Abstract
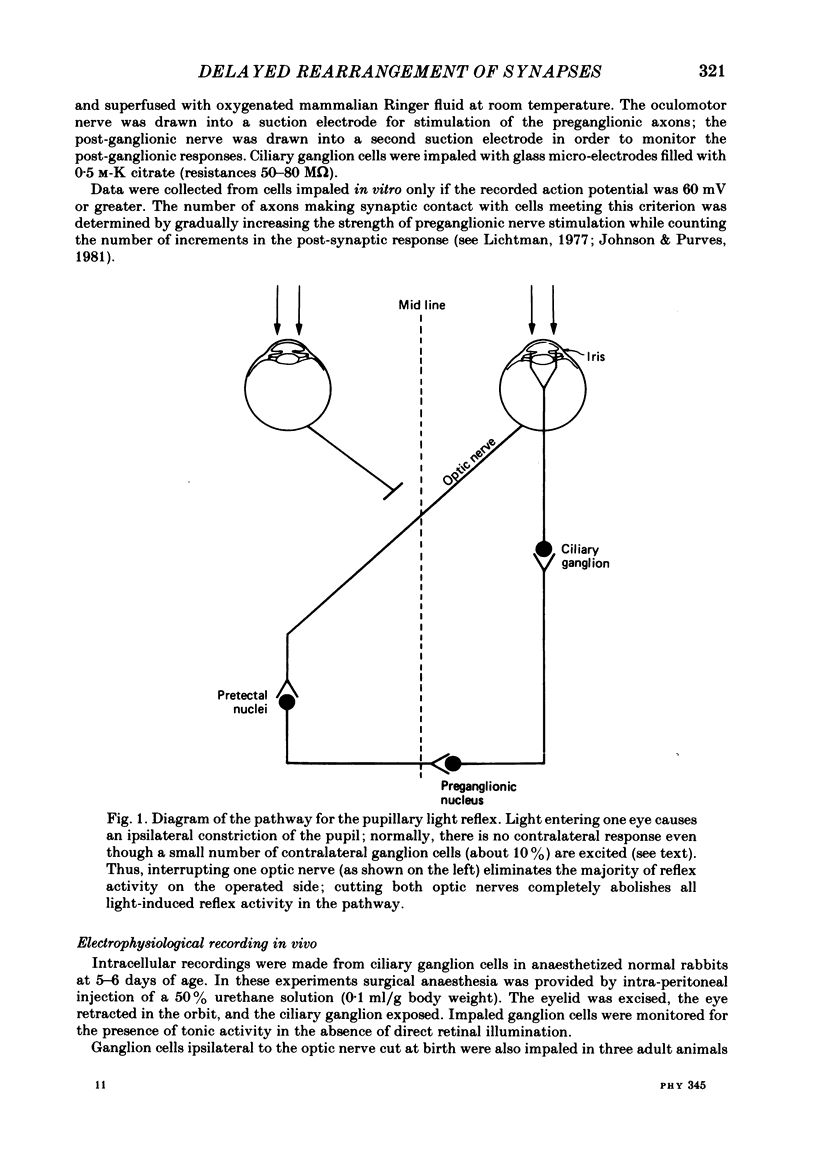
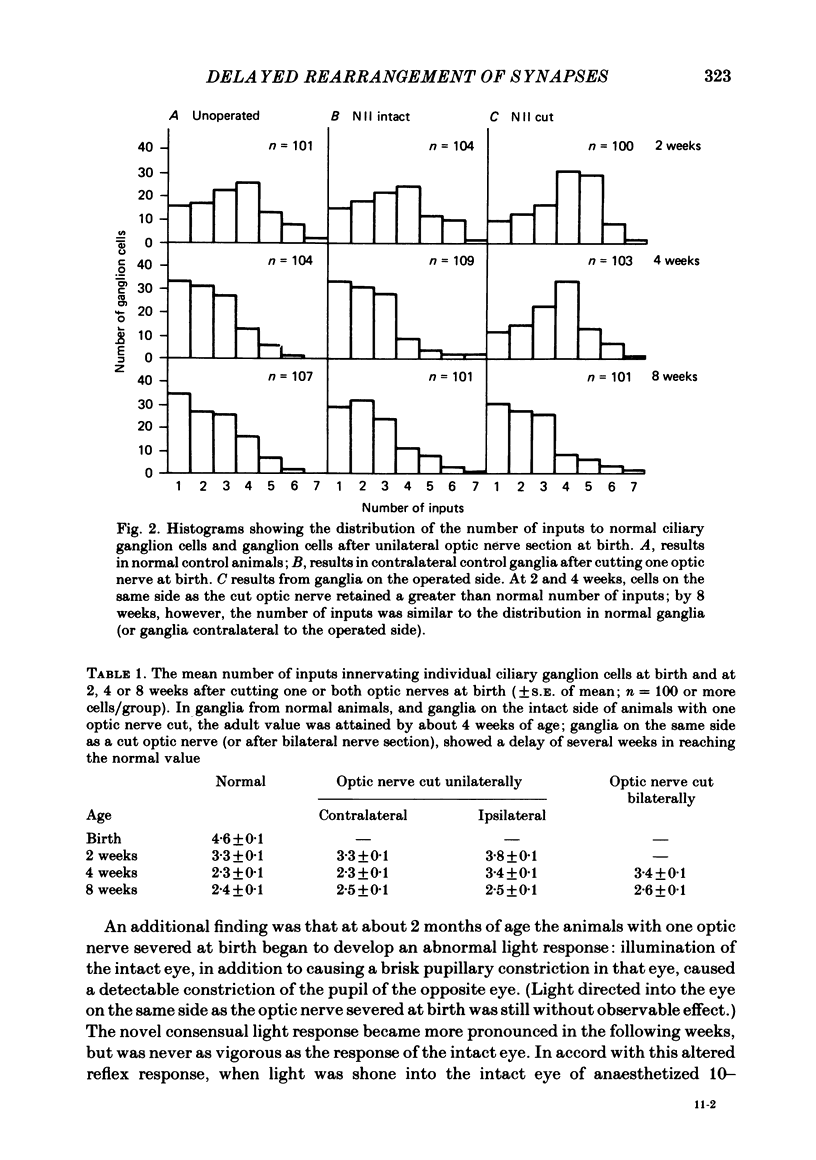
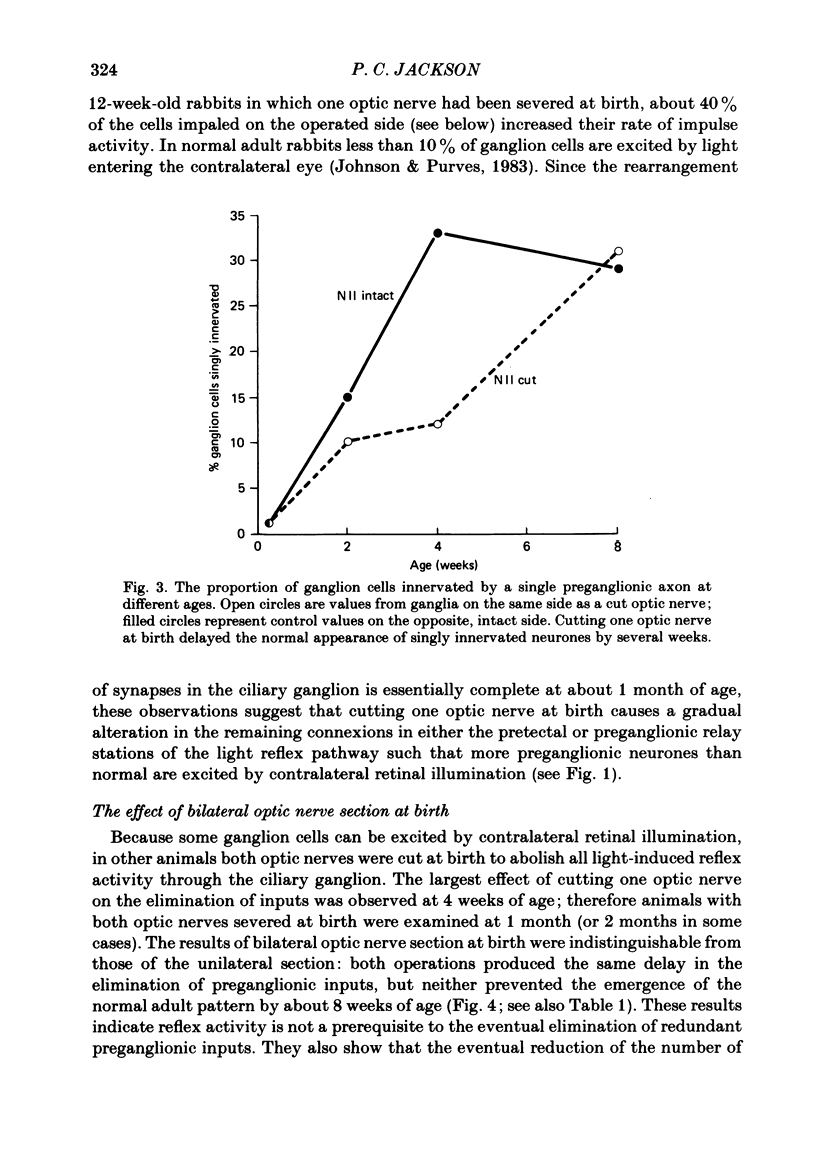
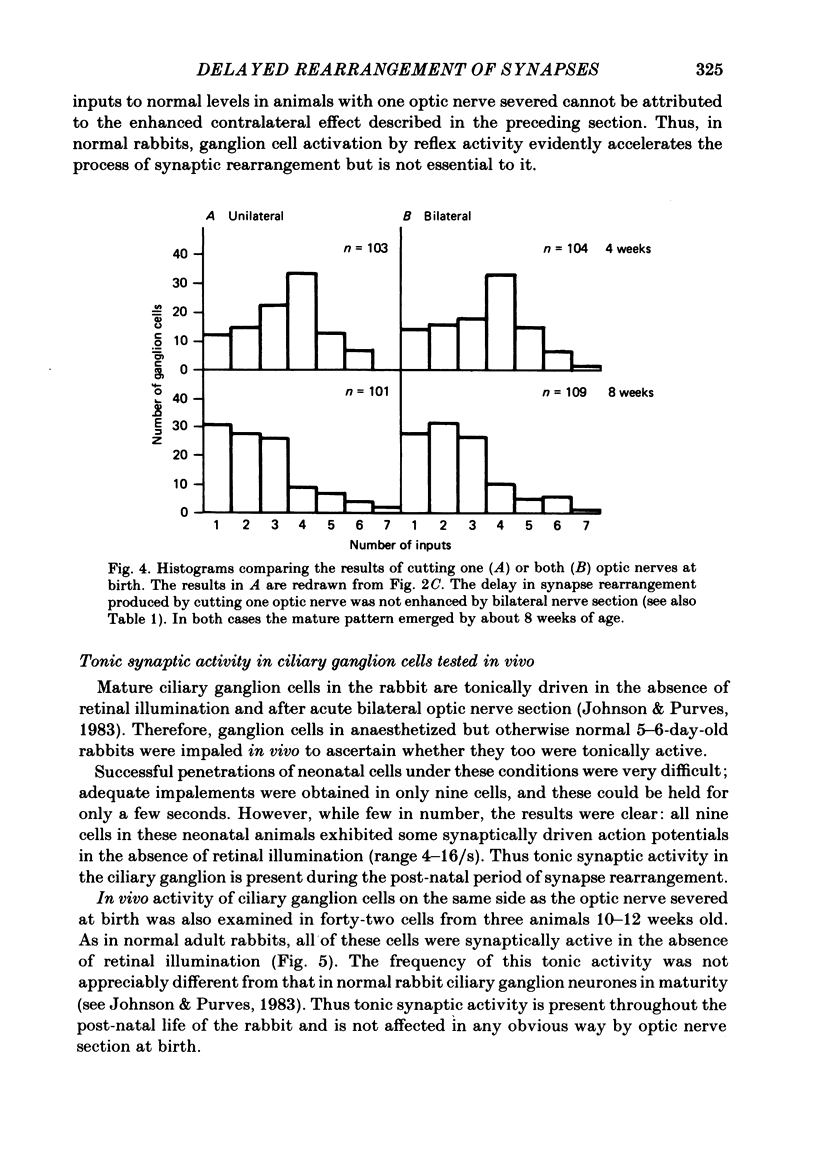
The number of preganglionic axons that innervate individual ciliary ganglion cells has been investigated after cutting one or both optic nerves in new-born rabbits. In agreement with previous work (Johnson & Purves, 1981), ciliary ganglion cells in normal rabbits lose, on average, about one-half of their inputs from preganglionic axons during the first month of post-natal life. Although severing one optic nerve at birth abolished the subsequent appearance of the direct pupillary light response on that side, ciliary ganglion cells of normal neonatal rabbits are driven synaptically in the absence of direct retinal illumination when tested in vivo. This tonic activity persisted for at least 3 months after interruption of the optic nerve at birth. Thus neonatal optic nerve section reduced, but did not eliminate, synaptic activation of ciliary ganglion cells. Optic nerve section at birth delayed the elimination of synaptic connexions in the ipsilateral but not the contralateral ciliary ganglion. Through the first 4 post-natal weeks ciliary ganglion