Abstract
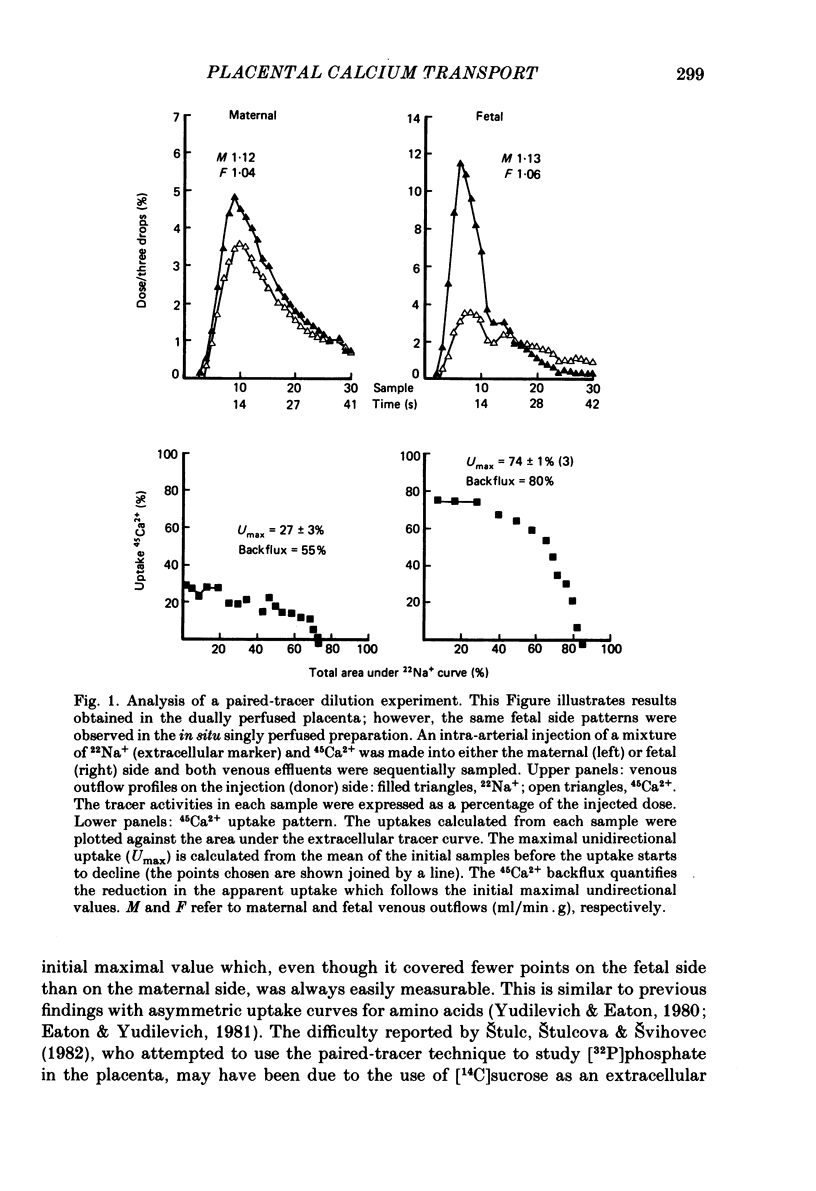
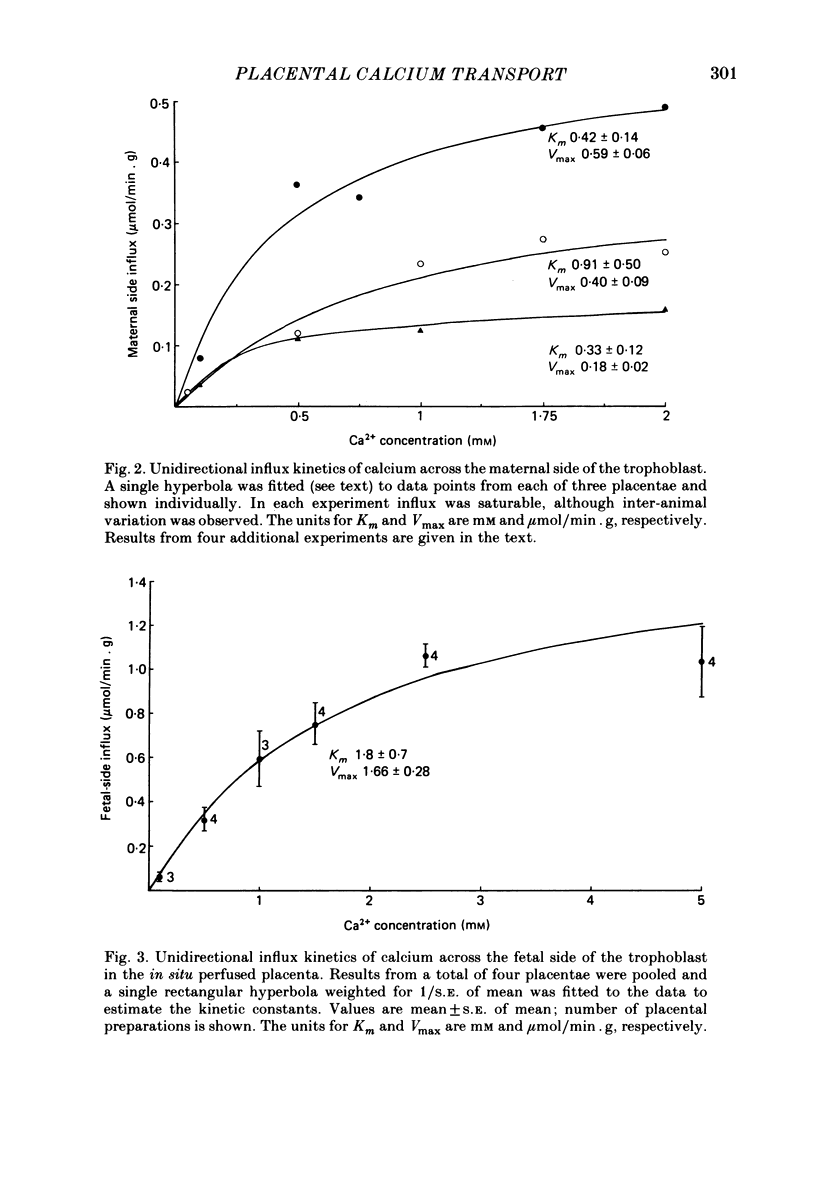
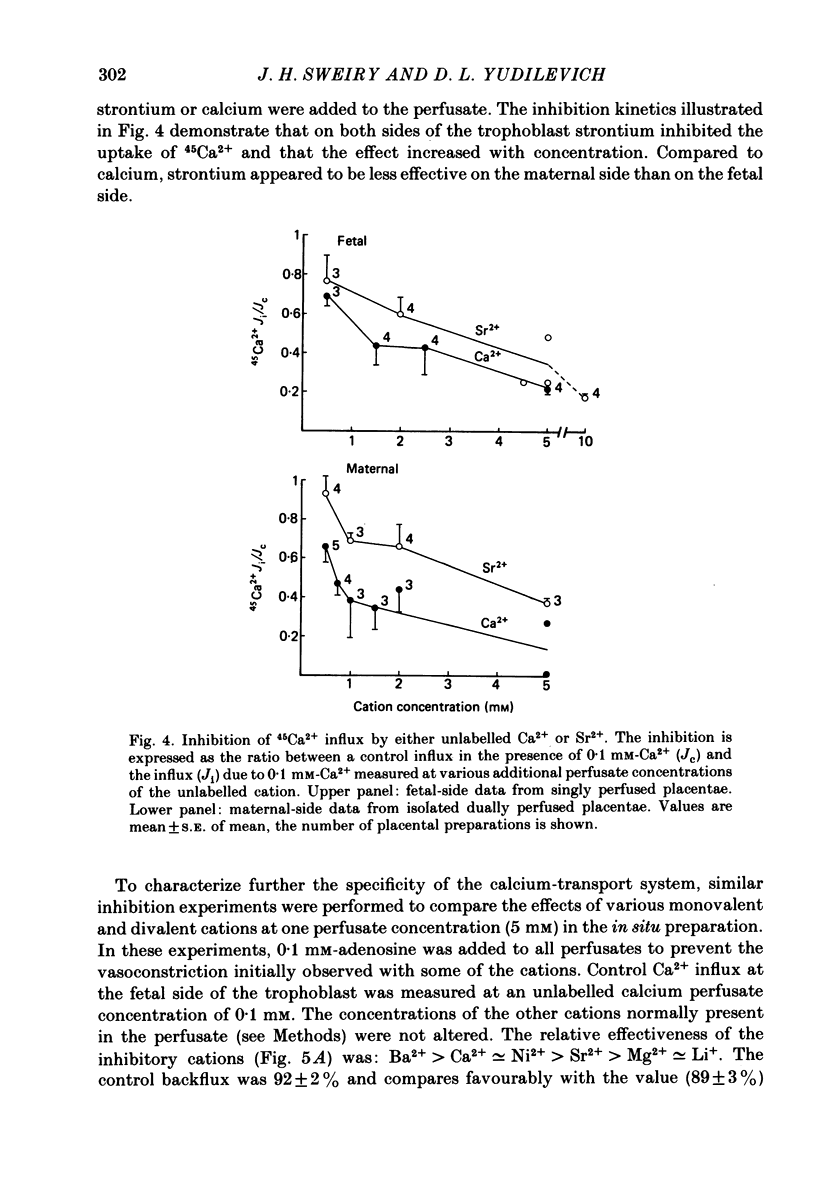
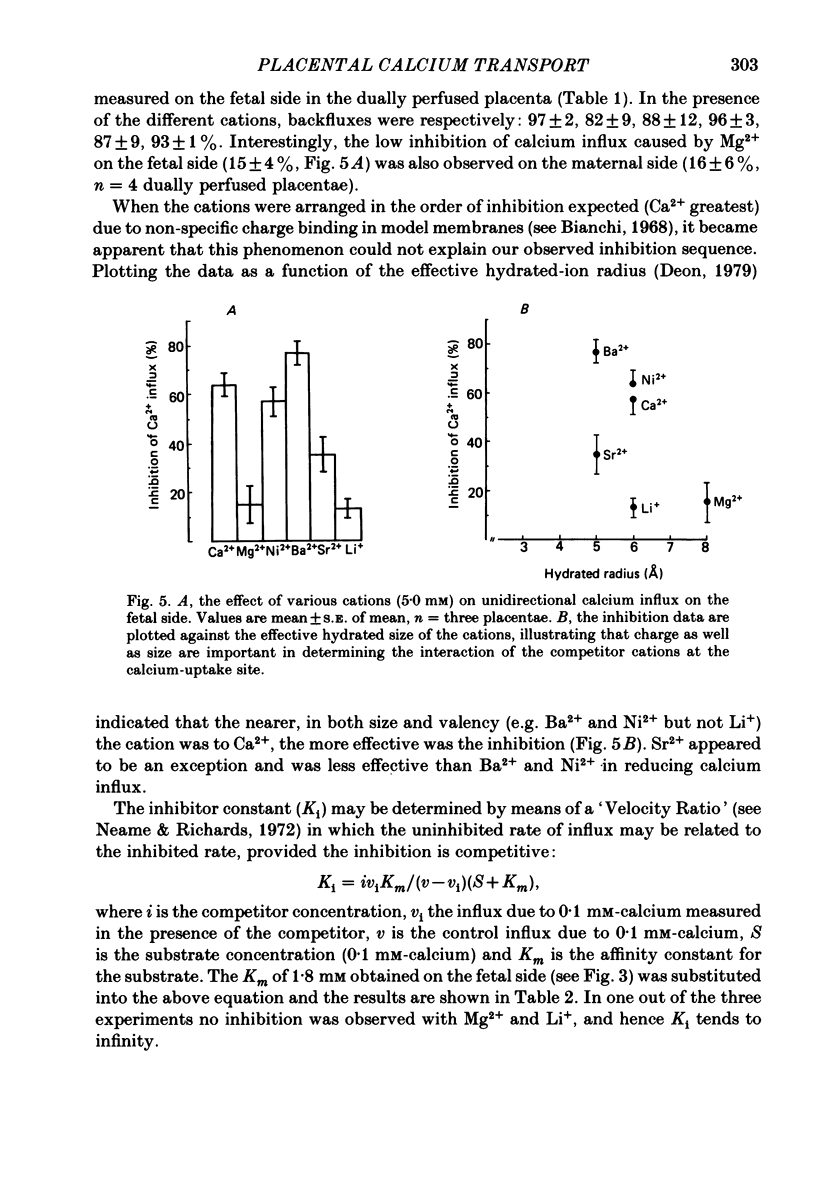
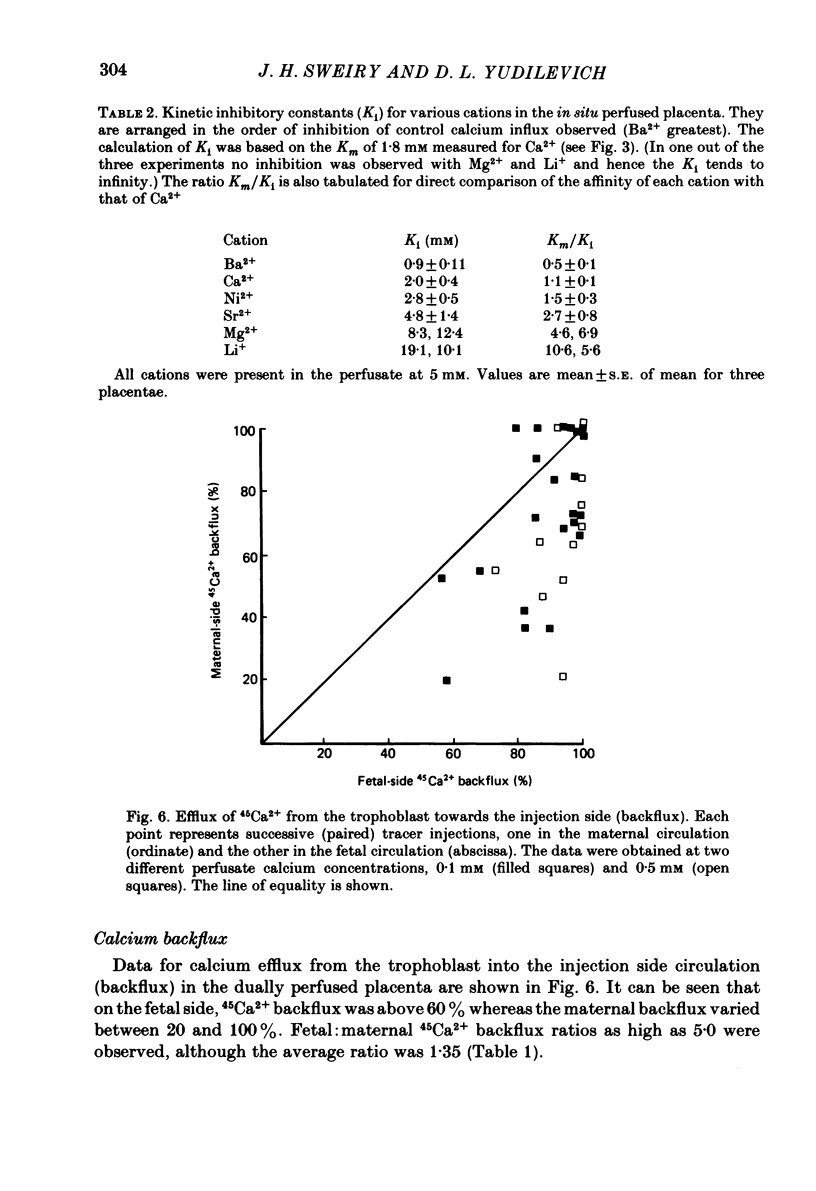
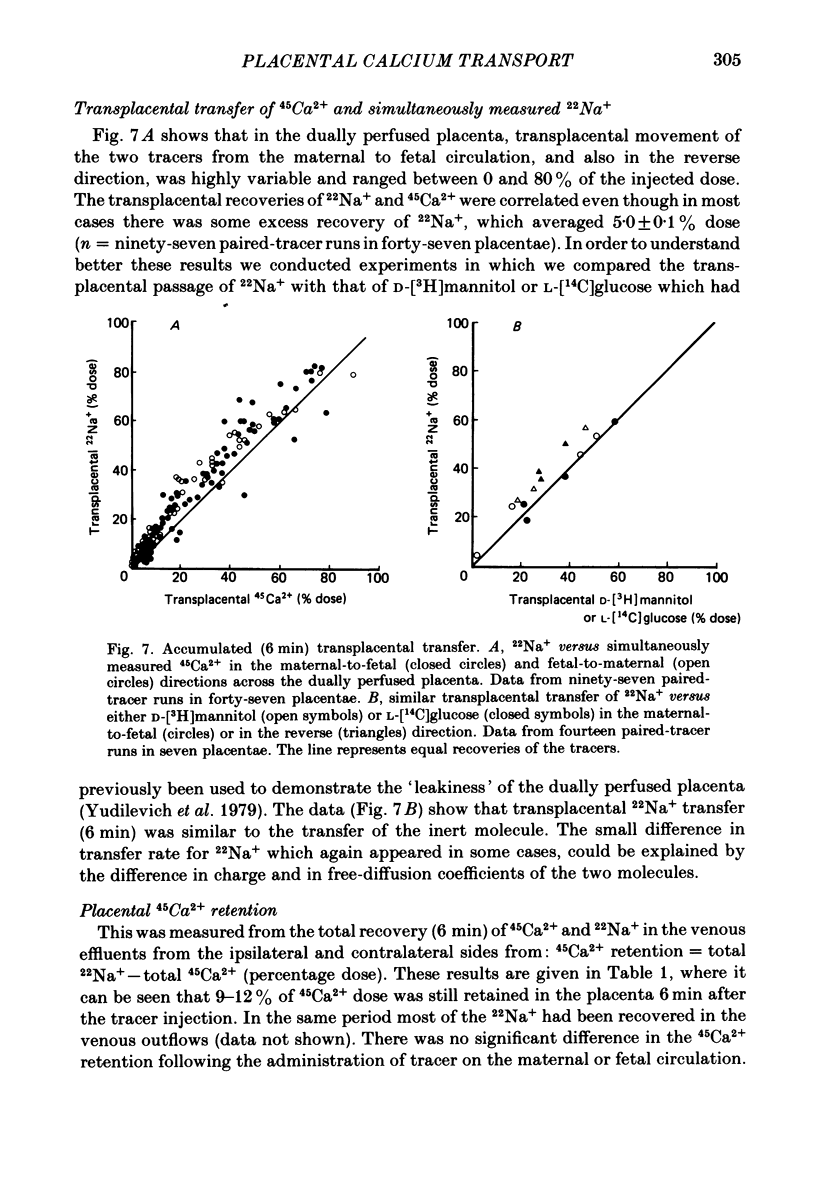
Unidirectional influx of calcium across maternal and fetal sides of the syncytiotrophoblast was investigated in the guinea-pig placenta by using a rapid (less than 30 s) paired-tracer dilution technique. Experiments were performed in an in situ placenta artificially perfused through the umbilical vessels or in an isolated placenta in which both the maternal and fetal circulations were perfused. At equimolar Ca2+ concentrations, unidirectional calcium influx was always significantly lower on the maternal side than on the fetal side. Saturation kinetics were observed: on the fetal side the estimated Km was 1.8 +/- 0.7 mM and Vmax was 1.66 +/- 0.28 mumol/min X g (mean +/- S.E. of mean) and on the maternal side Km ranged from 0.18 to 1.15 mM and Vmax ranged from 0.12 to 0.59 mumol/min X g. When the inhibition of calcium influx was investigated on the fetal side of the trophoblast by using competing cations, the following sequence was observed: Ba2+ greater than Ca2+ congruent to Ni2+ greater than Sr2+ greater than Mg2+ congruent to Li+. Efflux of 45Ca2+ from the trophoblast into the ipsilateral circulation (backflux) was