Abstract
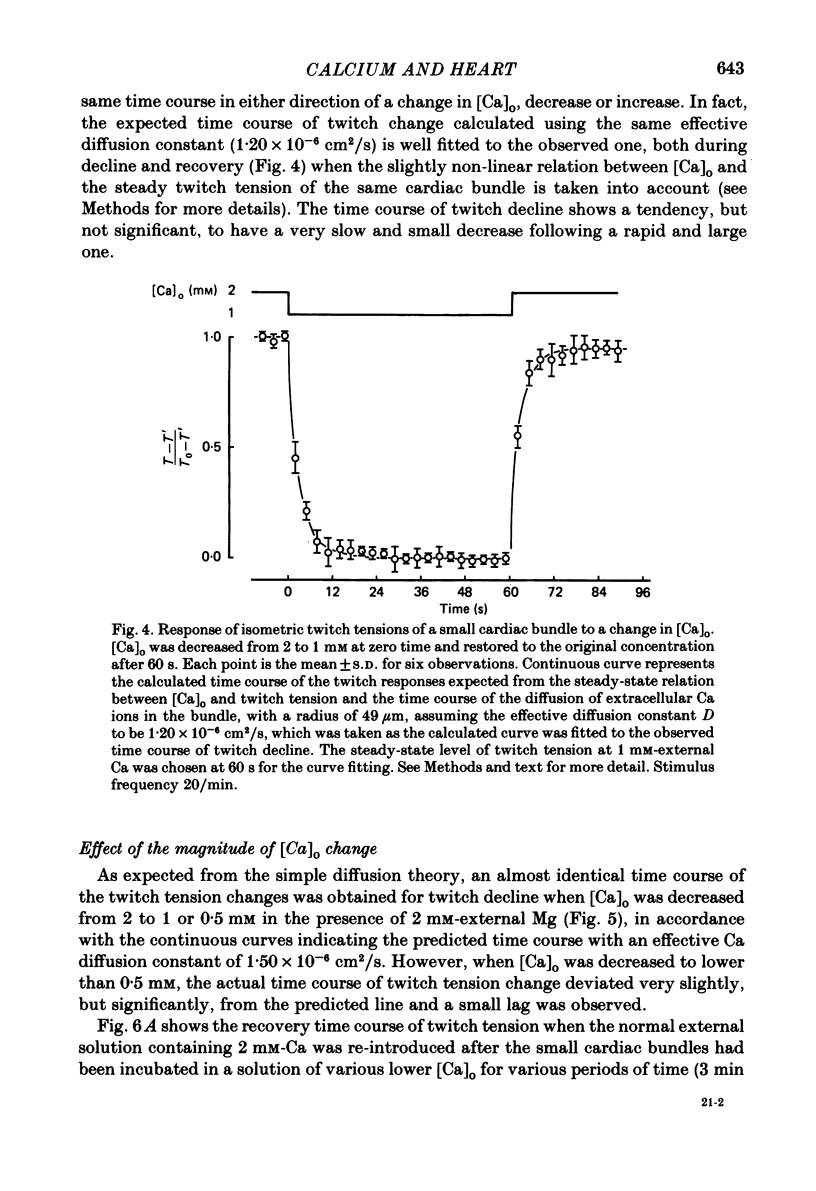
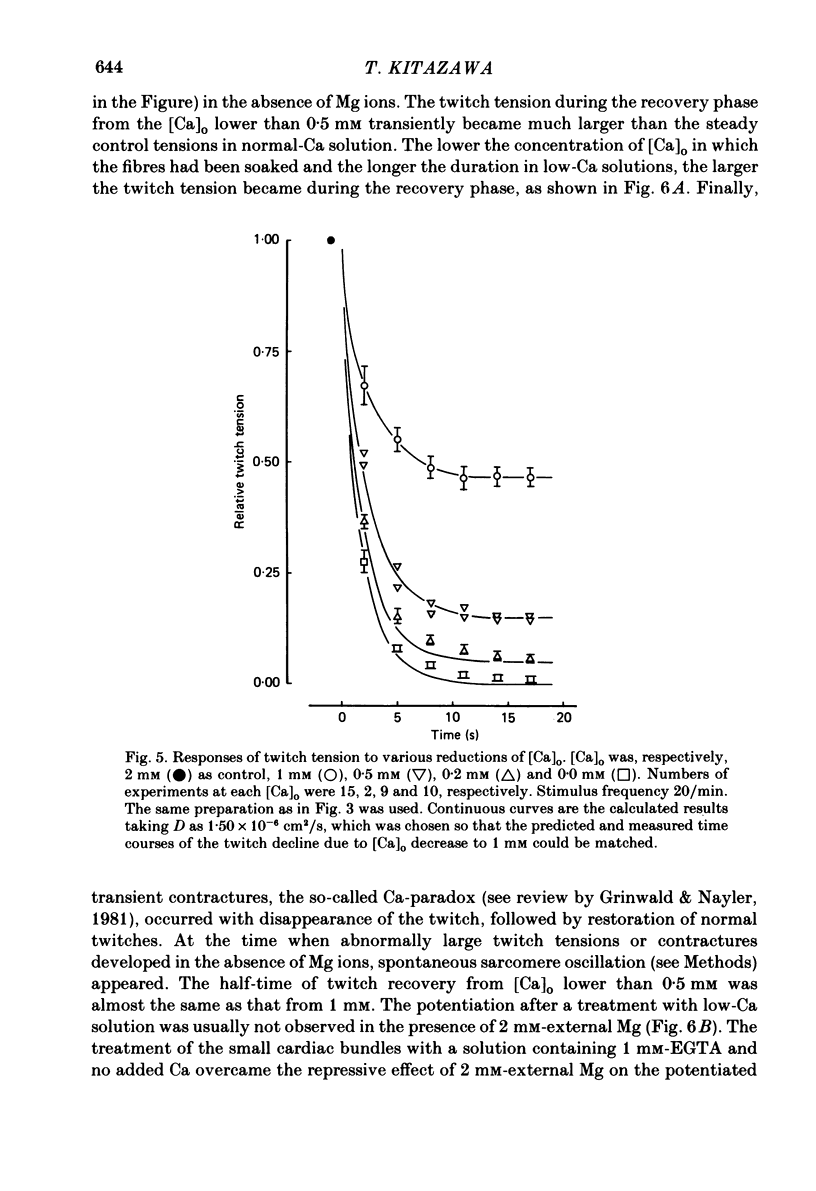
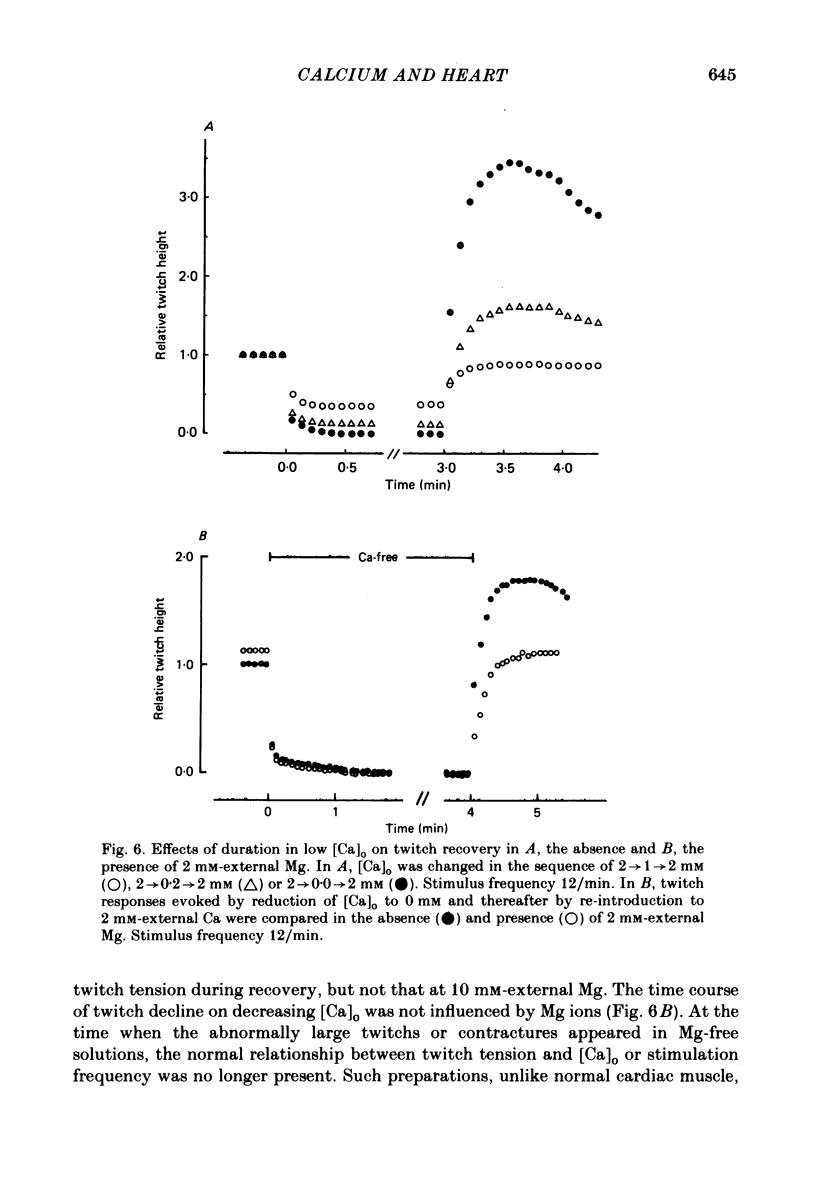
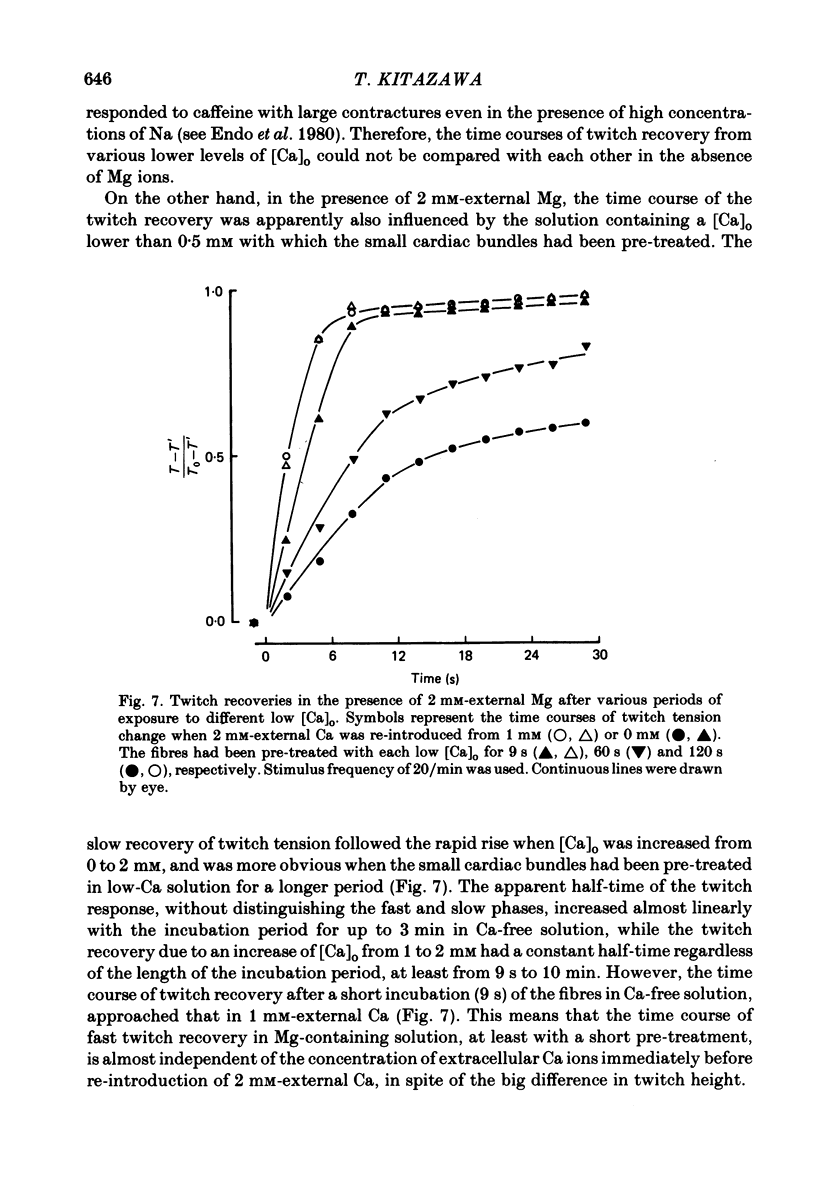
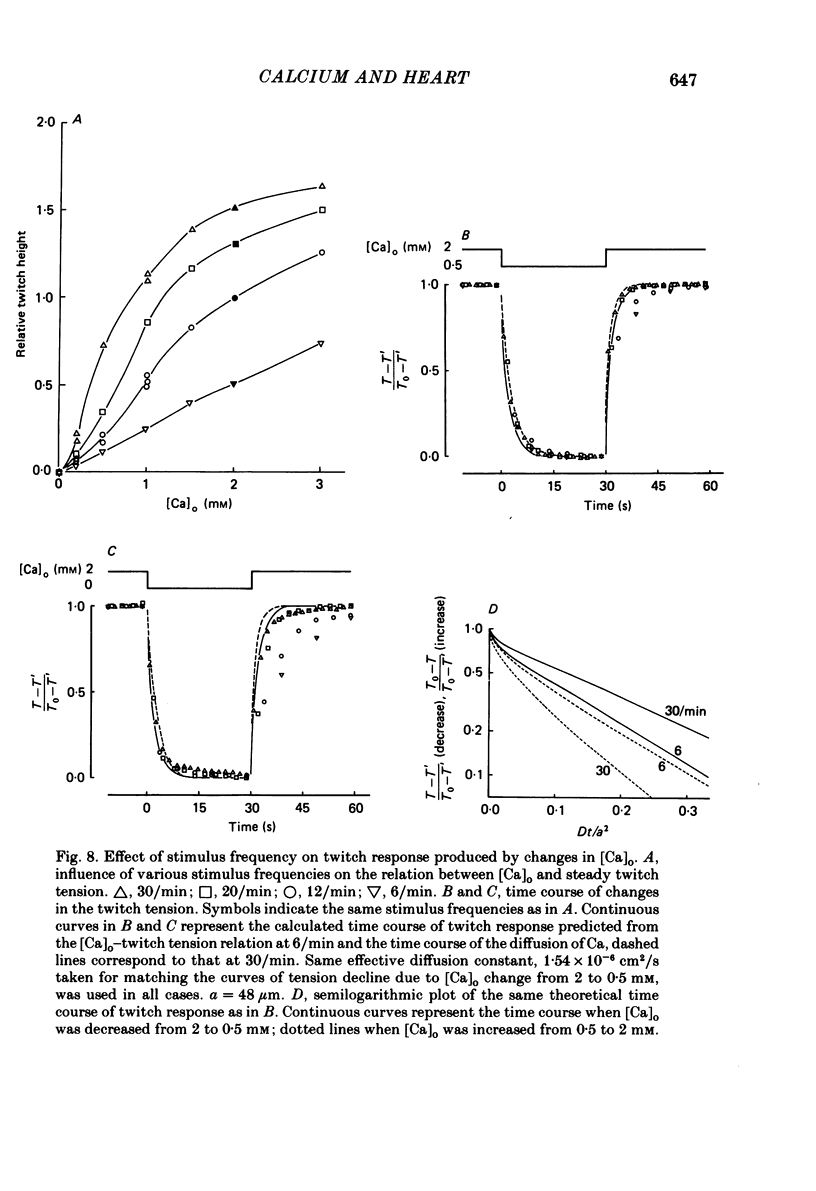
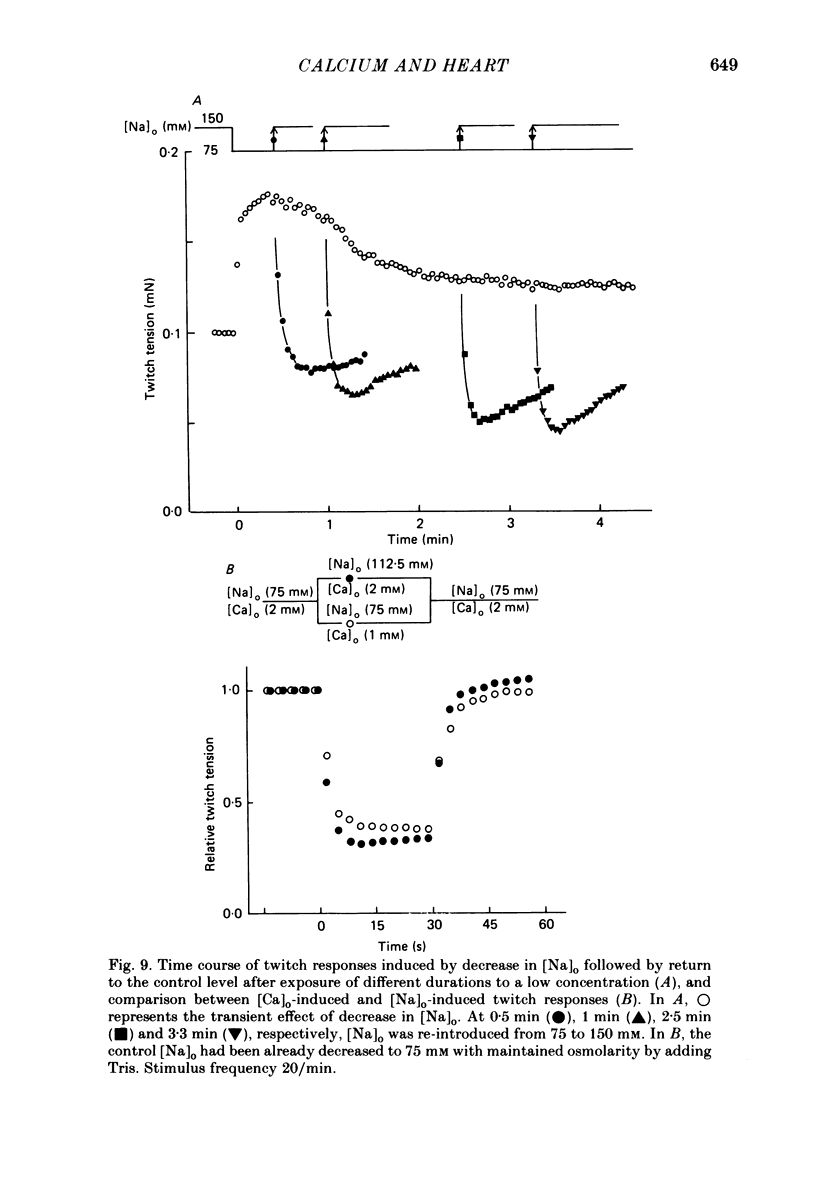
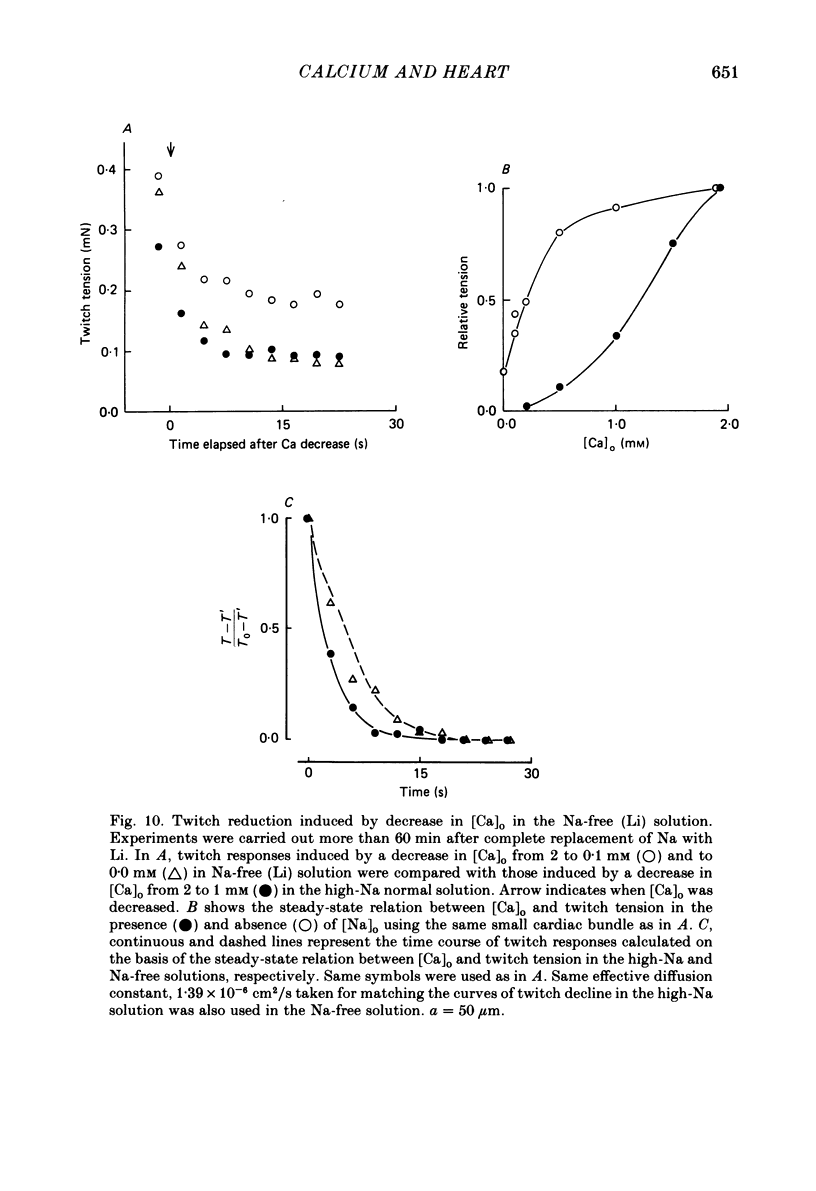
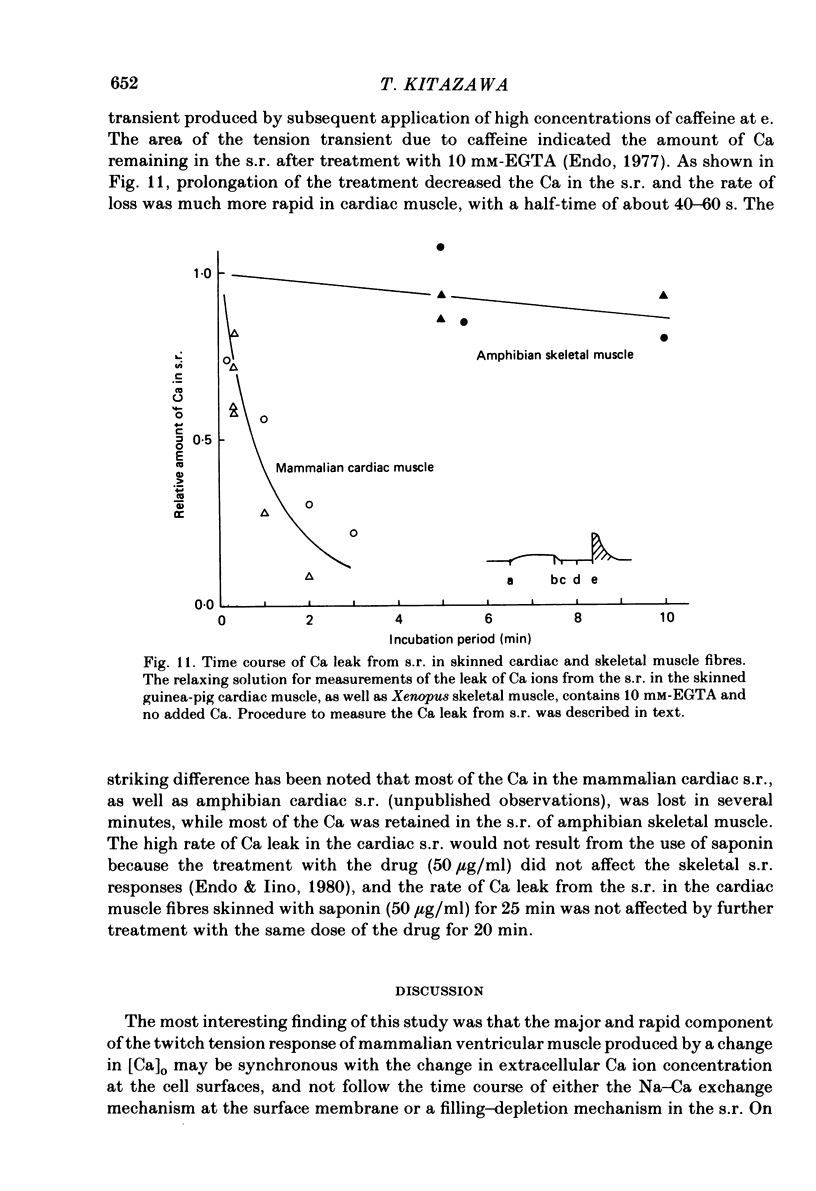
The problem of whether or not the alterations in twitch tension of mammalian cardiac muscle induced by changes in extracellular Ca concentration ([Ca]o) are due to the Na-Ca exchange process across the surface membrane and/or the changes in the amount of Ca in the sarcoplasmic reticulum has been re-examined by using thin bundles (70-120 micron diameter) dissected from guinea-pig papillary muscle. The observed time course of the change in the twitch tension due to a step change in [Ca]o was compared with that computed on a basis of the diffusion process of Ca ions in a circular cylinder and of the steady-state relation between [Ca]o and twitch tension. After a sudden decrease in [Ca]o from 2 mM to various lower concentrations, the isometric twitch tension of the thin bundles first fell rapidly and monotonically and then showed a much smaller and slower secondary fall. The correspondence of the observed time course of the rapid phase with the predicted time course and the observed half-time of the rapid phase ranging from 1.0 to 2.5 s indicate that the rapidity of the twitch response may be dominated by simple diffusion of Ca ions through the extracellular space. If so, the effective diffusion constant of Ca ions inside