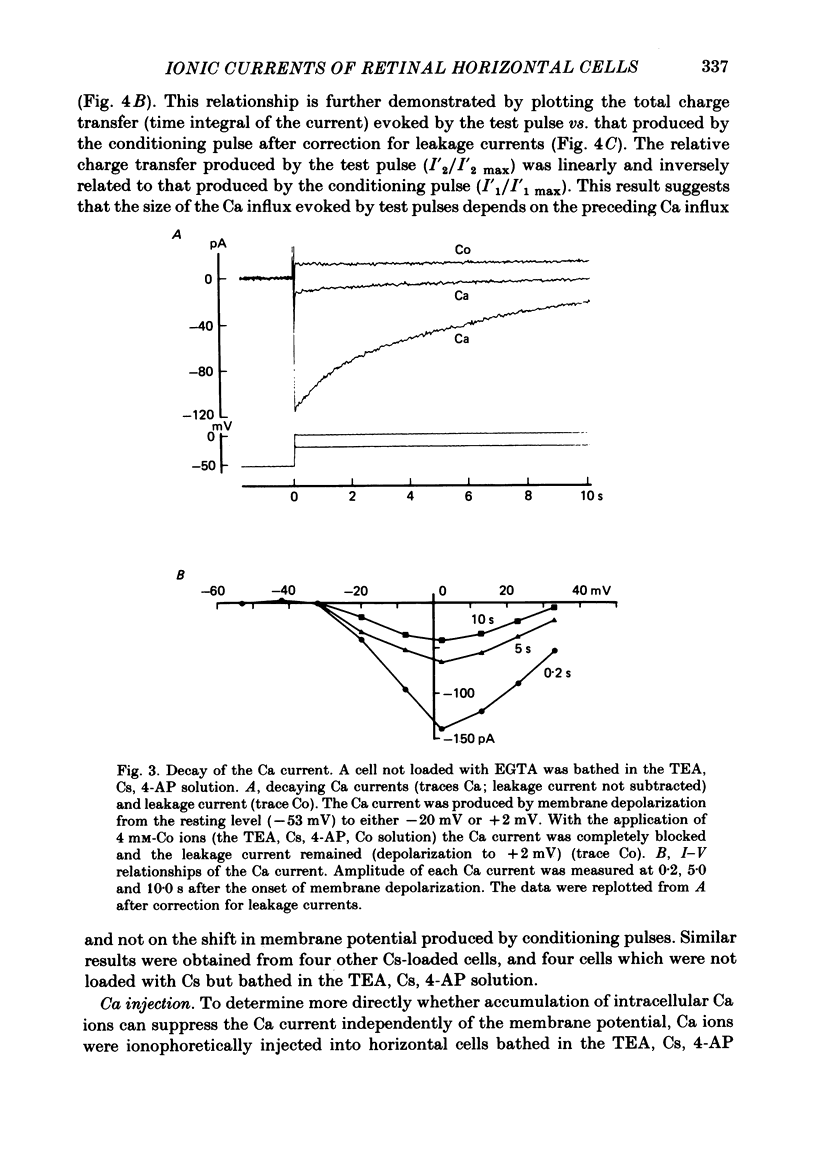
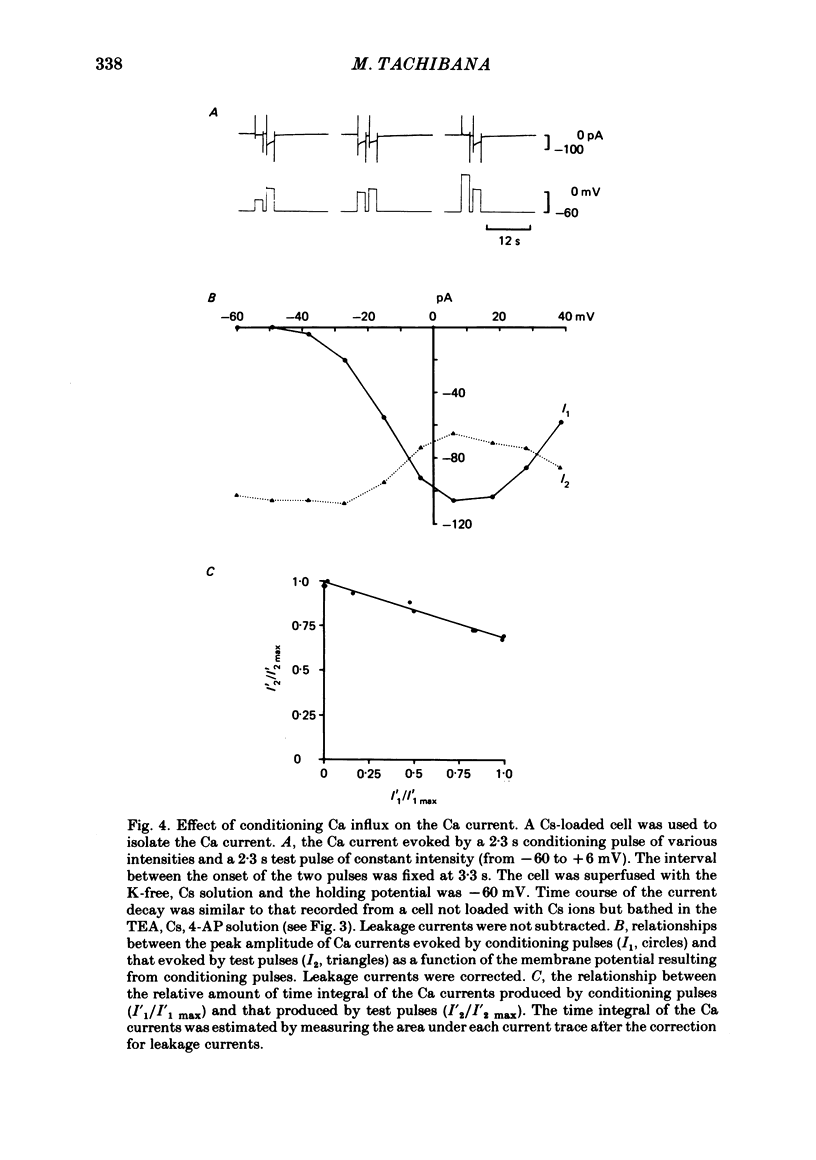
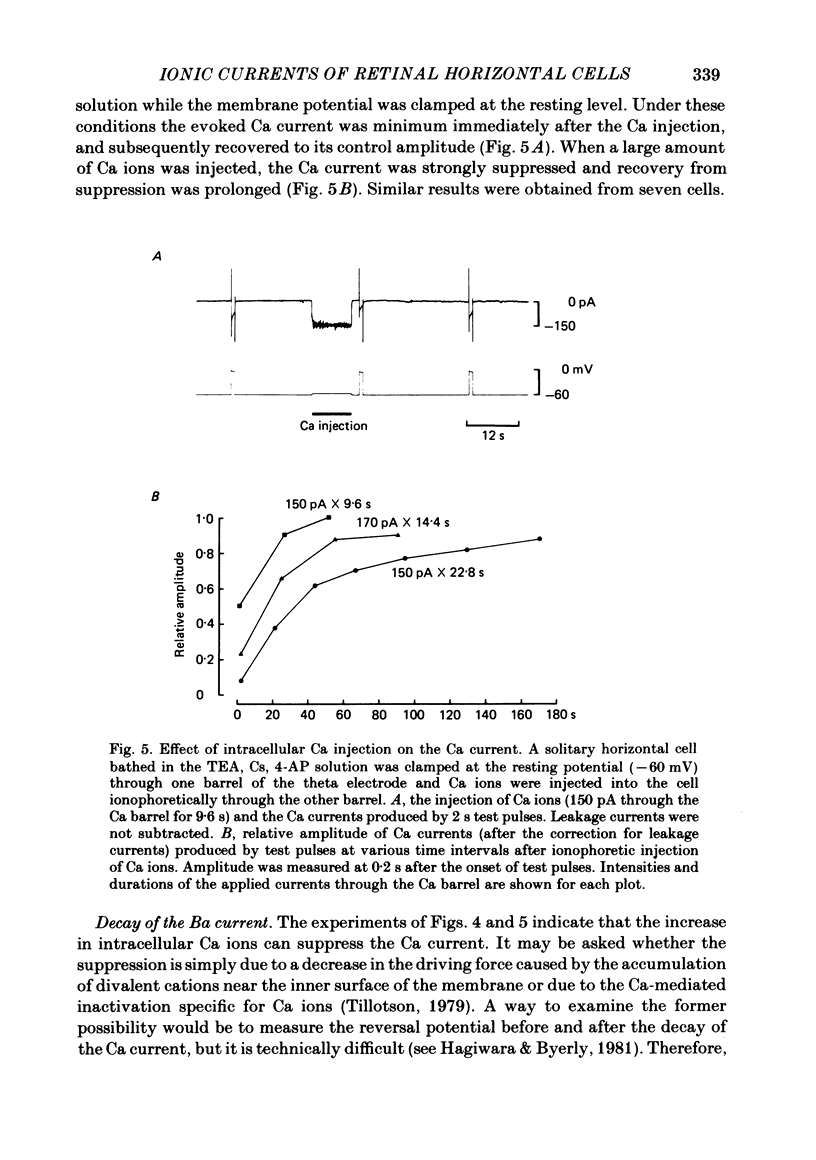
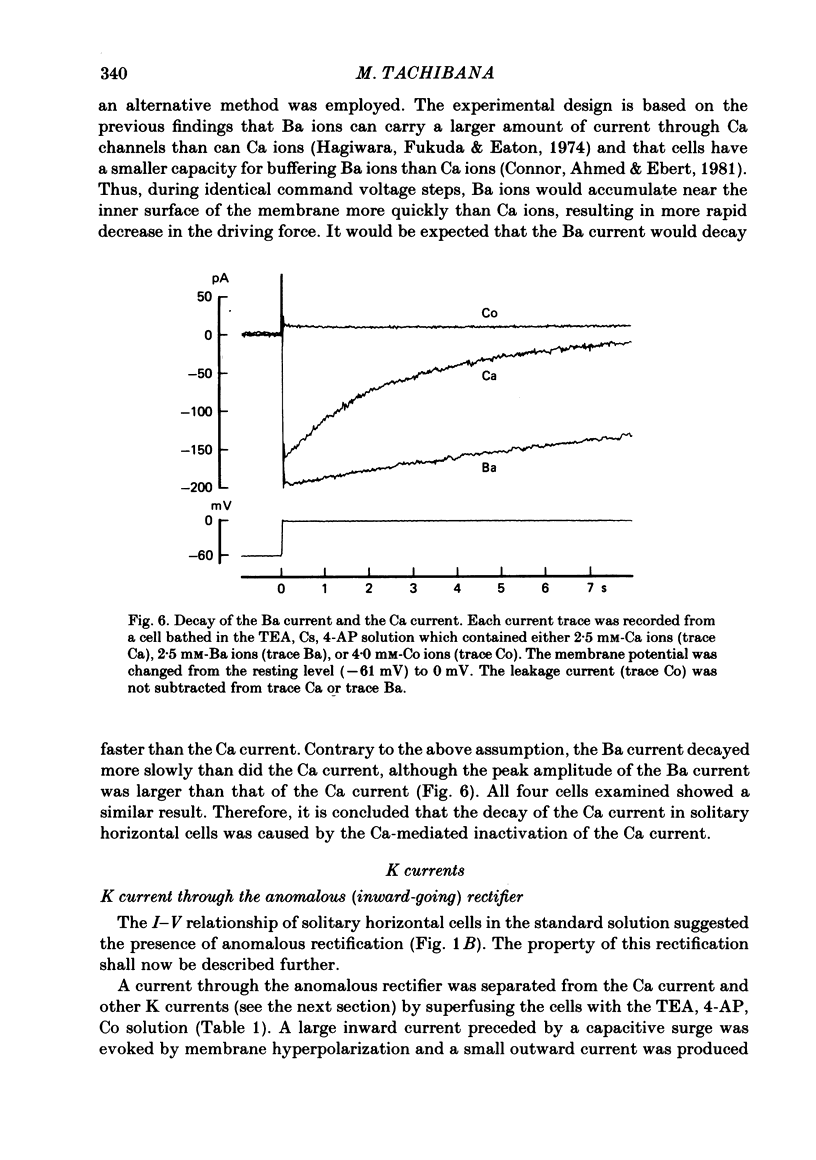
Abstract
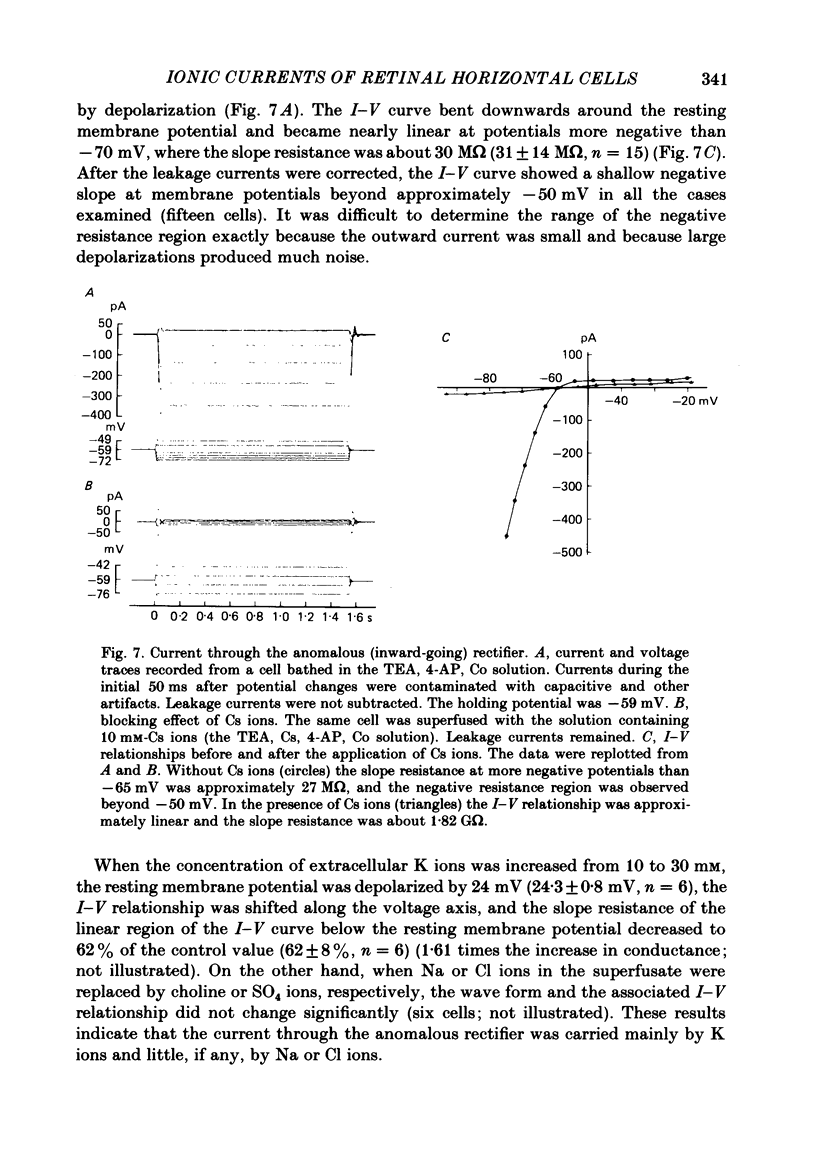
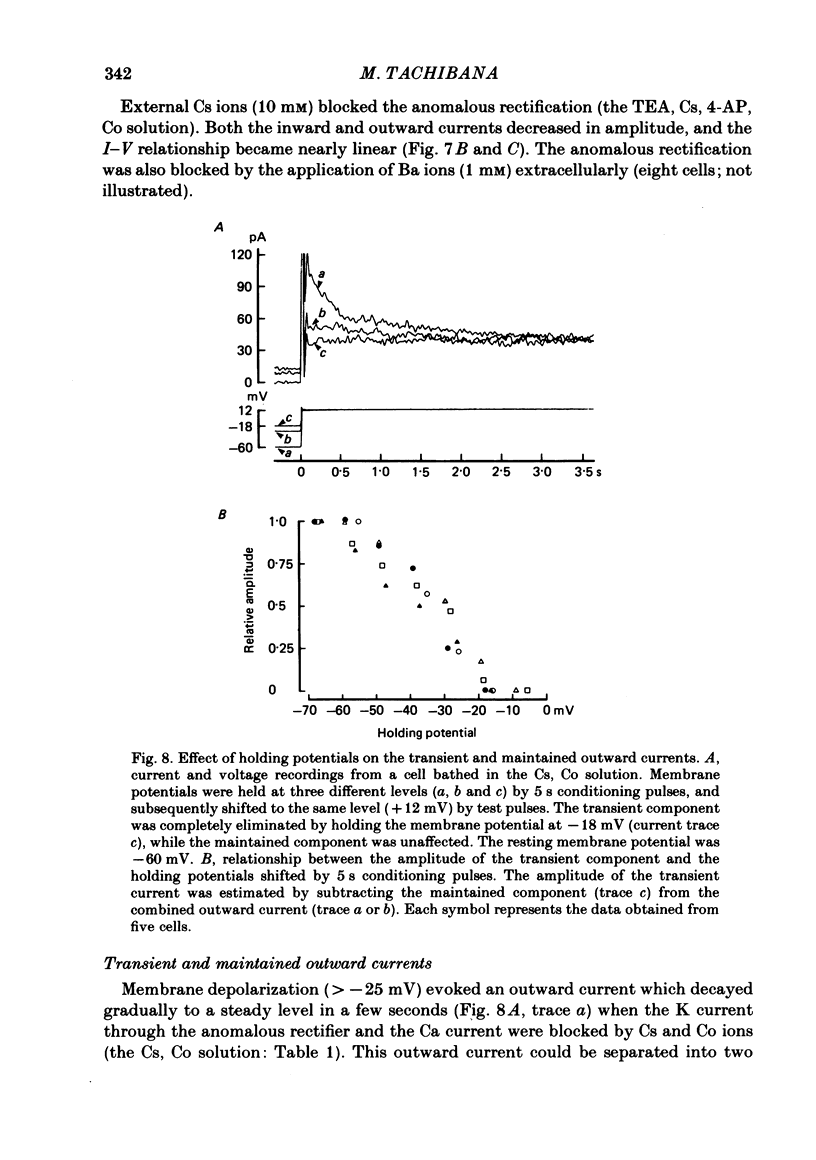
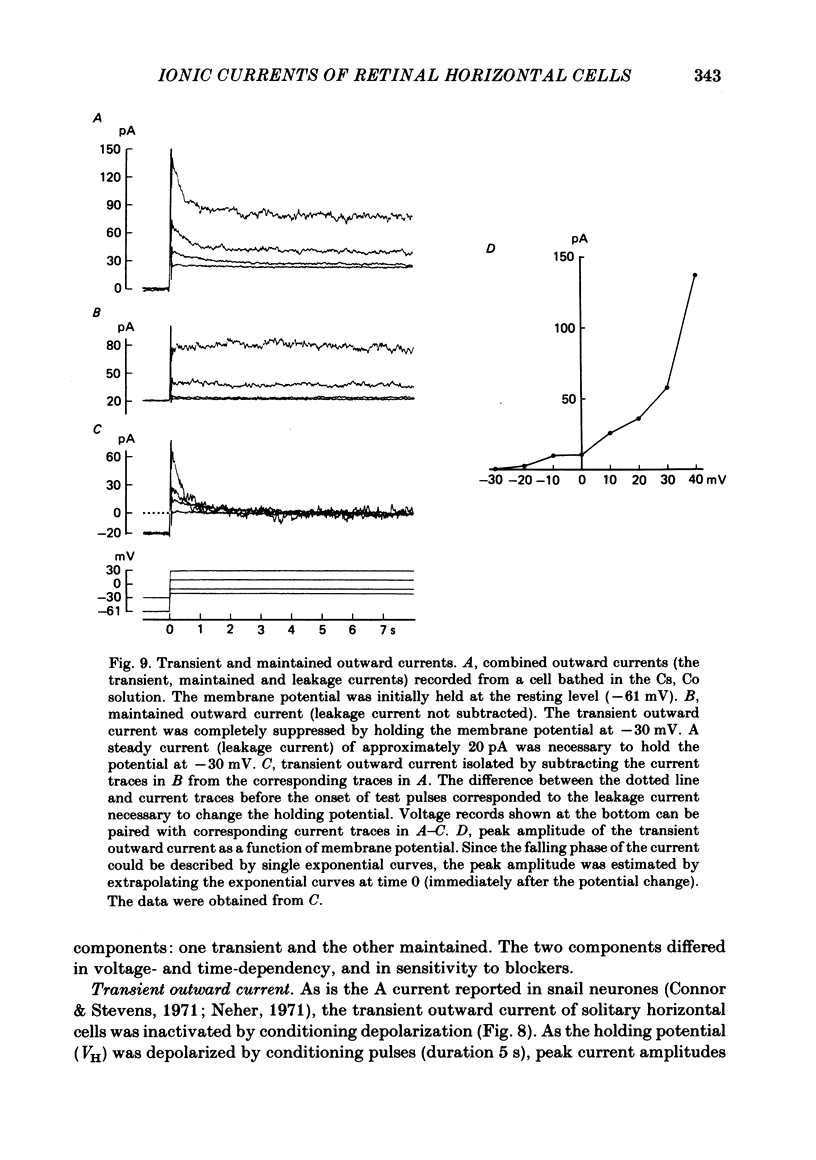
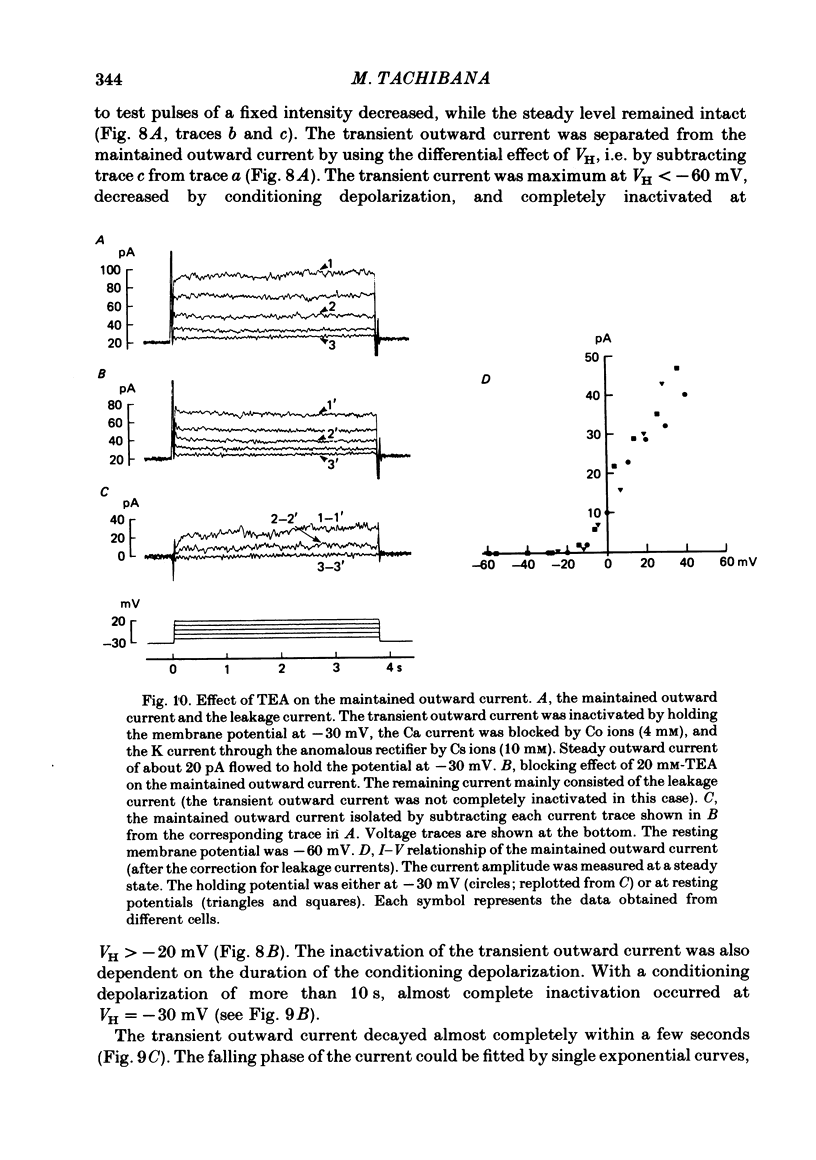
Solitary horizontal cells, dissociated from papain-treated goldfish retinas, produce action potentials and show a non-linear current-voltage relationship. Underlying ion-conductance mechanisms were analysed by a single-micro-electrode voltage-clamp technique. Pharmacological and ion-substitution experiments revealed that ionic currents could be separated into at least four voltage-dependent currents: a Ca current and three types of K currents. The Ca current was activated by membrane depolarization beyond -45 mV, reached a maximal value near 0 mV, and became smaller at more positive potentials. By extrapolation, the reversal potential was estimated to be approximately +50 mV. The Ca current was inactivated by accumulation of intracellular Ca ions but not by membrane depolarization. Co ions (4mM) blocked this current. The first type of K current showed anomalous (inward-going) rectification near the resting potential (congruent to -60 mV). Hyperpolarization from the resting level produced a large, almost steady inward current, while depolarization evoked only a small, steady outward current. The current-voltage relationship revealed a shallow