Abstract
The composition of afferent lymph draining into the canine popliteal lymph node was compared with that of the efferent lymph leaving the node. Both the protein and cellular composition were studied. In twenty-five greyhounds the protein concentration of efferent lymph was greater than that of afferent lymph collected from the same limb. Although the absolute level of protein varied greatly between dogs, in a particular animal there was a constant ratio between the protein content of afferent and efferent lymph. The concentration of protein in efferent lymph was approximately double that of afferent lymph. Chromatographic analysis of lymph and the use of radio-iodinated canine albumin indicated that the reason for the increased level of protein in the efferent lymph is that the popliteal node concentrates the protein in afferent lymph. Afferent lymph contained less than 3 X 10(3) cells/ml; efferent lymph contained between 0.5 X 10(6) and 4.3 X 10(6) cells/ml, 98% of which were lymphocytes. In different dogs there was no correlation between efferent lymphocyte density and afferent or efferent protein concentration; however, when an afferent lymphatic was perfused with solutions of different protein concentration, the lymphocyte number in the efferent fluid became greater as protein concentration in the afferent perfusate was increased. The concentrating effect of the node is discussed in terms of its significance to both fluid balance and immunological surveillance.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. D., Anderson A. O., Wyllie R. G. Specialized structure and metabolic activities of high endothelial venules in rat lymphatic tissues. Immunology. 1976 Sep;31(3):455–473. [PMC free article] [PubMed] [Google Scholar]
- Andrews P., Milsom D. W., Ford W. L. Migration of lymphocytes across specialized vascular endothelium. V. Production of a sulphated macromolecule by high endothelial cells in lymph nodes. J Cell Sci. 1982 Oct;57:277–292. doi: 10.1242/jcs.57.1.277. [DOI] [PubMed] [Google Scholar]