the bundles was 1.4 +/- 0.2 X 10(-6) cm2/s (mean +/- S.E., n = 9). The magnitude and direction of the step change in [Ca]o or the change at different stimulus frequencies gave rise to dissimilar time courses of the contractile change; the difference in the rapid time courses due to these factors could be explained by the simple diffusion models, but not in the much slower phase. The half-time for the Ca effect was the same as that for the rapid effect of Na ions in the external solutions. The time course of twitch decline due to [Ca]o decrease in the Na-free (Li) solution was identical to that predicted from the time course measured in the Na-rich solution and the steady-state relation between [Ca]o and tension in the Na-free solution. The half-time of Ca leak from the sarcoplasmic reticulum in the skinned cardiac muscle was 40-60 s in the presence of 10 mM-EGTA, much shorter than that of the Ca leak in the skinned amphibian skeletal muscle, but much longer than that of twitch responses due to step changes in [Ca]o in the intact cardiac muscle.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Blinks J. R. Calcium transients in aequorin-injected frog cardiac muscle. Nature. 1978 Jun 15;273(5663):509–513. doi: 10.1038/273509a0. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Blinks J. R., Prendergast F. G. Aequorin luminescence: relation of light emission to calcium concentration--a calcium-independent component. Science. 1977 Mar 11;195(4282):996–998. doi: 10.1126/science.841325. [DOI] [PubMed] [Google Scholar]