negative resistance region at membrane potentials beyond -50 mV. The current was blocked by Cs (10 mM) or Ba (1 mM) ions. The second type of K current (the transient outward current) was activated by membrane depolarization beyond -25 mV. The peak amplitude increased almost exponentially as the membrane was depolarized. During steady depolarization this current decayed exponentially (time constant congruent to 500 ms at +20 mV). The current was inactivated by conditioning depolarization (greater than 10 s) beyond -30 mV and blocked by 4-aminopyridine (10 mM). The third type of K current was the maintained outward current which was activated by membrane depolarization beyond -20 mV, increased to a steady level in a few hundred milliseconds, and showed little inactivation. The amplitude increased as the membrane was depolarized. The current was blocked by tetraethylammonium ions (20 mM). A Ca-mediated K current was not detected. Action potentials and the non-linear current-voltage relationship of solitary horizontal cells can be explained qualitatively by the combination of the four ionic currents.
Full text
PDF




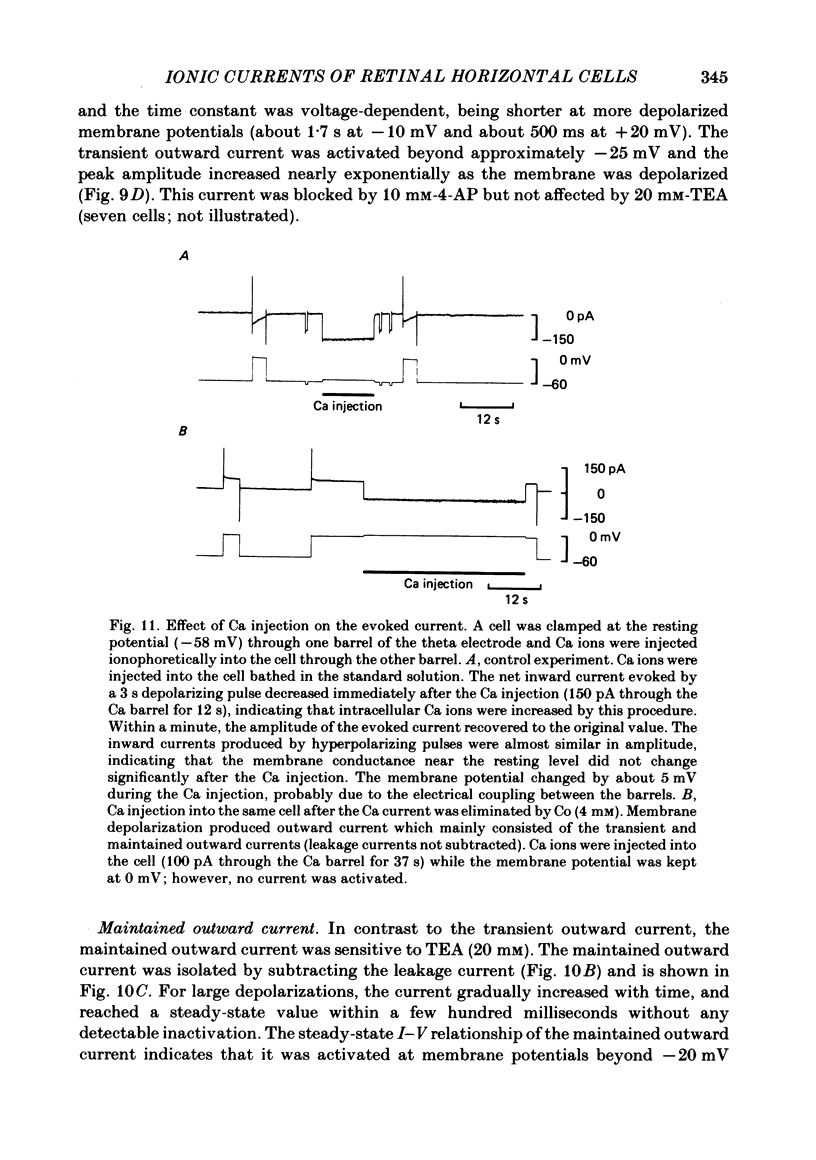






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anctil M., Ali M. A., Couillard P. Isolated retinal cells of some lower vertebrates. Rev Can Biol. 1973 Jun;32(2):107–119. [PubMed] [Google Scholar]
