Abstract
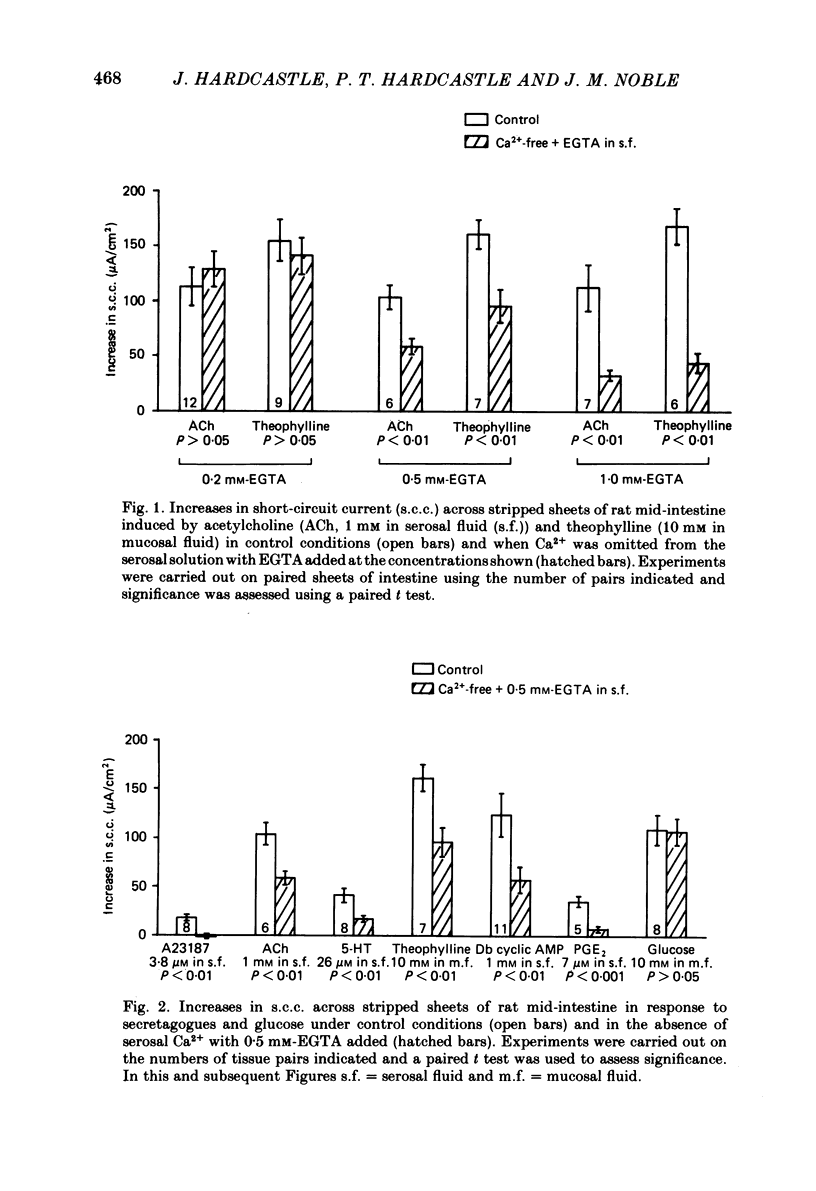
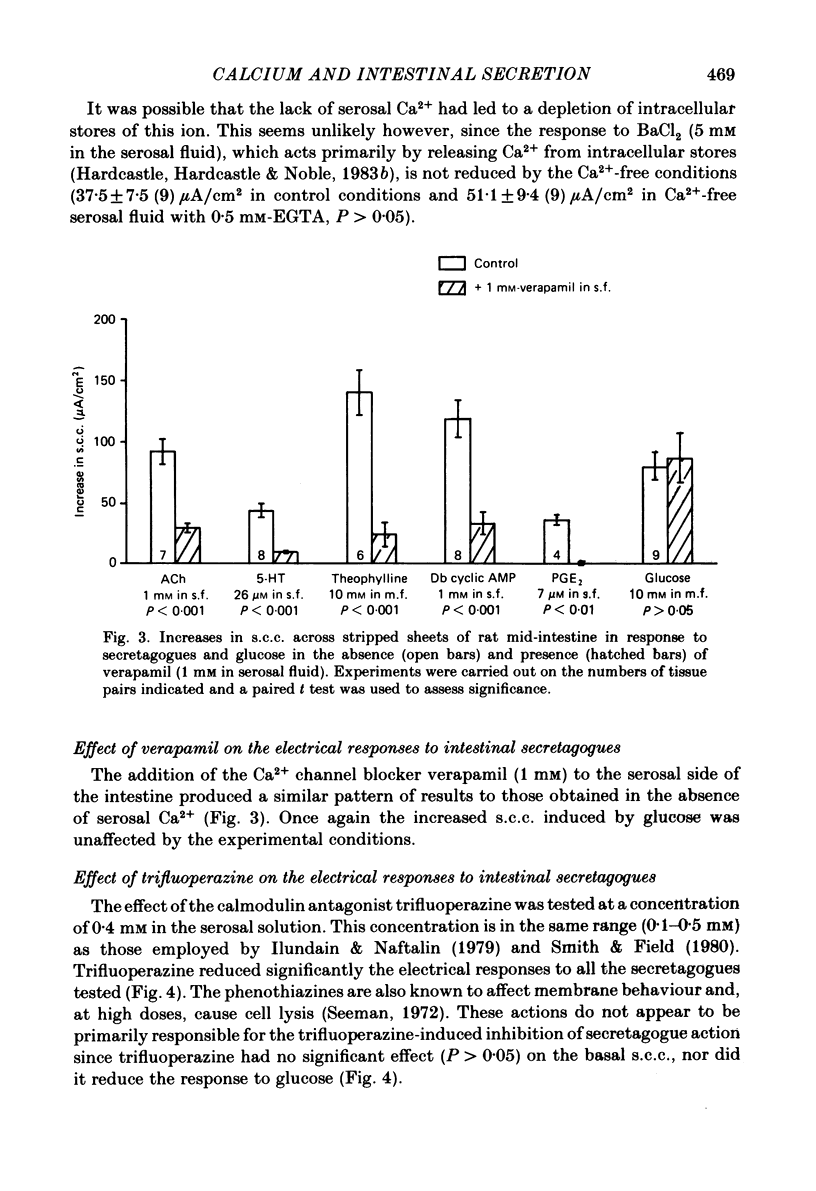
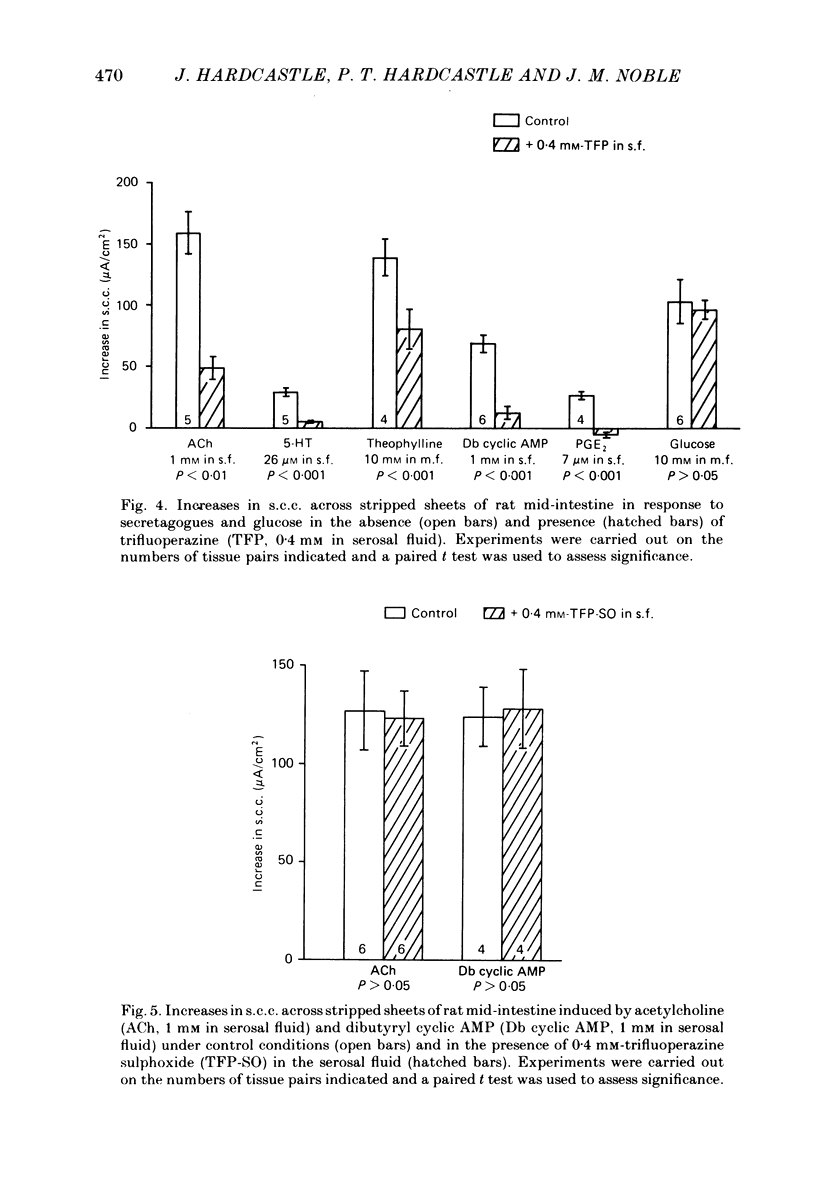
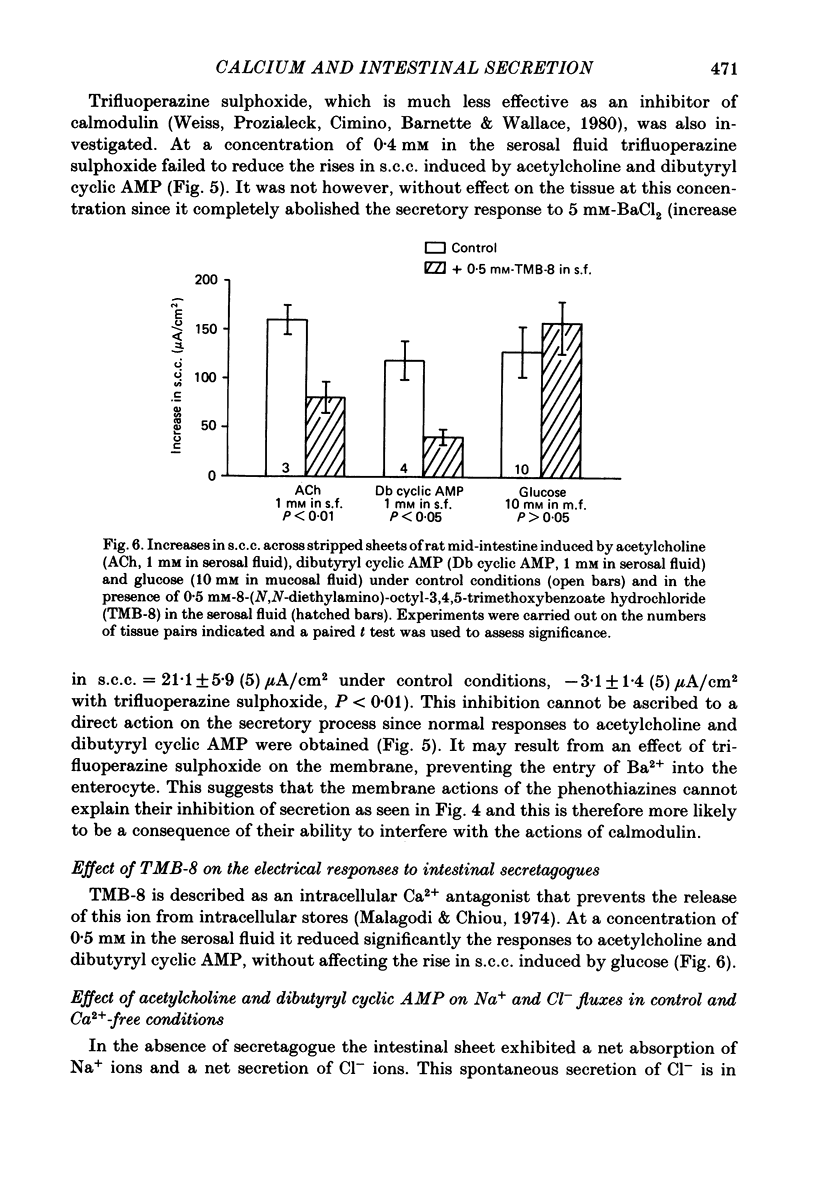
The involvement of Ca2+ in the regulation of intestinal secretion was investigated in stripped sheets of rat mid-intestine. Removal of serosal Ca2+ together with the addition of EGTA at concentrations of 0.5 and 1 mM inhibited the rise in short-circuit current (s.c.c.) induced by both acetylcholine and theophylline, a similar degree of inhibition being observed with both secretagogues. Ca2+-free serosal fluid with 0.5 mM-EGTA added reduced significantly the rises in s.c.c. induced by A23187, acetylcholine, 5-hydroxytryptamine, theophylline, dibutyryl cyclic AMP and prostaglandin E2, but not the increased s.c.c. associated with glucose absorption. The Ca2+ channel blocker verapamil produced similar results. The calmodulin antagonist trifluoperazine inhibited secretagogue action while its sulphoxide derivative was without effect at the same concentration. The intracellular Ca2+ antagonist TMB-8 reduced the increased s.c.c. observed with acetylcholine and dibutyryl cyclic AMP. The net Cl- secretion, but not the decreased mucosal-to-serosal Na+ flux, induced by acetylcholine was abolished in Ca2+-free conditions. There was no consistent effect on the reduction in the residual ion flux caused by acetylcholine. Absence of Ca2+ converted the stimulation of Cl- secretion induced by dibutyryl cyclic AMP observed