- BENACERRAF B., BIOZZI G., HALPERN B. N., STIFFEL C., MOUTON D. Phagocytosis of heat-denatured human serum albumin labelled with 131I and its use as a means of investigating liver blood flow. Br J Exp Pathol. 1957 Feb;38(1):35–48. [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- HALL J. G., MORRIS B. THE ORIGIN OF THE CELLS IN THE EFFERENT LYMPH FROM A SINGLE LYMPH NODE. J Exp Med. 1965 Jun 1;121:901–910. doi: 10.1084/jem.121.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAYERSON H. S., PATTERSON R. M., McKEE A., LEBRIE S. J., MAYERSON P. Permeability of lymphatic vessels. Am J Physiol. 1962 Jul;203:98–106. doi: 10.1152/ajplegacy.1962.203.1.98. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mikata A., Niki R. Permeability of postcapillary venules of the lymph node. An electron microscopic study. Exp Mol Pathol. 1971 Jun;14(3):289–305. doi: 10.1016/0014-4800(71)90002-5. [DOI] [PubMed] [Google Scholar]
- Pflug J. J., Calnan J. S. Lymphatics: normal anatomy in the dog hind leg. J Anat. 1969 Nov;105(Pt 3):457–465. [PMC free article] [PubMed] [Google Scholar]
- Pflug J. J., Calnan J. S. The normal anatomy of the lymphatic system in the human leg. Br J Surg. 1971 Dec;58(12):925–930. doi: 10.1002/bjs.1800581216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quin J. W., Shannon A. D. The influence of the lymph node on the protein concentration of efferent lymph leaving the node. J Physiol. 1977 Jan;264(2):307–321. doi: 10.1113/jphysiol.1977.sp011670. [DOI] [PMC free article] [PubMed] [Google Scholar]
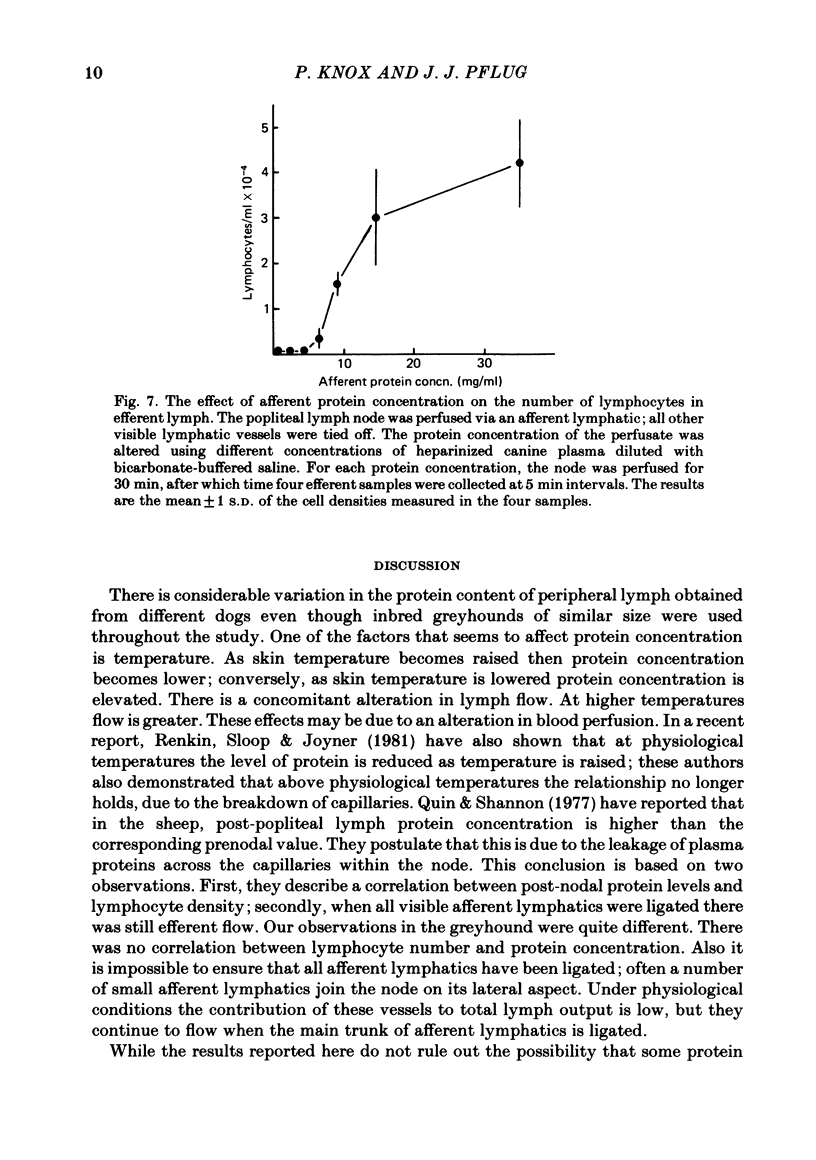
- Renkin E. M., Sloop C. S., Joyner W. L. Influence of venous congestion on blood-lymph transport of fluid and large molecules in the heat-injured dog's paw. Lymphology. 1981 Sep;14(3):113–117. [PubMed] [Google Scholar]
- SMITH C., HENON B. K. Histological and histochemical study of high endothelium of post-capillary veins of the lymph node. Anat Rec. 1959 Nov;135:207–213. doi: 10.1002/ar.1091350306. [DOI] [PubMed] [Google Scholar]
- Schoefl G. I. The migration of lymphocytes across the vascular endothelium in lymphoid tissue. A reexamination. J Exp Med. 1972 Sep 1;136(3):568–588. doi: 10.1084/jem.136.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk E. J., Orlic D., Reith E. J., Rhodin J. A. The ultrastructure of mouse lymph node venules and the passage of lymphocytes across their walls. J Ultrastruct Res. 1974 May;47(2):214–241. doi: 10.1016/s0022-5320(74)80071-7. [DOI] [PubMed] [Google Scholar]