- Bader C. R., Bertrand D., Schwartz E. A. Voltage-activated and calcium-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol. 1982 Oct;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., MacLeish P. R., Schwartz E. A. Responses to light of solitary rod photoreceptors isolated from tiger salamander retina. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3507–3511. doi: 10.1073/pnas.75.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D. Surface movements during the growth of single explanted neurons. Proc Natl Acad Sci U S A. 1970 Apr;65(4):905–910. doi: 10.1073/pnas.65.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977 Jan;40(1):53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzov A. L., Trifonov J. A. The response to electric stimulation of horizontal cells in the carp retina. Vision Res. 1968 Jul;8(7):817–822. doi: 10.1016/0042-6989(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Byzov A. L., Trifonov Y. A., Chailahian L. M., Golubtzov K. W. Amplification of graded potentials in horizontal cells of the retina. Vision Res. 1977 Feb;17(2):265–273. doi: 10.1016/0042-6989(77)90090-6. [DOI] [PubMed] [Google Scholar]
- Colburn T. R., Schwartz E. A. Linear voltage control of current passed through a micropipette with variable resistance. Med Biol Eng. 1972 Jul;10(4):504–509. doi: 10.1007/BF02474198. [DOI] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doroshenko P. A., Tsyndrenko A. Ia. Deistvie vnutrikletochnogo kal'tsiia na kal'tsievyi vkhodiashchii tok. Neirofiziologiia. 1978;10(2):203–205. [PubMed] [Google Scholar]