under control conditions to an enhancement of net Na+ and Cl- absorption. It is concluded that intestinal secretagogues, whether they act through cyclic AMP or not, require both internal and external sources of Ca2+ if they are to produce their full effects. Moreover, it appears that the nature of the response to dibutyryl cyclic AMP depends on the prevailing Ca2+ concentration.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton J. E., Field M. Ca ionophore-stimulated ion secretion in rabbit ileal mucosa: relation to actions of cyclic 3',5'-AMP and carbamylcholine. J Membr Biol. 1977 Jun 30;35(2):159–173. doi: 10.1007/BF01869947. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Feinman R. D., Detwiler T. C. Inhibition of platelet secretion by an antagonist of intracellular calcium. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1462–1467. doi: 10.1016/s0006-291x(76)80178-7. [DOI] [PubMed] [Google Scholar]
- Corbett C. L., Isaacs P. E., Riley A. K., Turnberg L. A. Human intestinal ion transport in vitro. Gut. 1977 Feb;18(2):136–140. doi: 10.1136/gut.18.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M., Asarkof N., Pike G. Calcium dependence of serotonin-induced changes in rabbit ileal electrolyte transport. J Clin Invest. 1980 Aug;66(2):341–352. doi: 10.1172/JCI109862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donowitz M. Ca2+ in the control of active intestinal Na and Cl transport: involvement in neurohumoral action. Am J Physiol. 1983 Aug;245(2):G165–G177. doi: 10.1152/ajpgi.1983.245.2.G165. [DOI] [PubMed] [Google Scholar]