rapid (20-100% in 6 min) and asymmetric since the fetal:maternal ratio was 1.35 +/- 0.11 (mean +/- S.E. of mean) in the presence of 0.1 mM-Ca2+. In the dually perfused placenta, transplacental transfer (6 min) of 45Ca2+ varied over a wide range (0-80%); however, it was similar to that of the extracellular reference tracer, 22Na+, in either maternal-to-fetal or fetal-to-maternal directions. It is suggested that this is a consequence of the 'leakiness' of the dually perfused placenta since the transplacental transfer of 22Na+ and D-[3H]mannitol (or L-[14C]glucose) measured simultaneously were also variable but similar. Transplacental transfer of 45Ca2+ could not be used to characterize specific calcium-transport mechanisms, whereas highly sensitive trophoblast uptake measurements were provided by the single-circulation, paired-tracer technique. Our findings suggest the presence of a specific carrier-mediated transport system for calcium on both maternal and fetal surfaces of the trophoblast. The asymmetries in unidirectional influx into the trophoblast and rapid backflux indicate a mechanism by which the net transfer of calcium from the maternal to the fetal circulation is maintained in favour of the fetus.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAWDEN J. W., WOLKOFF A. S., FLOWERS C. E. MATERNAL-FETAL BLOOD CALCIUM RELATIONSHIPS IN SHEEP. Obstet Gynecol. 1965 Apr;25:548–552. [PubMed] [Google Scholar]