- Dowling J. E., Brown J. E., Major D. Synapses of horizontal cells in rabbit and cat retinas. Science. 1966 Sep 30;153(3744):1639–1641. doi: 10.1126/science.153.3744.1639. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Werblin F. S. Organization of retina of the mudpuppy, Necturus maculosus. I. Synaptic structure. J Neurophysiol. 1969 May;32(3):315–338. doi: 10.1152/jn.1969.32.3.315. [DOI] [PubMed] [Google Scholar]
- Drujan B. D., Svaetichin G. Characterization of different classes of isolated retinal cells. Vision Res. 1972 Nov;12(11):1777–1784. doi: 10.1016/0042-6989(72)90068-5. [DOI] [PubMed] [Google Scholar]
- Ehinger B., Falck B., Laties A. M. Adrenergic neurons in teleost retina. Z Zellforsch Mikrosk Anat. 1969 May 23;97(2):285–297. doi: 10.1007/BF00344763. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Lisman J. E. Membrane conductances of photoreceptors. Prog Biophys Mol Biol. 1981;37(2):91–147. doi: 10.1016/0079-6107(82)90021-9. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18(1):61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Yoshida S., Yoshii M. Transient and delayed potassium currents in the egg cell membrane of the coelenterate, Renilla koellikeri. J Physiol. 1981 Sep;318:123–141. doi: 10.1113/jphysiol.1981.sp013854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Yoshii M. Effects of internal potassium and sodium on the anomalous rectification of the starfish egg as examined by internal perfusion. J Physiol. 1979 Jul;292:251–265. doi: 10.1113/jphysiol.1979.sp012849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity of Na and K channels of nerve membranes. Membranes. 1975;3:255–323. [PubMed] [Google Scholar]
