Abstract
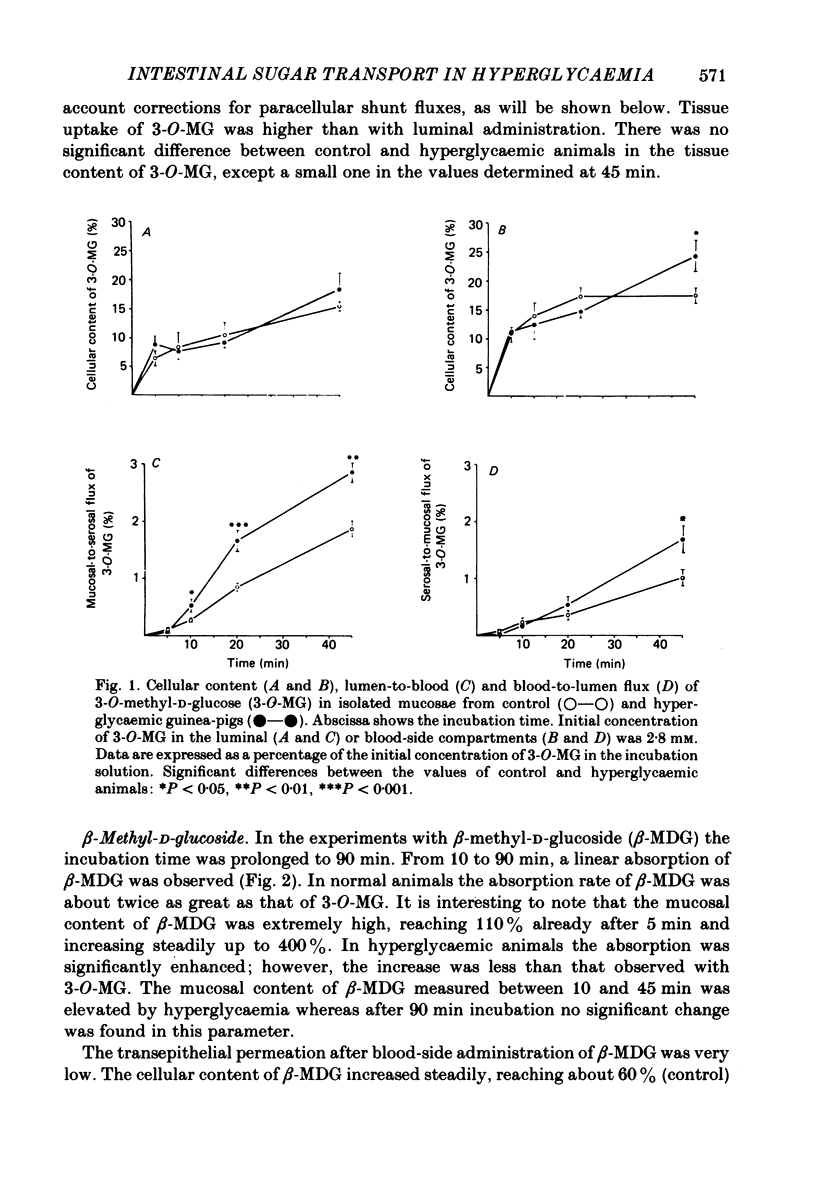
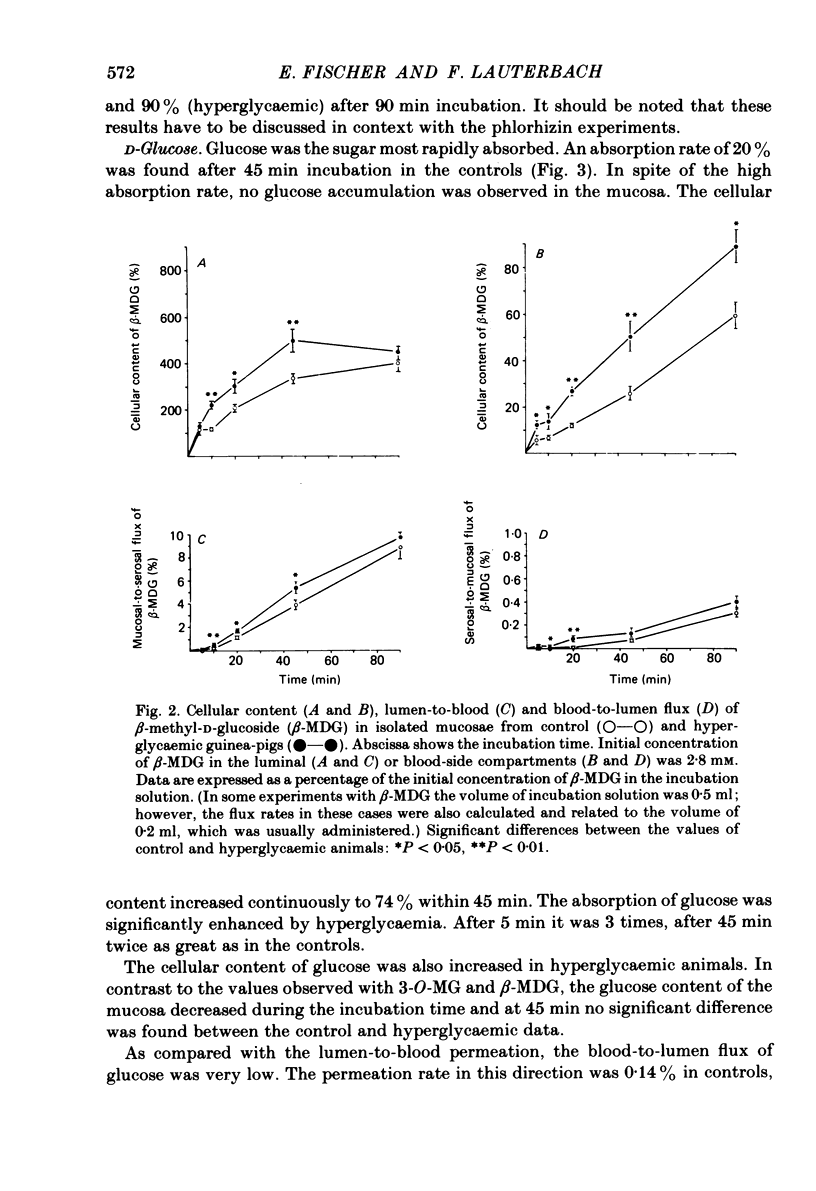
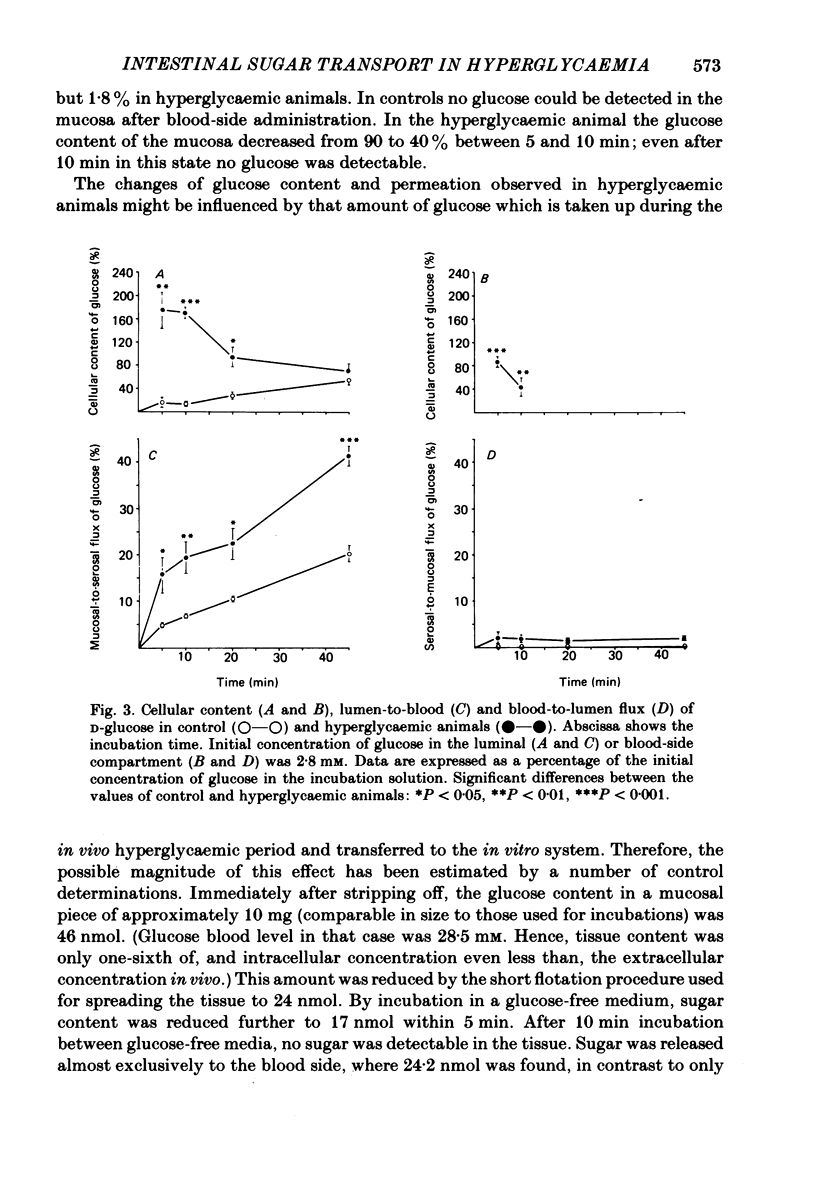
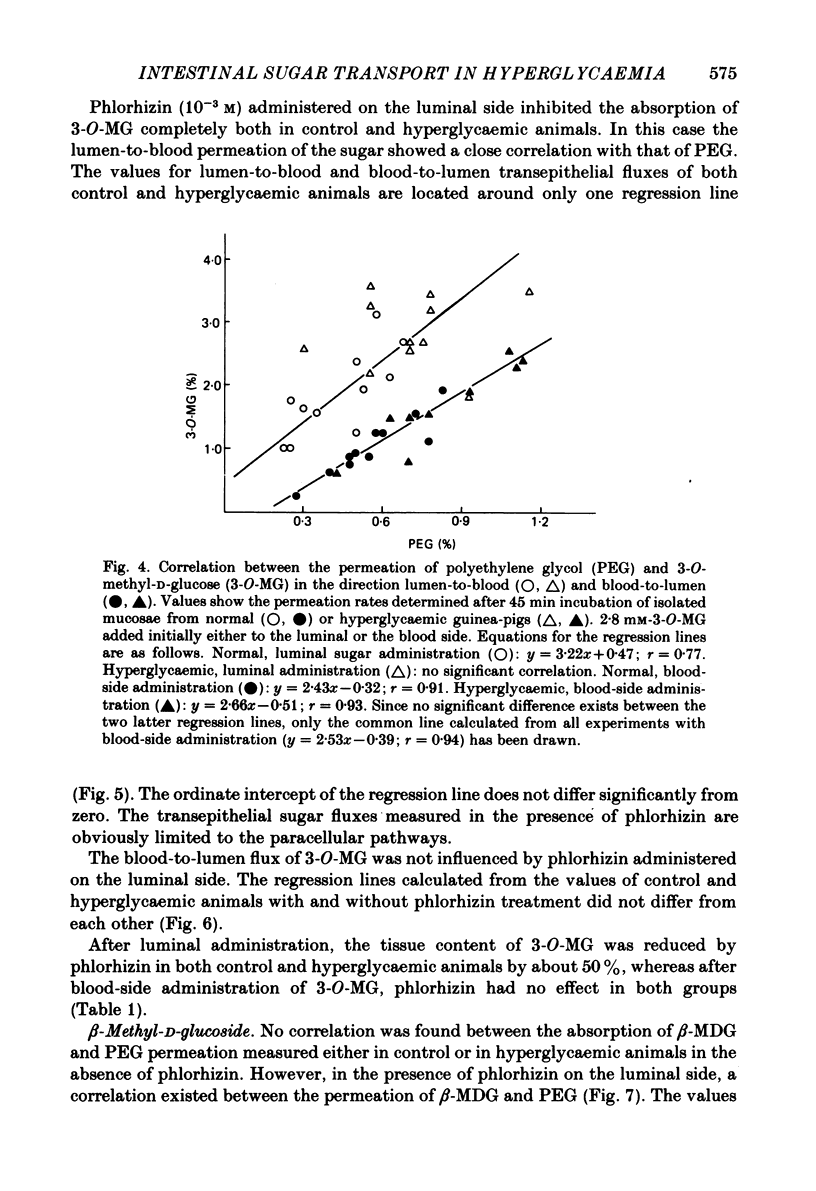
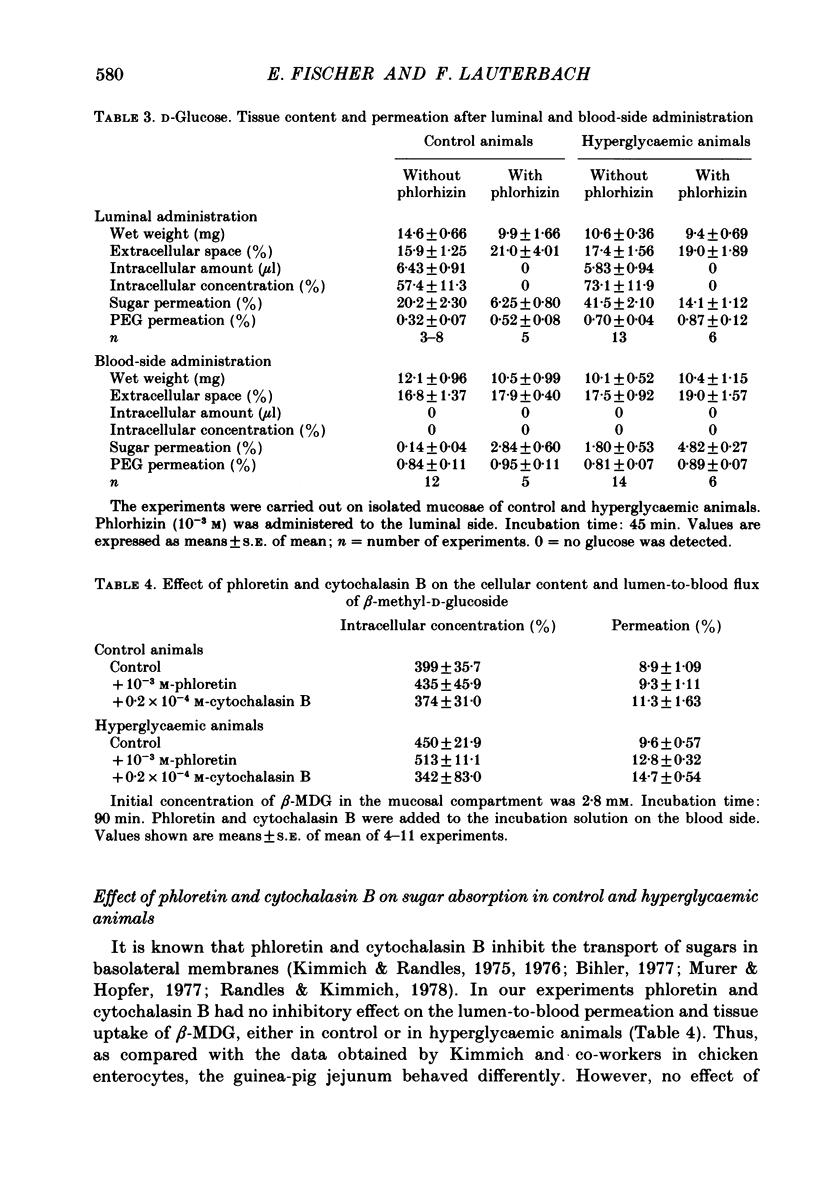
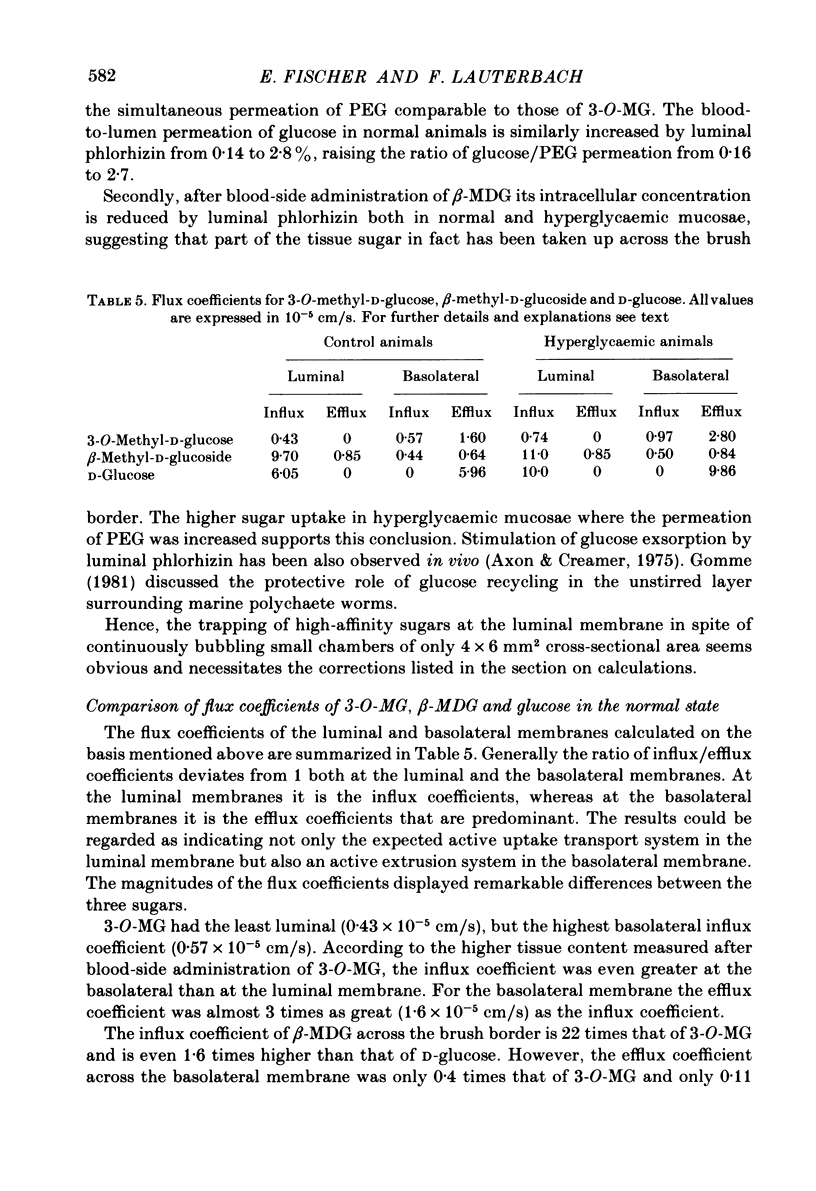
The effect of hyperglycaemia on sugar transport was studied by comparing transepithelial permeation and tissue content of 3-O-methyl-D-glucose (3-O-MG), beta-methyl-D-glucoside (beta-MDG) and D-glucose in isolated mucosae of guinea-pig jejunum mounted in a flux chamber. Sugars were administered either to the luminal or the blood side of mucosae prepared either from normal animals or those maintained in a hyperglycaemic state by I.V. glucose infusion for 12 h. In control animals, absorptive sugar fluxes increased in the order glucose greater than beta-MDG greater than 3-O-MG. Only beta-MDG was accumulated in the tissue beyond the medium concentration. Permeation of 3-O-MG and beta-MDG in the direction blood-to-lumen was mainly paracellular as indicated by the strict correlation with the simultaneous permeation of polyethylene glycol (mol. wt. 900). Luminal addition of 10(-3) M-phlorhizin increased permeation and decreased tissue content of beta-MDG and D-glucose when administered on the blood side, suggesting that these sugars are recaptured at the brush border even from vigorously mixed solutions. For flux coefficient calculation the preparation was regarded as a three-compartment system. With all three sugars, the influx coefficient was higher at the luminal, but lower at the basolateral membrane than the corresponding efflux coefficient. 