- Bawden J. W., Wolkoff A. S. Fetal blood calcium responses to maternal calcium infusion in sheep. Am J Obstet Gynecol. 1967 Sep 1;99(1):55–60. doi: 10.1016/s0002-9378(16)34490-8. [DOI] [PubMed] [Google Scholar]
- Bers D. M., Langer G. A. Uncoupling cation effects on cardiac contractility and sarcolemmal Ca2+ binding. Am J Physiol. 1979 Sep;237(3):H332–H341. doi: 10.1152/ajpheart.1979.237.3.H332. [DOI] [PubMed] [Google Scholar]
- Bradbury M. W., Hedley R. Porosity of the guinea-pig placenta and its increase by perfusion [proceedings]. J Physiol. 1979 Jun;291:28P–28P. [PubMed] [Google Scholar]
- Bustamante J. C., Mann G. E., Yudilevich D. L. Specificity of neutral amino acid uptake at the basolateral side of the epithelium in the cat salivary gland in situ. J Physiol. 1981;313:65–79. doi: 10.1113/jphysiol.1981.sp013651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen M. H., Leichtweiss H. P., Schröder H. Lactate carriers in the artificially perfused human term placenta. Placenta. 1983 Apr-Jun;4(2):165–174. doi: 10.1016/s0143-4004(83)80029-0. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. The statistical analysis of enzyme kinetic data. Adv Enzymol Relat Areas Mol Biol. 1967;29:1–32. doi: 10.1002/9780470122747.ch1. [DOI] [PubMed] [Google Scholar]