- Donowitz M., Fogel R., Battisti L., Asarkof N. The neurohumoral secretagogues carbachol, substance P and neurotensin increase Ca++ influx and calcium content in rabbit ileum. Life Sci. 1982 Nov 1;31(18):1929–1937. doi: 10.1016/0024-3205(82)90031-5. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A. Active chloride secretion by rabbit colon: calcium-dependent stimulation by ionophore A23187. J Membr Biol. 1977 Jun 30;35(2):175–187. doi: 10.1007/BF01869948. [DOI] [PubMed] [Google Scholar]
- Garcia R., Laychock S. G., Rubin R. P. Inhibition of dibutyryl cyclic AMP induced steroidogenesis in rat adrenocortical cells by the putative calcium antagonist TMB-8. J Steroid Biochem. 1982 Feb;16(2):317–322. doi: 10.1016/0022-4731(82)90183-2. [DOI] [PubMed] [Google Scholar]
- Hardcastle J., Hardcastle P. T., Noble J. M. The effect of barium chloride on intestinal secretion in the rat. J Physiol. 1983 Nov;344:69–80. doi: 10.1113/jphysiol.1983.sp014924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardcastle J., Hardcastle P. T., Read N. W., Redfern J. S. The action of loperamide in inhibiting prostaglandin-induced intestinal secretion in the rat. Br J Pharmacol. 1981 Nov;74(3):563–569. doi: 10.1111/j.1476-5381.1981.tb10465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper A. A., Macdonald A. G., Wann K. T. The effect of temperature on the nerve-blocking action of benzyl alcohol on the squid giant axon. J Physiol. 