine serum albumins on a column of Sephadex. Biochim Biophys Acta. 1966 May 26;121(1):21–25. doi: 10.1016/0304-4165(66)90344-8. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. Quantitation of conformational changes on chemical modification of proteins: use of succinylated proteins as a model. Arch Biochem Biophys. 1967 Sep;121(3):652–664. doi: 10.1016/0003-9861(67)90050-1. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U. Molecular aspects of ligand binding to serum albumin. Pharmacol Rev. 1981 Mar;33(1):17–53. [PubMed] [Google Scholar]
- Levick J. R., Michel C. C. The effect of bovine albumin on the permeability of frog mesenteric capillaries. Q J Exp Physiol Cogn Med Sci. 1973 Jan;58(1):87–97. doi: 10.1113/expphysiol.1973.sp002194. [DOI] [PubMed] [Google Scholar]
- Levick J. R., Michel C. C. The permeability of individually perfused frog mesenteric capillaries to T1824 and T1824-albumin as evidence for a large pore system. Q J Exp Physiol Cogn Med Sci. 1973 Jan;58(1):67–85. doi: 10.1113/expphysiol.1973.sp002192. [DOI] [PubMed] [Google Scholar]
- Mason J. C., Curry F. E., Michel C. C. The effects of proteins upon the filtration coefficient of individually perfused frog mesenteric capillaries. Microvasc Res. 1977 Mar;13(2):185–202. doi: 10.1016/0026-2862(77)90084-x. [DOI] [PubMed] [Google Scholar]
- Michel C. C. Filtration coefficients and osmotic reflexion coefficients of the walls of single frog mesenteric capillaries. J Physiol. 1980 Dec;309:341–355. doi: 10.1113/jphysiol.1980.sp013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C., Mason J. C., Curry F. E., Tooke J. E., Hunter P. J. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1974 Oct;59(4):283–309. doi: 10.1113/expphysiol.1974.sp002275. [DOI] [PubMed] [Google Scholar]
- Michel C. C., Phillips M. E. The effects of Ficoll 70 on bovine serum albumin on the permeability properties of individually perfused frog mesenteric capillaries [proceedings]. J Physiol. 1979 Jun;291:39P–39P. [PubMed] [Google Scholar]
- Michel C. C. The measurement of permeability in single capillaries. Arch Int Physiol Biochim. 1978 Aug;86(3):657–667. doi: 10.3109/13813457809055934. [DOI] [PubMed] [Google Scholar]
- Myhre K., Steen J. B. The effect of plasma proteins on the capillary permeability in the rete mirabile of the eel (Anguilla vulgaris L.). Acta Physiol Scand. 1977 Jan;99(1):98–104. doi: 10.1111/j.1748-1716.1977.tb10357.x. [DOI] [PubMed] [Google Scholar]
- Reed R. G., Putnam F. W., Peters T., Jr Sequence of residues 400--403 of bovine serum albumin. Biochem J. 1980 Dec 1;191(3):867–868. doi: 10.1042/bj1910867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe B., Folkow B. Capillary permeability to albumin in normotensive and spontaneously hypertensive rats. Acta Physiol Scand. 1977 Sep;101(1):72–83. doi: 10.1111/j.1748-1716.1977.tb05985.x. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E. Circulating proteins and macromolecular transport across continuous, nonfenestrated endothelium. Ann N Y Acad Sci. 1982;401:25–37. doi: 10.1111/j.1749-6632.1982.tb25704.x. [DOI] [PubMed] [Google Scholar]
- Schneeberger E. E., Hamelin M. Interaction of serum proteins with lung endothelial glycocalyx: its effect on endothelial permeability. Am J Physiol. 1984 Aug;247(2 Pt 2):H206–H217. doi: 10.1152/ajpheart.1984.247.2.H206. [DOI] [PubMed] [Google Scholar]
- Toi K., Bynum E., Norris E., Itano H. A. Studies on the chemical modification of arginine. I. The reaction of 1,2-cyclohexanedione with arginine and arginyl residues of proteins. J Biol Chem. 1967 Mar 10;242(5):1036–1043. [PubMed] [Google Scholar]
- Turner M. R., Clough G., Michel C. C. The effects of cationised ferritin and native ferritin upon the filtration coefficient of single frog capillaries. Evidence that proteins in the endothelial cell coat influence permeability. Microvasc Res. 1983 Mar;25(2):205–222. doi: 10.1016/0026-2862(83)90016-x. [DOI] [PubMed] [Google Scholar]