- Allen D. G., Jewell B. R., Wood E. H. Studies of the contractility of mammalian myocardium at low rates of stimulation. J Physiol. 1976 Jan;254(1):1–17. doi: 10.1113/jphysiol.1976.sp011217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D. G., Kurihara S. The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle. J Physiol. 1982 Jun;327:79–94. doi: 10.1113/jphysiol.1982.sp014221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey L. E., Ong S. D., Queen G. M. Calcium movement during contraction in the cat heart. J Mol Cell Cardiol. 1972 Apr;4(2):121–138. doi: 10.1016/0022-2828(72)90070-3. [DOI] [PubMed] [Google Scholar]
- Beeler G. W., Jr, Reuter H. Membrane calcium current in ventricular myocardial fibres. J Physiol. 1970 Mar;207(1):191–209. doi: 10.1113/jphysiol.1970.sp009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D. M., Langer G. A. Uncoupling cation effects on cardiac contractility and sarcolemmal Ca2+ binding. Am J Physiol. 1979 Sep;237(3):H332–H341. doi: 10.1152/ajpheart.1979.237.3.H332. [DOI] [PubMed] [Google Scholar]
- Brady A. J., Tan S. T., Ricchiuti N. V. Contractile force measured in unskinned isolated adult rat heart fibres. Nature. 1979 Dec 13;282(5740):728–729. doi: 10.1038/282728a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Lee K. S., Powell T. Sodium current in single rat heart muscle cells. J Physiol. 1981 Sep;318:479–500. doi: 10.1113/jphysiol.1981.sp013879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Chapman R. A., Leoty C. Proceedings: A method of mounting, perfusing and recording the tension generated by isolated mammalian cardiac trabeculae which overcomes their susceptibility to mechanical damage. J Physiol. 