1983 May;338:51–60. doi: 10.1113/jphysiol.1983.sp014659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel K. A. Intestinal ion transport: effect of norepinephrine, pilocarpine, and atropine. Am J Physiol. 1976 Jul;231(1):252–257. doi: 10.1152/ajplegacy.1976.231.1.252. [DOI] [PubMed] [Google Scholar]
- Ilundain A., Naftalin R. J. Role of Ca(2+)-dependent regulator protein in intestinal secretion. Nature. 1979 May 31;279(5712):446–448. doi: 10.1038/279446a0. [DOI] [PubMed] [Google Scholar]
- Malagodi M. H., Chiou C. Y. Pharmacological evaluation of a new Ca2+ antagonist, 8-(N,N-diethylamino)-octyl-3,4,5-trimethoxybenzoate hydrochloride (TMB-8): studies in smooth muscles. Eur J Pharmacol. 1974 Jun;27(1):25–33. doi: 10.1016/0014-2999(74)90198-8. [DOI] [PubMed] [Google Scholar]
- Munck B. G. Effects of sugar and amino acid transport on transepithelial fluxes of sodium and chloride of short circuited rat jejunum. J Physiol. 1972 Jun;223(3):699–717. doi: 10.1113/jphysiol.1972.sp009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck B. G. Interactions between lysine, Na+ and Cl- transport in rat jejunum. Biochim Biophys Acta. 1970 Jun 2;203(3):424–433. doi: 10.1016/0005-2736(70)90182-3. [DOI] [PubMed] [Google Scholar]
- Munck B. G., Schultz S. G. Properties of the passive conductance pathway across in vitro rat jejunum. J Membr Biol. 1974;16(2):163–174. doi: 10.1007/BF01872412. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Smith P. L., Field M. In vitro antisecretory effects of trifluoperazine and other neuroleptics in rabbit and human small intestine. Gastroenterology. 1980 Jun;78(6):1545–1553. [PubMed] [Google Scholar]