3-O-MG displayed the highest basolateral influx coefficient of all three sugars, being even higher than its luminal influx coefficient. The luminal influx coefficient of beta-MDG was 22 times greater, and its basolateral efflux coefficient 2.5 times less than the corresponding values for 3-O-MG, resulting in cellular beta-MDG accumulation. D-Glucose was suited best for transepithelial transport, having a luminal influx coefficient only 1.6 times less, and a basolateral efflux coefficient almost 10 times greater than those for beta-MDG. Prolonged hyperglycaemia increased the lumen-to-blood permeation of all three sugars 1.3-2-fold. No significant differences in tissue content to control values were observed after 45 min (3-O-MG, D-glucose) or 90 min (beta-MDG) incubation. Therefore, flux coefficients increased by the same factors in luminal and basolateral membranes, i.e. 1.7, 1.3 and 1.7 for 3-O-MG, beta-MDG and D-glucose, respectively. These results indicate that changes in both the luminal and basolateral membranes play a role in the increase of sugar transport in hyperglycaemia and that a regulatory mechanism might exist between the transport systems located in both membranes.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrad A. D., Lawrence A. L., Hazelwood R. L. Fasting and alloxan diabetes effects of intestinal transport of monosaccharides. Am J Physiol. 1970 Oct;219(4):860–864. doi: 10.1152/ajplegacy.1970.219.4.860. [DOI] [PubMed] [Google Scholar]
- Axon A. T., Creamer B. The exsorption characteristics of various sugars. Gut. 1975 Feb;16(2):99–104. doi: 10.1136/gut.16.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C. A., Parsons D. S. Effects of vascular perfusion on the accumulation, distribution and transfer of 3-O-methyl-D-glucose within and across the small intestine. J Physiol. 1978 Jan;274:17–36. doi: 10.1113/jphysiol.1978.sp012131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd C. A., Parsons D. S. Movements of monosaccharides between blood and tissues of vascularly perfused small intestine. J Physiol. 1979 Feb;287:371–391. doi: 10.1113/jphysiol.1979.sp012665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronk J. R., Ingham P. A. Sugar transfer from the lumen of the rat small intestine to the vascular bed. J Physiol. 1979 Apr;289:99–113. doi: 10.1113/jphysiol.1979.sp012727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE R. K. An effect of alloxan-diabetes on the active transport of sugars by rat small intestine, in vitro. Biochem Biophys Res Commun. 1961 Apr 28;4:436–440. doi: 10.1016/0006-291x(61)90304-7. [DOI] [PubMed] [Google Scholar]