cells on the operated side had, on average, more than the normal number of inputs from preganglionic axons. By 8 weeks of age, however, the normal number of connexions was established in spite of the unilateral visual deprivation. The elimination of connexions in ganglia on the unoperated side followed a normal time course. The effect of bilateral optic nerve section on synaptic rearrangement was the same as the ipsilateral effect after cutting only one optic nerve. It is concluded that the rate of synaptic rearrangement in this ganglion is slowed by a chronic reduction of synaptic activity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz W. J., Caldwell J. H., Ribchester R. R. The effects of partial denervation at birth on the development of muscle fibres and motor units in rat lumbrical muscle. J Physiol. 1980 Jun;303:265–279. doi: 10.1113/jphysiol.1980.sp013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Holland R. L., Hopkins W. G. Restoration of focal multiple innervation in rat muscles by transmission block during a critical stage of development. J Physiol. 1981 Sep;318:355–364. doi: 10.1113/jphysiol.1981.sp013869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crepel F., Mariani J., Delhaye-Bouchaud N. Evidence for a multiple innervation of Purkinje cells by climbing fibers in the immature rat cerebellum. J Neurobiol. 1976 Nov;7(6):567–578. doi: 10.1002/neu.480070609. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Binocular interaction in striate cortex of kittens reared with artificial squint. J Neurophysiol. 1965 Nov;28(6):1041–1059. doi: 10.1152/jn.1965.28.6.1041. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N., LeVay S. Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):377–409. doi: 10.1098/rstb.1977.0050. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Purves D. Apportionment of the terminals from single preganglionic axons to target neurones in the rabbit ciliary ganglion. J Physiol. 1983 May;338:259–275. doi: 10.1113/jphysiol.1983.sp014672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume R. I., Purves D. Geometry of neonatal neurones and the regulation of synapse elimination. Nature. 1981 Oct 8;293(5832):469–471. doi: 10.1038/293469a0. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Thompson W., Kuffler D. P. The formation and maintenance of synaptic connections as illustrated by studies of the neuromuscular junction. Prog Brain Res. 1978;48:3–19. doi: 10.1016/S0079-6123(08)61012-2. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Purves D. Post-natal reduction of neural unit size in the rabbit ciliary ganglion. J Physiol. 1981 Sep;318:143–159. doi: 10.1113/jphysiol.1981.sp013855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Purves D. Tonic and reflex synaptic activity recorded in ciliary ganglion cells of anaesthetized rabbits. J Physiol. 1983 Jun;339:599–613. doi: 10.1113/jphysiol.1983.sp014737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol. 1980 Apr;301:213–228. doi: 10.1113/jphysiol.1980.sp013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Redfern P. A. Neuromuscular transmission in new-born rats. J Physiol. 1970 Aug;209(3):701–709. doi: 10.1113/jphysiol.1970.sp009187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J., Stryker M. P. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol. 1978 Aug;281:267–283. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W., Kuffler D. P., Jansen J. K. The effect of prolonged, reversible block of nerve impulses on the elimination of polyneuronal innervation of new-born rat skeletal muscle fibers. Neuroscience. 1979;4(2):271–281. doi: 10.1016/0306-4522(79)90088-5. [DOI] [PubMed] [Google Scholar]