- Croley T. E. The intracellular localization of calcium within the mature human placental barrier. Am J Obstet Gynecol. 1973 Dec 1;117(7):926–932. doi: 10.1016/0002-9378(73)90063-x. [DOI] [PubMed] [Google Scholar]
- Delivoria-Papadopoulos M., Battaglia F. C., Bruns P. D., Meschia G. Total, protein-bound, and ultrafilterable calcium in maternal and fetal plasmas. Am J Physiol. 1967 Aug;213(2):363–366. doi: 10.1152/ajplegacy.1967.213.2.363. [DOI] [PubMed] [Google Scholar]
- Eaton B. M., Mann G. E., Yudilevich D. L. Transport specificity for neutral and basic amino acids at maternal and fetal interfaces of the guinea-pig placenta. J Physiol. 1982 Jul;328:245–258. doi: 10.1113/jphysiol.1982.sp014262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton B. M., Yudilevich D. L. Uptake and asymmetric efflux of amino acids at maternal and fetal sides of placenta. Am J Physiol. 1981 Sep;241(3):C106–C112. doi: 10.1152/ajpcell.1981.241.3.C106. [DOI] [PubMed] [Google Scholar]
- Faber J. J., Hart F. M. Transfer of charged and uncharged molecules in the placenta of the rabbit. Am J Physiol. 1967 Oct;213(4):890–894. doi: 10.1152/ajplegacy.1967.213.4.890. [DOI] [PubMed] [Google Scholar]
- Hedley R., Bradbury M. W. Transport of polar non-electrolytes across the intact and perfused guinea-pig placenta. Placenta. 1980 Oct-Dec;1(4):277–285. doi: 10.1016/s0143-4004(80)80029-4. [DOI] [PubMed] [Google Scholar]