- CRANE R. K. An effect of alloxan-diabetes on the active transport of sugars by rat small intestine, in vitro. Biochem Biophys Res Commun. 1961 Apr 28;4:436–440. doi: 10.1016/0006-291x(61)90304-7. [DOI] [PubMed] [Google Scholar]
- Caspary W. F. Effect of insulin and experimental diabetes mellitus on the digestive-absorptive function of the small intestine. Digestion. 1973 Oct;9(3):248–263. doi: 10.1159/000197452. [DOI] [PubMed] [Google Scholar]
- Caspary W. F., Rhein A. M., Creutzfeldt W. Increase of intestinal brush border hydrolases in mucosa of streptozotocin-diabetic rats. Diabetologia. 1972 Dec;8(6):412–414. doi: 10.1007/BF01212169. [DOI] [PubMed] [Google Scholar]
- Csáky T. Z., Fischer E. Induction of an intestinal epithelial sugar transport system by high blood sugar. Experientia. 1977 Feb 15;33(2):223–224. doi: 10.1007/BF02124079. [DOI] [PubMed] [Google Scholar]
- Csáky T. Z., Fischer E. Intestinal sugar transport in experimental diabetes. Diabetes. 1981 Jul;30(7):568–574. doi: 10.2337/diab.30.7.568. [DOI] [PubMed] [Google Scholar]
- Esposito G., Faelli A., Tosco M., Capraro V. Hyperglycemia and net transintestinal glucose and sodium transport in the rat. Pflugers Arch. 1981 May;390(2):202–206. doi: 10.1007/BF00590208. [DOI] [PubMed] [Google Scholar]
- Flores P., Schedl H. P. Intestinal transport of 3-O-methyl-D-glucose in the normal and alloxan-diabetic rat. Am J Physiol. 1968 Apr;214(4):725–729. doi: 10.1152/ajplegacy.1968.214.4.725. [DOI] [PubMed] [Google Scholar]