- Johnston D., Lam D. M. Regenerative and passive membrane properties of isolated horizontal cells from a teleost retina. Nature. 1981 Jul 30;292(5822):451–454. doi: 10.1038/292451a0. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971 Feb;213(1):95–105. doi: 10.1113/jphysiol.1971.sp009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Shimazaki H. Effects of external ions on the synaptic transmission from photorecptors to horizontal cells in the carp retina. J Physiol. 1975 Nov;252(2):509–522. doi: 10.1113/jphysiol.1975.sp011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A. Effects of calcium and calcium-chelating agents on the inward and outward current in the membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):569–580. doi: 10.1113/jphysiol.1977.sp011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M. Biosynthesis of acetylcholine in turtle photoreceptors. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1987–1991. doi: 10.1073/pnas.69.7.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Steinman L. The uptake of ( - 3 H) aminobutyric acid in the goldfish retina. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2777–2781. doi: 10.1073/pnas.68.11.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam D. M., Su Y. Y., Swain L., Marc R. E., Brandon C., Wu J. Y. Immunocytochemical localisation of L-glutamic acid decarboxylase in the goldfish retina. Nature. 1979 Apr 5;278(5704):565–567. doi: 10.1038/278565a0. [DOI] [PubMed] [Google Scholar]
- MACNICHOL E. J., SVAETICHIN G. Electric responses from the isolated retinas of fishes. Am J Ophthalmol. 1958 Sep;46(3 Pt 2):26–46. doi: 10.1016/0002-9394(58)90053-9. [DOI] [PubMed] [Google Scholar]
- Marc R. E., Stell W. K., Bok D., Lam D. M. GABA-ergic pathways in the goldfish retina. J Comp Neurol. 1978 Nov 15;182(2):221–244. doi: 10.1002/cne.901820204. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W. The sensitivity of Helix aspersa neurones to injected calcium ions. J Physiol. 1974 Mar;237(2):259–277. doi: 10.1113/jphysiol.1974.sp010481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki S. I., Ohmori H., Sasaki S. Potassium rectifications of the starfish oocyte membrane and their changes during oocyte maturation. J Physiol. 1975 Mar;246(1):55–78. doi: 10.1113/jphysiol.1975.sp010880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. The generation and spread of S-potentials in fish (Cyprinidae). J Physiol. 1967 Sep;192(2):437–461. doi: 10.1113/jphysiol.1967.sp008308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka K. I. The horizontal cells. Vision Res. 1972 Apr;12(4):573–588. doi: 10.1016/0042-6989(72)90153-8. [DOI] [PubMed] [Google Scholar]
