Abstract
Simultaneous recordings were made from fusimotor axons in the central ends of filaments of the masseter nerve, and from masseter and temporalis spindle afferents in the mesencephalic nucleus of the fifth cranial nerve in lightly anaesthetized cats. Fusimotor and alpha-motor units in the masseter nerve were differentiated on the basis of their response to passive ramp and hold stretches applied to the jaw. Spindle afferents were identified as primary or secondary according to their dynamic index after administration of suxamethonium. The activity of a given fusimotor unit during reflex movements of the jaw followed one of two distinct patterns: so-called 'tonic' units showed a general increase in activity during a movement, without detailed relation to lengthening or shortening, while 'modulated' units displayed a striking modulation of their activity with shortening, and were usually silent during subsequent lengthening. Comparison of the simultaneously recorded fusimotor and spindle afferent activity suggests that modulated units may be representative of a population of static fusimotor neurones, and tonic units of a population of dynamic fusimotor neurones. In these lightly anaesthetized animals, both primary and secondary spindle afferents showed increased firing during muscle shortening as well as during lengthening. This increase during shortening is not usually seen in conscious animals and reasons are given for the view that it is due to greater depression of alpha-motor activity than of static fusimotor activity during anaesthesia. The results are discussed in relation to the theories of 'alpha-gamma co-activation' and of 'servo-assistance'; and it is suggested that static fusimotor neurones provide a 'temporal template' of the intended movement, while dynamic fusimotor neurones set the required dynamic sensitivity to deviations from the intended movement pattern.