- Gilles-Baillien M. Transepithelial fluxes of amino acids and metabolism in the tortoise intestinal mucosa. Arch Int Physiol Biochim. 1980 Feb;88(1):15–23. doi: 10.3109/13813458009080854. [DOI] [PubMed] [Google Scholar]
- Gomme J. Recycling of D-glucose in collagenous cuticle: A means of nutrient conservation? J Membr Biol. 1981;62(1-2):47–52. doi: 10.1007/BF01870198. [DOI] [PubMed] [Google Scholar]
- Hanson P. J., Parsons S. Metabolism and transport of glutamine and glucose in vascularly perfused small intestine rat. Biochem J. 1977 Sep 15;166(3):509–519. doi: 10.1042/bj1660509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U. Diabetes mellitus: changes in the transport properties of isolated intestinal microvillous membranes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2027–2031. doi: 10.1073/pnas.72.6.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson I. T., Bronk J. R. Electron microscope autoradiography of a diffusible intracellular constituent, using freeze-dried frozen sections of mammalian intestinal epithelium. J Microsc. 1979 Mar;115(2):187–194. doi: 10.1111/j.1365-2818.1979.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. 2-Deoxyglucose transport by intestinal epithelial cells isolated from the chick. J Membr Biol. 1976 Jun 30;27(4):363–379. doi: 10.1007/BF01869146. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J. A Na+-independent, phloretin-sensitive monosaccharide transport system in isolated intestinal epithelial cells. J Membr Biol. 1975 Aug 11;23(1):57–76. doi: 10.1007/BF01870244. [DOI] [PubMed] [Google Scholar]