- Law R. O. Techniques and applications of extracellular space determination in mammalian tissues. Experientia. 1982 Apr 15;38(4):411–421. doi: 10.1007/BF01952615. [DOI] [PubMed] [Google Scholar]
- Leichtweiss H. P., Schröder H. Uber den Glucosetransport durch die isolierte, beiderseits künstlich perfundierte Meerschweinchenplacenta. Pflugers Arch. 1971;325(2):139–148. doi: 10.1007/BF00587004. [DOI] [PubMed] [Google Scholar]
- MONEY W. L., DANCIS J. Technique for the in situ study of placental transport in the pregnant guinea pig. Am J Obstet Gynecol. 1960 Aug;80:209–214. doi: 10.1016/0002-9378(60)90114-9. [DOI] [PubMed] [Google Scholar]
- McKercher H. G., Derewlany L. O., Radde I. C. Placental calcium and phosphorus transfer in the guinea pig: lack of effect of modulators of Ca-ATPase and alkaline phosphatase activity. Can J Physiol Pharmacol. 1983 Nov;61(11):1354–1360. doi: 10.1139/y83-194. [DOI] [PubMed] [Google Scholar]
- Miller R. K., Berndt W. O. Evidence for Mg 2+ -dependent, Na + + K + -activated ATPase and Ca 2+ -ATPase in the human term placenta. Proc Soc Exp Biol Med. 1973 May;143(1):118–122. [PubMed] [Google Scholar]
- Moore C. L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem Biophys Res Commun. 1971 Jan 22;42(2):298–305. doi: 10.1016/0006-291x(71)90102-1. [DOI] [PubMed] [Google Scholar]