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group III muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:275–292. doi: 10.1113/jphysiol.1983.sp014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenteng K., Morimoto T., Taylor A. Fusimotor activity in masseter nerve of the cat during reflex jaw movements. J Physiol. 1980 Aug;305:415–431. doi: 10.1113/jphysiol.1980.sp013373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appenteng K., Prochazka A., Proske U., Wand P. Effect of fusimotor stimulation on ia discharge during shortening of cat soleus muscle at different speeds. J Physiol. 1982 Aug;329:509–526. doi: 10.1113/jphysiol.1982.sp014316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessou P., Laporte Y., Pagès B. Frequencygrams of spindle primary endings elicited by stimulation of static and dynamic fusimotor fibres. J Physiol. 1968 May;196(1):47–63. doi: 10.1113/jphysiol.1968.sp008493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWE A., MATTHEWS P. B. THE EFFECTS OF STIMULATION OF STATIC AND DYNAMIC FUSIMOTOR FIBRES ON THE RESPONSE TO STRETCHING OF THE PRIMARY ENDINGS OF MUSCLE SPINDLES. J Physiol. 1964 Oct;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody F. W., Harrison L. M., Taylor A. Analysis of activity of muscle spindles of the jaw-closing muscles during normal movements in the cat. J Physiol. 1975 Dec;253(2):565–582. doi: 10.1113/jphysiol.1975.sp011207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cody F. W., Lee R. W., Taylor A. A functional analysis of the components of the mesencephalic nucleus of the fifth nerve in the cat. J Physiol. 