- Lal D., Schedl H. P. Intestinal adaptation in diabetes: amino acid absorption. Am J Physiol. 1974 Oct;227(4):827–831. doi: 10.1152/ajplegacy.1974.227.4.827. [DOI] [PubMed] [Google Scholar]
- Lauterbach F. Passive permeabilities of luminal and basolateral membranes in the isolated mucosal epithelium of guinea pig small intestine. Naunyn Schmiedebergs Arch Pharmacol. 1977 Apr;297(3):201–212. doi: 10.1007/BF00509262. [DOI] [PubMed] [Google Scholar]
- Levinson R. A., Englert E., Jr Intestinal absorption of sugars, water and sodium in alloxan diabetic rats. Diabetes. 1970 Oct;19(10):683–687. doi: 10.2337/diab.19.10.683. [DOI] [PubMed] [Google Scholar]
- Luppa D., Hönicke S., Reissig D., Müller F. Der Einfluss des Alloxandiabetes auf die (Na+ + K+)-aktivierbare ATPase in der Bürstensaummembran der Rattendünndarmmukosa. Acta Biol Med Ger. 1978;37(1):39–47. [PubMed] [Google Scholar]
- Olsen W. A., Rosenberg I. H. Intestinal transport of sugars and amino acids in diabetic rats. J Clin Invest. 1970 Jan;49(1):96–105. doi: 10.1172/JCI106227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randles J., Kimmich G. A. Effects of phloretin and theophylline on 3-O-methylglucose transport by intestinal epithelial cells. Am J Physiol. 1978 Mar;234(3):C64–C72. doi: 10.1152/ajpcell.1978.234.3.C64. [DOI] [PubMed] [Google Scholar]