1976 Jun;258(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- Crevey B. J., Langer G. A., Frank J. S. Role of Ca2+ in maintenance of rabbit myocardial cell membrane structural and functional integrity. J Mol Cell Cardiol. 1978 Dec;10(12):1081–1100. doi: 10.1016/0022-2828(78)90354-1. [DOI] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Changes in the intracellular sodium activity of sheep heart Purkinje fibres produced by calcium and other divalent cations. J Physiol. 1978 Apr;277:437–453. doi: 10.1113/jphysiol.1978.sp012283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann N Y Acad Sci. 1978 Apr 28;307:491–522. doi: 10.1111/j.1749-6632.1978.tb41979.x. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozzard H. A. Heart: excitation-contraction coupling. Annu Rev Physiol. 1977;39:201–220. doi: 10.1146/annurev.ph.39.030177.001221. [DOI] [PubMed] [Google Scholar]
- Gordon A. M., Huxley A. F., Julian F. J. Tension development in highly stretched vertebrate muscle fibres. J Physiol. 1966 May;184(1):143–169. doi: 10.1113/jphysiol.1966.sp007908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinwald P. M., Nayler W. G. Calcium entry in the calcium paradox. J Mol Cell Cardiol. 1981 Oct;13(10):867–880. doi: 10.1016/0022-2828(81)90286-8. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Klöckner U. Glycocalyx is not required for show inward calcium current in isolated rat heart myocytes. Nature. 