1972 Oct;226(1):249–261. doi: 10.1113/jphysiol.1972.sp009983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin G. M., Luschei E. S. Discharge of spindle afferents from jaw-closing muscles during chewing in alert monkeys. J Neurophysiol. 1975 May;38(3):560–571. doi: 10.1152/jn.1975.38.3.560. [DOI] [PubMed] [Google Scholar]
- Hulliger M. The responses of primary spindle afferents to fusimotor stimulation at constant and abruptly changing rates. J Physiol. 1979 Sep;294:461–482. doi: 10.1113/jphysiol.1979.sp012941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Morimoto T., Kawamura Y. Response characteristics an classification of muscle spindles of the masseter muscle in the cat. Exp Neurol. 1981 Nov;74(2):548–560. doi: 10.1016/0014-4886(81)90190-4. [DOI] [PubMed] [Google Scholar]
- Kato T., Kawamura Y., Morimoto T. Branching of muscle spindle afferents of jaw closing muscles in the cat. J Physiol. 1982 Feb;323:483–495. doi: 10.1113/jphysiol.1982.sp014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C. R., Smith A., Luschei E. S. Discharge characteristics and stretch sensitivity of jaw muscle afferents in the monkey during controlled isometric bites. J Neurophysiol. 1981 Jul;46(1):130–142. doi: 10.1152/jn.1981.46.1.130. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Position and velocity sensitivity of muscle spindles in the cat. 3. Static fusimotor single-fibre activation of primary and secondary endings. Acta Physiol Scand. 1968 Sep-Oct;74(1):30–49. doi: 10.1111/j.1748-1716.1968.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Lennerstrand G., Thoden U. Position and velocity sensitivity of muscle spindles in the cat. II. Dynamic fusimotor single-fibre activation of primary endings. Acta Physiol Scand. 1968 Sep-Oct;74(1):16–29. doi: 10.1111/j.1748-1716.1968.tb04211.x. [DOI] [PubMed] [Google Scholar]