- Ockleford C. D., Whyte A. Differeniated regions of human placental cell surface associated with exchange of materials between maternal and foetal blood: coated vesicles. J Cell Sci. 1977 Jun;25:293–312. doi: 10.1242/jcs.25.1.293. [DOI] [PubMed] [Google Scholar]
- PAYNE J. M., SANSOM B. F. THE SUSCEPTIBILITY OF VARIOUS GROUPS OF RATS TO EXPERIMENTAL HYPOCALCAEMIA. J Physiol. 1963 Oct;168:554–563. doi: 10.1113/jphysiol.1963.sp007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M., Connor J. D., Crawford I. L. Permeability changes in the blood-brain barrier: causes and consequences. CRC Crit Rev Toxicol. 1975 Jan;3(2):159–199. doi: 10.3109/10408447509079857. [DOI] [PubMed] [Google Scholar]
- Reynolds M. L., Young M. The transfer of free alpha-amino nitrogen across the placental membrane in the guinea-pig. J Physiol. 1971 May;214(3):583–597. doi: 10.1113/jphysiol.1971.sp009450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi B., Macintyre J. D., Gárdos G. Kinetics of active calcium transport in inside-out red cell membrane vesicles. FEBS Lett. 1978 May 1;89(1):78–82. doi: 10.1016/0014-5793(78)80526-2. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Möhlen K. H., Challier J. C., Dancis J. Transfer of glutamic acid across the human placenta perfused in vitro. Br J Obstet Gynaecol. 1979 Apr;86(4):299–306. doi: 10.1111/j.1471-0528.1979.tb11260.x. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F., Bonting S. L. Transport adenosine triphosphatases: properties and functions. Physiol Rev. 1981 Jan;61(1):1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- Shami Y., Messer H. H., Copp D. H. Calcium binding to placental plasma membranes as measured by rate of diffusion in a flow dialysis system. Biochim Biophys Acta. 1974 Mar 29;339(3):323–333. doi: 10.1016/0005-2736(74)90159-x. [DOI] [PubMed] [Google Scholar]
- Shami Y., Radde I. C. Calcium-stimulated ATPase of guinea pig placenta. Biochim Biophys Acta. 1971 Dec 3;249(2):345–352. doi: 10.1016/0005-2736(71)90113-1. [DOI] [PubMed] [Google Scholar]
- Stulc J., Stulcová B., Svihovec J. Uptake of inorganic phosphate by the maternal border of the guinea pig placenta. Pflugers Arch. 1982 Dec;395(4):326–330. doi: 10.1007/BF00580797. [DOI] [PubMed] [Google Scholar]
- Suki W. N. Calcium transport in the nephron. Am J Physiol. 1979 Jul;237(1):F1–F6. doi: 10.1152/ajprenal.1979.237.1.F1. [DOI] [PubMed] [Google Scholar]
- Van Rossum G. D. Net movements of calcium and magnesium in slices of rat liver. J Gen Physiol. 1970 Jan;55(1):18–32. doi: 10.1085/jgp.55.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsett J. A., Lessard J. L. Characteristics of the microvillus brush border of human placenta: insulin receptor localization in brush border membranes. Endocrinology. 1978 Oct;103(4):1458–1468. doi: 10.1210/endo-103-4-1458. [DOI] [PubMed] [Google Scholar]