1980 Mar 27;284(5754):358–360. doi: 10.1038/284358a0. [DOI] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T. Physiological significance of Ca uptake by mitochondria in the heart in comparison with that by cardiac sarcoplasmic reticulum. J Biochem. 1976 Nov;80(5):1129–1147. doi: 10.1093/oxfordjournals.jbchem.a131369. [DOI] [PubMed] [Google Scholar]
- Krueger J. W., Pollack G. H. Myocardial sarcomere dynamics during isometric contraction. J Physiol. 1975 Oct;251(3):627–643. doi: 10.1113/jphysiol.1975.sp011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUTTGAU H. C., NIEDERGERKE R. The antagonism between Ca and Na ions on the frog's heart. J Physiol. 1958 Oct 31;143(3):486–505. doi: 10.1113/jphysiol.1958.sp006073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel E., Niedergerke R., Page S. Analysis of a rapid twitch facilitation in the frog heart. Proc R Soc Lond B Biol Sci. 1975 Jun 17;189(1097):577–590. doi: 10.1098/rspb.1975.0073. [DOI] [PubMed] [Google Scholar]
- Langer G. A. Events at the cardiac sarcolemma: localization and movement of contractile-dependent calcium. Fed Proc. 1976 May 1;35(6):1274–1278. [PubMed] [Google Scholar]
- Langer G. A. Ionic basis of myocardial contractility. Annu Rev Med. 1977;28:13–20. doi: 10.1146/annurev.me.28.020177.000305. [DOI] [PubMed] [Google Scholar]
- Langer G. A. The role of calcium in the control of myocardial contractility: an update. J Mol Cell Cardiol. 1980 Mar;12(3):231–239. doi: 10.1016/0022-2828(80)90037-1. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Uhm D. Y., Dresdner K. Sodium-calcium exchange in rabbit heart muscle cells: direct measurement of sarcoplasmic Ca2+ activity. Science. 