- Loeb G. E., Duysens J. Activity patterns in individual hindlimb primary and secondary muscle spindle afferents during normal movements in unrestrained cats. J Neurophysiol. 1979 Mar;42(2):420–440. doi: 10.1152/jn.1979.42.2.420. [DOI] [PubMed] [Google Scholar]
- Lund J. P., Smith A. M., Sessle B. J., Murakami T. Activity of trigeminal alpha- and gamma-motoneurons and muscle afferents during performance of a biting task. J Neurophysiol. 1979 May;42(3):710–725. doi: 10.1152/jn.1979.42.3.710. [DOI] [PubMed] [Google Scholar]
- Matsunami K., Kubota K. Muscle afferents of trigeminal mesencephalic tract nucleus and mastication in chronic monkeys. Jpn J Physiol. 1972 Oct;22(5):545–555. doi: 10.2170/jjphysiol.22.545. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Evolving views on the internal operation and functional role of the muscle spindle. J Physiol. 1981 Nov;320:1–30. doi: 10.1113/jphysiol.1981.sp013931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill E. G., Ainsworth A. Glass-coated platinum-plated tungsten microelectrodes. Med Biol Eng. 1972 Sep;10(5):662–672. doi: 10.1007/BF02476084. [DOI] [PubMed] [Google Scholar]
- Morimoto T., Inoue H., Kawamura Y. Diameter spectra of sensory and motor fibers in nerves to jaw-closing and jaw-opening muscles in the cat. Jpn J Physiol. 1982;32(2):171–182. doi: 10.2170/jjphysiol.32.171. [DOI] [PubMed] [Google Scholar]
- Phillips C. G. The Ferrier lecture, 1968. Motor apparatus of the baboon's hand. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):141–174. doi: 10.1098/rspb.1969.0044. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Stephens J. A., Wand P. Muscle spindle discharge in normal and obstructed movements. J Physiol. 