- Naka K. Functional organization of catfish retina. J Neurophysiol. 1977 Jan;40(1):26–43. doi: 10.1152/jn.1977.40.1.26. [DOI] [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton A. L., Spekreijse H., Wolbarsht M. L., Wagner H. G. Receptive field organization of the S-potential. Science. 1968 May 31;160(3831):1021–1022. doi: 10.1126/science.160.3831.1021. [DOI] [PubMed] [Google Scholar]
- O'Lague P. H., Potter D. D., Furshpan E. J. Studies on rat sympathetic neurons developing in cell culture. I. Growth characteristics and electrophysiological properties. Dev Biol. 1978 Dec;67(2):384–403. doi: 10.1016/0012-1606(78)90208-7. [DOI] [PubMed] [Google Scholar]
- Pinto L. H., Pak W. L. Light-induced changes in photoreceptor membrane resistance and potential in Gecko retinas. I. Preparations treated to reduce lateral interactions. J Gen Physiol. 1974 Jul;64(1):26–48. doi: 10.1085/jgp.64.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L. H., Pak W. L. Light-induced changes in photoreceptor membrane resistance and potential in Gecko retinas. II. Preparations with active lateral interactions. J Gen Physiol. 1974 Jul;64(1):49–69. doi: 10.1085/jgp.64.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B. Calcium current inactivation in identified neurones of Helix aspersa. J Physiol. 1981 Dec;321:273–285. doi: 10.1113/jphysiol.1981.sp013983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol. 1982 Feb;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMITA T. Electrical activity in the vertebrate retina. J Opt Soc Am. 1963 Jan;53:49–57. doi: 10.1364/josa.53.000049. [DOI] [PubMed] [Google Scholar]