1980 Aug 8;209(4457):699–701. doi: 10.1126/science.7394527. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Trypsin inhibits the action of tetrodotoxin on neurones. Nature. 1977 Feb 24;265(5596):751–753. doi: 10.1038/265751a0. [DOI] [PubMed] [Google Scholar]
- Marban E., Rink T. J., Tsien R. W., Tsien R. Y. Free calcium in heart muscle at rest and during contraction measured with Ca2+ -sensitive microelectrodes. Nature. 1980 Aug 28;286(5776):845–850. doi: 10.1038/286845a0. [DOI] [PubMed] [Google Scholar]
- Morad M., Goldman Y. E., Trentham D. R. Rapid photochemical inactivation of Ca2+-antagonists shows that Ca2+ entry directly activates contraction in frog heart. Nature. 1983 Aug 18;304(5927):635–638. doi: 10.1038/304635a0. [DOI] [PubMed] [Google Scholar]
- Nakajima S., Nakajima Y., Bastian J. Effects of sudden changes in external sodium concentration on twitch tension in isolated muscle fibers. J Gen Physiol. 1975 Apr;65(4):459–482. doi: 10.1085/jgp.65.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Endo M. Release of calcium induced by 'depolarisation' of the sarcoplasmic reticulum membrane. Nat New Biol. 1973 Dec 19;246(155):216–218. doi: 10.1038/newbio246216a0. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. The ionic nature of slow inward current and its relation to contraction. Pflugers Arch. 1972;334(1):24–38. doi: 10.1007/BF00585998. [DOI] [PubMed] [Google Scholar]
- Page S. G., Niedergerke R. Structures of physiological interest in the frog heart ventricle. J Cell Sci. 1972 Jul;11(1):179–203. doi: 10.1242/jcs.11.1.179. [DOI] [PubMed] [Google Scholar]