1979 Feb;287:57–66. doi: 10.1113/jphysiol.1979.sp012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Discharges of single hindlimb afferents in the freely moving cat. J Neurophysiol. 1976 Sep;39(5):1090–1104. doi: 10.1152/jn.1976.39.5.1090. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Westerman R. A., Ziccone S. P. Ia afferent activity during a variety of voluntary movements in the cat. J Physiol. 1977 Jun;268(2):423–448. doi: 10.1113/jphysiol.1977.sp011864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessle B. J. Identification of alpha and gamma trigeminal motoneurons and effects of stimulation of amygdala, cerebellum, and cerebral cortex. Exp Neurol. 1977 Feb;54(2):303–322. doi: 10.1016/0014-4886(77)90272-2. [DOI] [PubMed] [Google Scholar]
- Taylor A. A technique for recording normal jaw movements in conscious cats. Med Biol Eng. 1969 Jan;7(1):89–90. doi: 10.1007/BF02474674. [DOI] [PubMed] [Google Scholar]
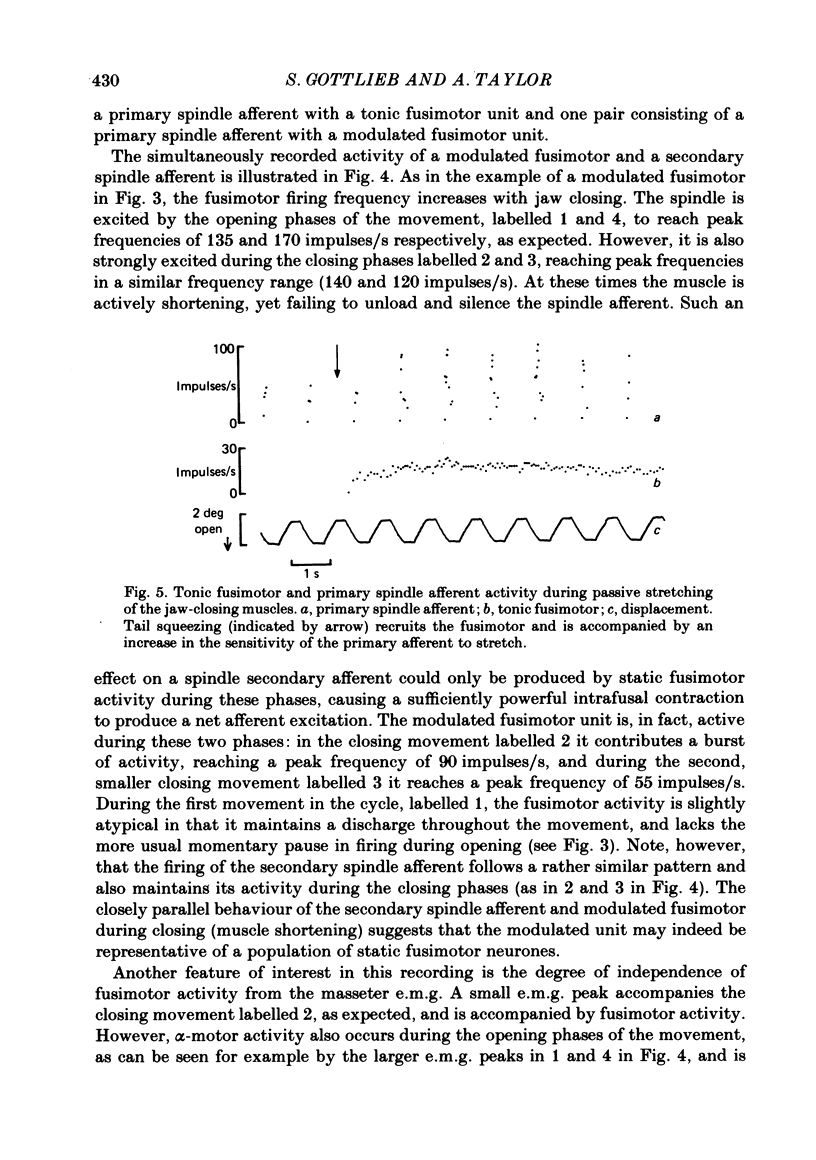
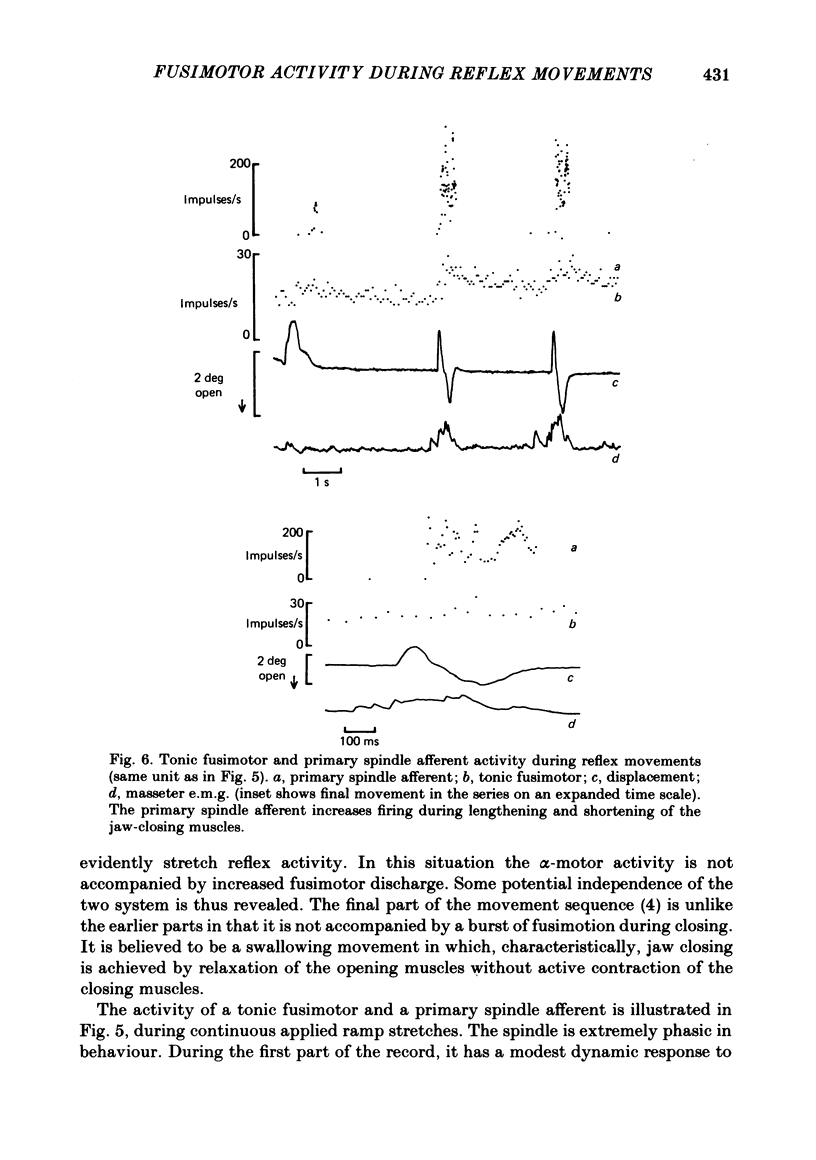
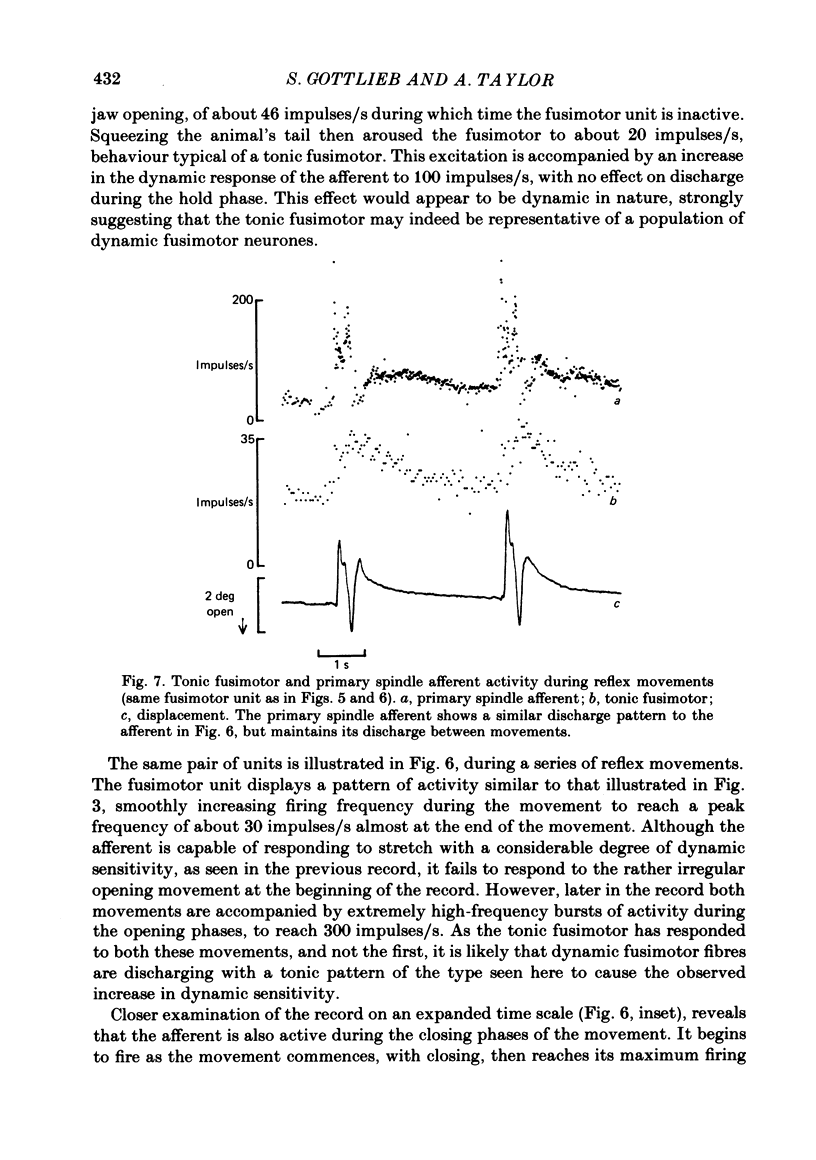
- Taylor A., Appenteng K., Morimoto T. Proprioceptive input from the jaw muscles and its influence on lapping, chewing, and posture. Can J Physiol Pharmacol. 1981 Jul;59(7):636–644. doi: 10.1139/y81-098. [DOI] [PubMed] [Google Scholar]
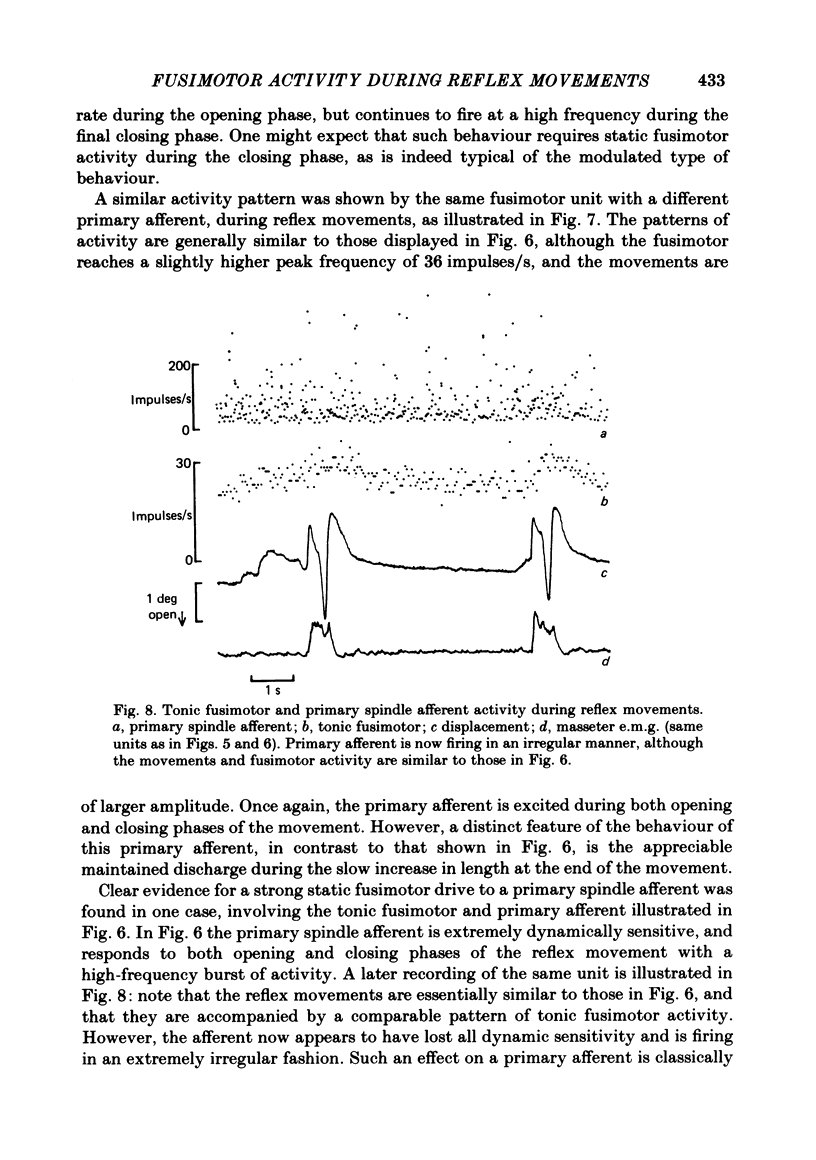
- Taylor A., Cody F. W. Jaw muscle spindle activity in the cat during normal movements of eating and drinking. Brain Res. 1974 May 17;71(2-3):523–530. doi: 10.1016/0006-8993(74)90996-2. [DOI] [PubMed] [Google Scholar]
- Taylor A., Davey M. R. Behaviour of jaw muscle stretch receptors during active and passive movements in the cat. Nature. 1968 Oct 19;220(5164):301–302. doi: 10.1038/220301a0. [DOI] [PubMed] [Google Scholar]