- Whitsett J. A., Tsang R. C. Calcium uptake and binding by membrane fractions of human placenta: ATP-dependent calcium accumulation. Pediatr Res. 1980 May;14(5):769–775. doi: 10.1203/00006450-198005000-00012. [DOI] [PubMed] [Google Scholar]
- Wilson P. W., Lawson D. E. Calcium binding activity by chick intestinal brush-border membrane vesicles. Pflugers Arch. 1980 Dec;389(1):69–74. doi: 10.1007/BF00587930. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Eaton B. M. Amino acid carriers at maternal and fetal surfaces of the placenta by single circulation paired-tracer dilution. Kinetics of phenylalanine transport. Biochim Biophys Acta. 1980 Feb 28;596(2):315–319. doi: 10.1016/0005-2736(80)90364-8. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Eaton B. M., Short A. H., Leichtweiss H. P. Glucose carriers at maternal and fetal sides of the trophoblast in guinea pig placenta. Am J Physiol. 1979 Nov;237(5):C205–C212. doi: 10.1152/ajpcell.1979.237.5.C205. [DOI] [PubMed] [Google Scholar]
- Yudilevich D. L., Mann G. E. Unidirectional uptake of substrates at the blood side of secretory epithelia: stomach, salivary gland, pancreas. Fed Proc. 1982 Dec;41(14):3045–3053. [PubMed] [Google Scholar]
- van Kreel B. K., van Dijk J. P. Mechanisms involved in the transfer of calcium across the isolated guinea pigs placenta. J Dev Physiol. 1983 Jun;5(3):155–165. [PubMed] [Google Scholar]