- Philipson K. D., Nishimoto A. Y. Efflux of Ca2+ from cardiac sarcolemmal vesicles. Influence of external Ca2+ and Na+. J Biol Chem. 1981 Apr 25;256(8):3698–3702. [PubMed] [Google Scholar]
- Pitts B. J. Stoichiometry of sodium-calcium exchange in cardiac sarcolemmal vesicles. Coupling to the sodium pump. J Biol Chem. 1979 Jul 25;254(14):6232–6235. [PubMed] [Google Scholar]
- Powell T., Twist V. W. A rapid technique for the isolation and purification of adult cardiac muscle cells having respiratory control and a tolerance to calcium. Biochem Biophys Res Commun. 1976 Sep 7;72(1):327–333. doi: 10.1016/0006-291x(76)90997-9. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich T. L., Langer G. A. A comparison of excitation-contraction coupling in heart and skeletal muscle: an examination of "calcium-induced calcium-release". J Mol Cell Cardiol. 1975 Oct;7(10):747–765. doi: 10.1016/0022-2828(75)90041-3. [DOI] [PubMed] [Google Scholar]
- Saari J. T., Johnson J. A. Decay of calcium content and contractile force in the rabbit heart. Am J Physiol. 1971 Dec;221(6):1572–1575. doi: 10.1152/ajplegacy.1971.221.6.1572. [DOI] [PubMed] [Google Scholar]
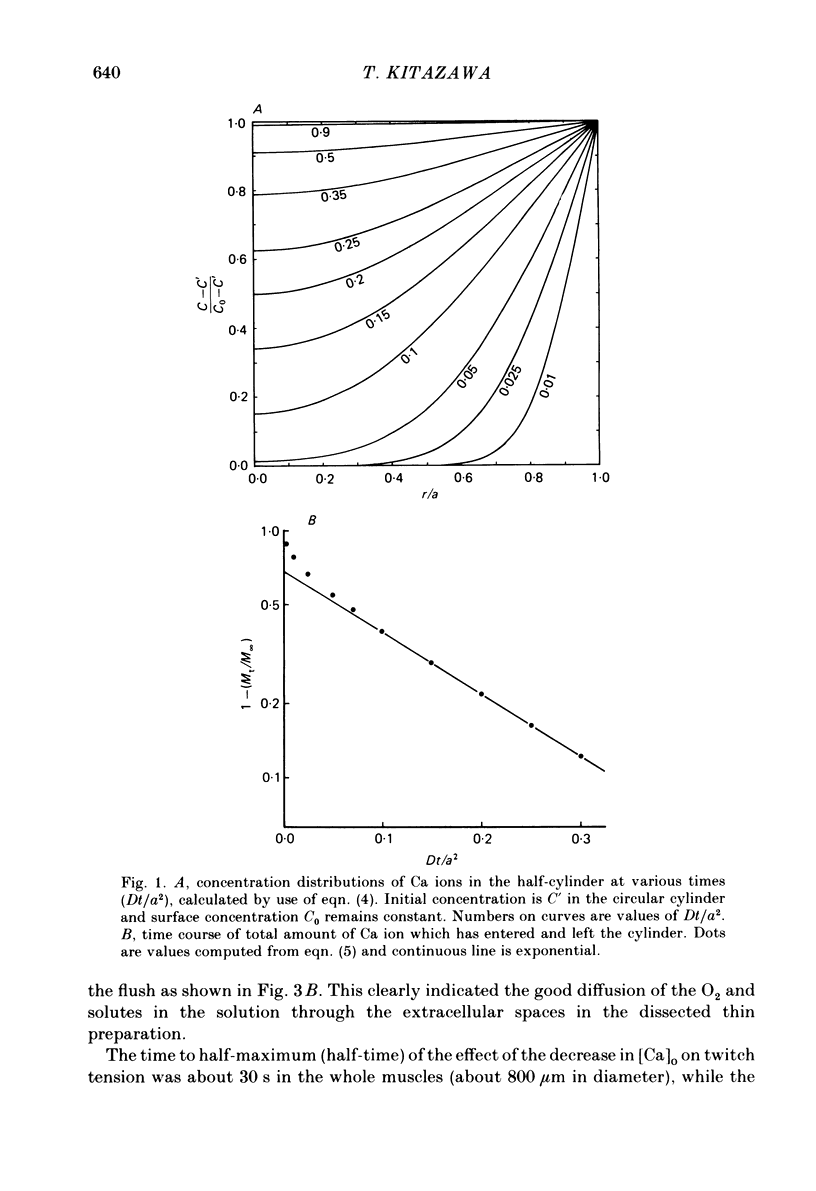
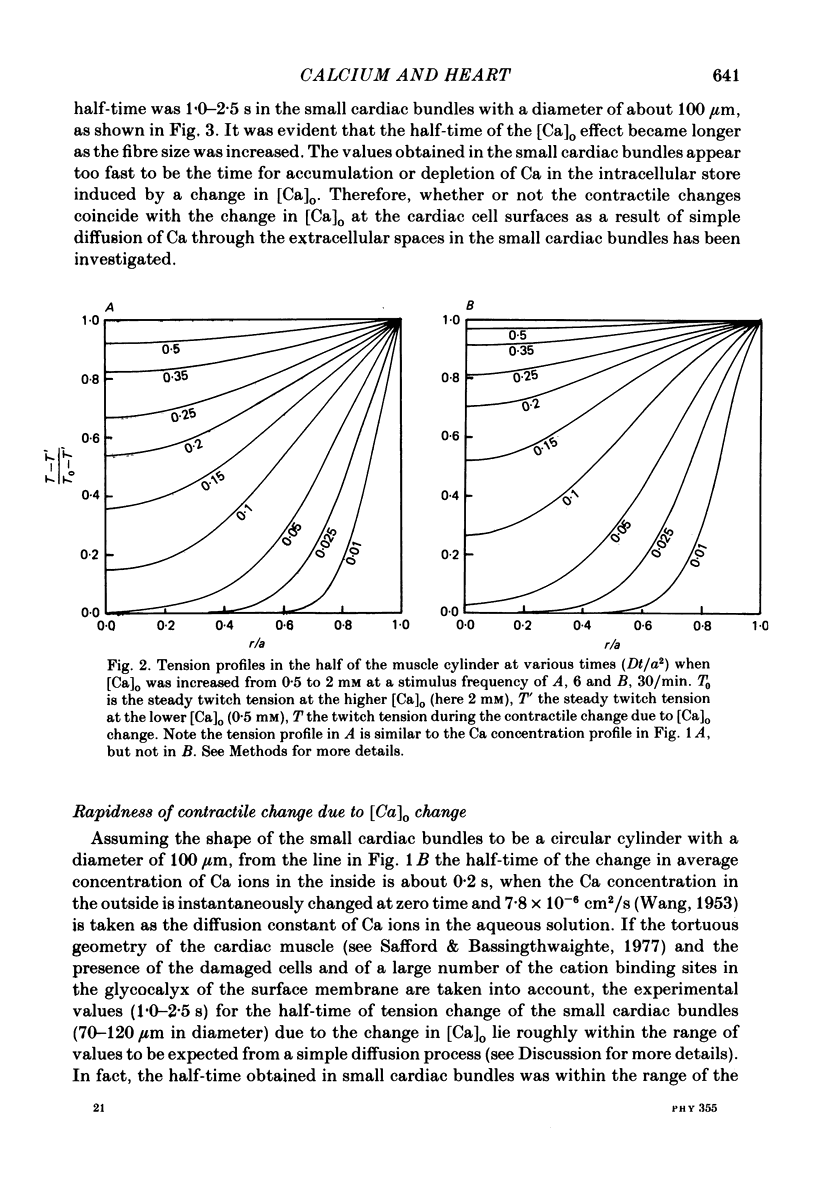
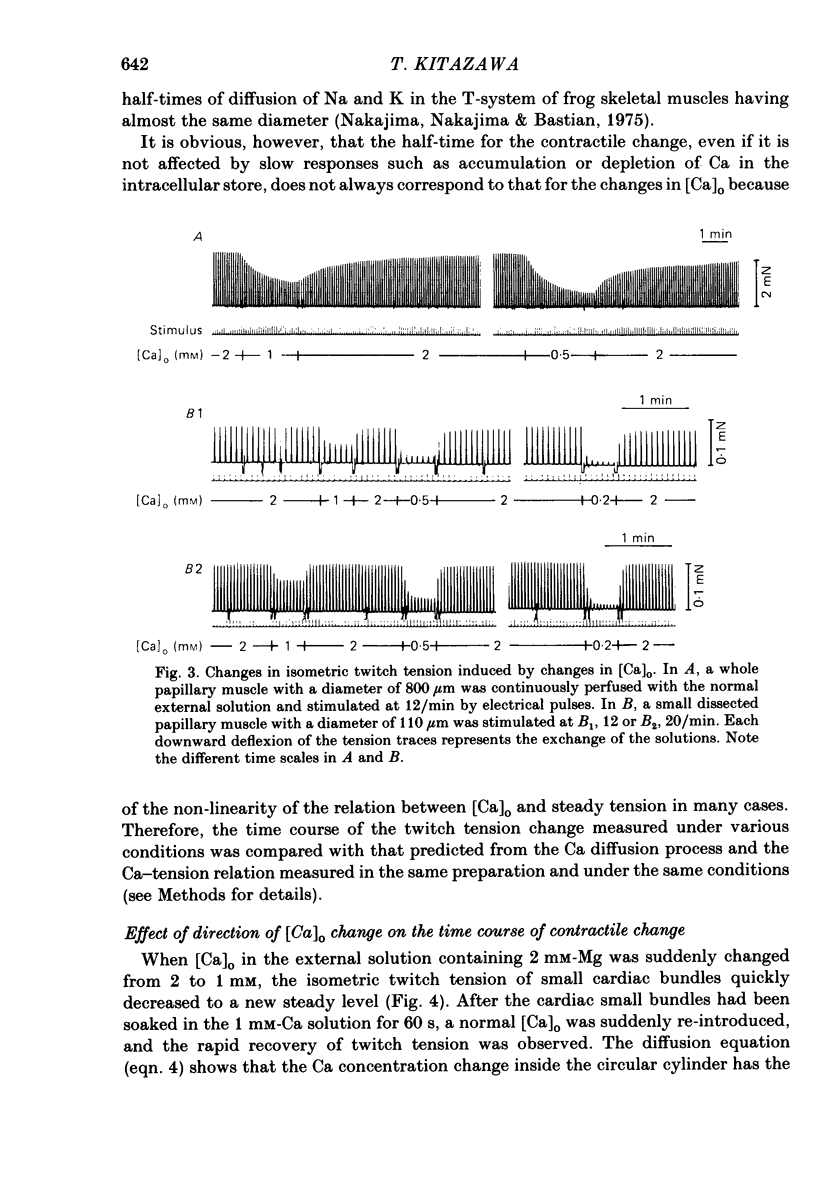