- Rerup C. C. Drugs producing diabetes through damage of the insulin secreting cells. Pharmacol Rev. 1970 Dec;22(4):485–518. [PubMed] [Google Scholar]
- Schedl H. P., Wilson H. D. Effects of diabetes on intestinal growth and hexose transport in the rat. Am J Physiol. 1971 Jun;220(6):1739–1745. doi: 10.1152/ajplegacy.1971.220.6.1739. [DOI] [PubMed] [Google Scholar]
- Sund R. B., Lauterbach F. Intestinal secretion of sulphanilic acid by the isolated mucosa of guinea pig jejunum. Acta Pharmacol Toxicol (Copenh) 1978 Nov;43(5):331–338. doi: 10.1111/j.1600-0773.1978.tb02275.x. [DOI] [PubMed] [Google Scholar]
- Turnheim K., Frizzell R. A., Schultz S. G. Effects of anions on amiloride-sensitive, active sodium transport across rabbit colon, in vitro. Evidence for "trans-inhibition" of the Na entry mechanism. J Membr Biol. 1977 Oct 3;37(1):63–84. doi: 10.1007/BF01940924. [DOI] [PubMed] [Google Scholar]
- Turnheim K., Frizzell R. A., Schultz S. G. Interaction between cell sodium and the amiloride-sensitive sodium entry step in rabbit colon. J Membr Biol. 1978 Mar 10;39(2-3):233–256. doi: 10.1007/BF01870333. [DOI] [PubMed] [Google Scholar]
- VARMA S. D., BANERJEE S. Intestinal absorption of glucose in normal and diabetic rats: the effect of ethylene diamine tetra acetate (Edta). Indian J Med Res. 1963 May;51:507–511. [PubMed] [Google Scholar]