- Smith R. J., Iden S. S. Phorbol myristate acetate-induced release of granule enzymes from human neutrophils: inhibition by the calcium antagonist, 8-(N,N-diethylamino)-octyl 3,4,5-trimethoxybenzoate hydrochloride. Biochem Biophys Res Commun. 1979 Nov 14;91(1):263–271. doi: 10.1016/0006-291x(79)90612-0. [DOI] [PubMed] [Google Scholar]
- Tai Y. H., Decker R. A. Mechanisms of electrolyte transport in rat ileum. Am J Physiol. 1980 Mar;238(3):G208–G212. doi: 10.1152/ajpgi.1980.238.3.G208. [DOI] [PubMed] [Google Scholar]
- Taylor A., Windhager E. E. Possible role of cytosolic calcium and Na-Ca exchange in regulation of transepithelial sodium transport. Am J Physiol. 1979 Jun;236(6):F505–F512. doi: 10.1152/ajprenal.1979.236.6.F505. [DOI] [PubMed] [Google Scholar]
- Trotter J. A., Quintana R. L. Inhibition of macrophage spreading by antagonists of cellular calcium. FEBS Lett. 1981 Jun 29;129(1):29–32. doi: 10.1016/0014-5793(81)80747-8. [DOI] [PubMed] [Google Scholar]
- Weiss B., Prozialeck W., Cimino M., Barnette M. S., Wallace T. L. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. W., Dobbins J. W., Binder H. J. Role of calcium in the regulation of colonic secretion in the rat. Am J Physiol. 1983 May;244(5):G552–G560. doi: 10.1152/ajpgi.1983.244.5.G552. [DOI] [PubMed] [Google Scholar]