- Safford R. E., Bassingthwaighte J. B. Calcium diffusion in transient and steady states in muscle. Biophys J. 1977 Oct;20(1):113–136. doi: 10.1016/S0006-3495(77)85539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine K. I., Serena S. D., Langer G. A. Kinetic localization of contractile calcium in rabbit myocardium. Am J Physiol. 1971 Nov;221(5):1408–1417. doi: 10.1152/ajplegacy.1971.221.5.1408. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Gonzalez-Serratos H. G., Shuman H., McClellan G., Somlyo A. P. Calcium release and ionic changes in the sarcoplasmic reticulum of tetanized muscle: an electron-probe study. J Cell Biol. 1981 Sep;90(3):577–594. doi: 10.1083/jcb.90.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch J. H., Fung L. K., Hom P. M., Langer G. A. Transient and steady-state effects of sodium and calcium on myocardial contractile response. J Mol Cell Cardiol. 1979 Feb;11(2):137–148. doi: 10.1016/0022-2828(79)90459-0. [DOI] [PubMed] [Google Scholar]
- WINEGRAD S., SHANES A. M. Calcium flux and contractility in guinea pig atria. J Gen Physiol. 1962 Jan;45:371–394. doi: 10.1085/jgp.45.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G. Calcium transients during excitation-contraction coupling in mammalian heart: aequorin signals of canine Purkinje fibers. Science. 1980 Mar 7;207(4435):1085–1087. doi: 10.1126/science.7355274. [DOI] [PubMed] [Google Scholar]
- Wood E. H., Heppner R. L., Weidmann S. Inotropic effects of electric currents. I. Positive and negative effects of constant electric currents or current pulses applied during cardiac action potentials. II. Hypotheses: calcium movements, excitation-contraction coupling and inotropic effects. Circ Res. 1969 Mar;24(3):409–445. doi: 10.1161/01.res.24.3.409. [DOI] [PubMed] [Google Scholar]