- Tachibana M. Membrane properties of solitary horizontal cells isolated from goldfish retina. J Physiol. 1981 Dec;321:141–161. doi: 10.1113/jphysiol.1981.sp013976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yoshii M. Effects of internal free calcium upon the sodium and calcium channels in the tunicate egg analysed by the internal perfusion technique. J Physiol. 1978 Jun;279:519–549. doi: 10.1113/jphysiol.1978.sp012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda J., Kujiraoka T. Analyses of bipolar cell responses elicited by polarization of horizontal cells. J Gen Physiol. 1982 Jan;79(1):131–145. doi: 10.1085/jgp.79.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifonov I. U. Izuchenie sinapticheskoi peredachi mezhdu fotoretseptorom i gorizontal'noi kletkoi pri pomoshchi élektricheskikh razdrazhenii setchatki. Biofizika. 1968 Sep-Oct;13(5):809–817. [PubMed] [Google Scholar]
- Werblin F. S., Dowling J. E. Organization of the retina of the mudpuppy, Necturus maculosus. II. Intracellular recording. J Neurophysiol. 1969 May;32(3):339–355. doi: 10.1152/jn.1969.32.3.339. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Dowling J. E. Synaptic relationships in the plexiform layers of carp retina. Z Zellforsch Mikrosk Anat. 1969;100(1):60–82. doi: 10.1007/BF00343821. [DOI] [PubMed] [Google Scholar]
- Yamada E., Ishikawa T. The fine structure of the horizontal cells in some vertebrate retinae. Cold Spring Harb Symp Quant Biol. 1965;30:383–392. doi: 10.1101/sqb.1965.030.01.038. [DOI] [PubMed] [Google Scholar]