Abstract
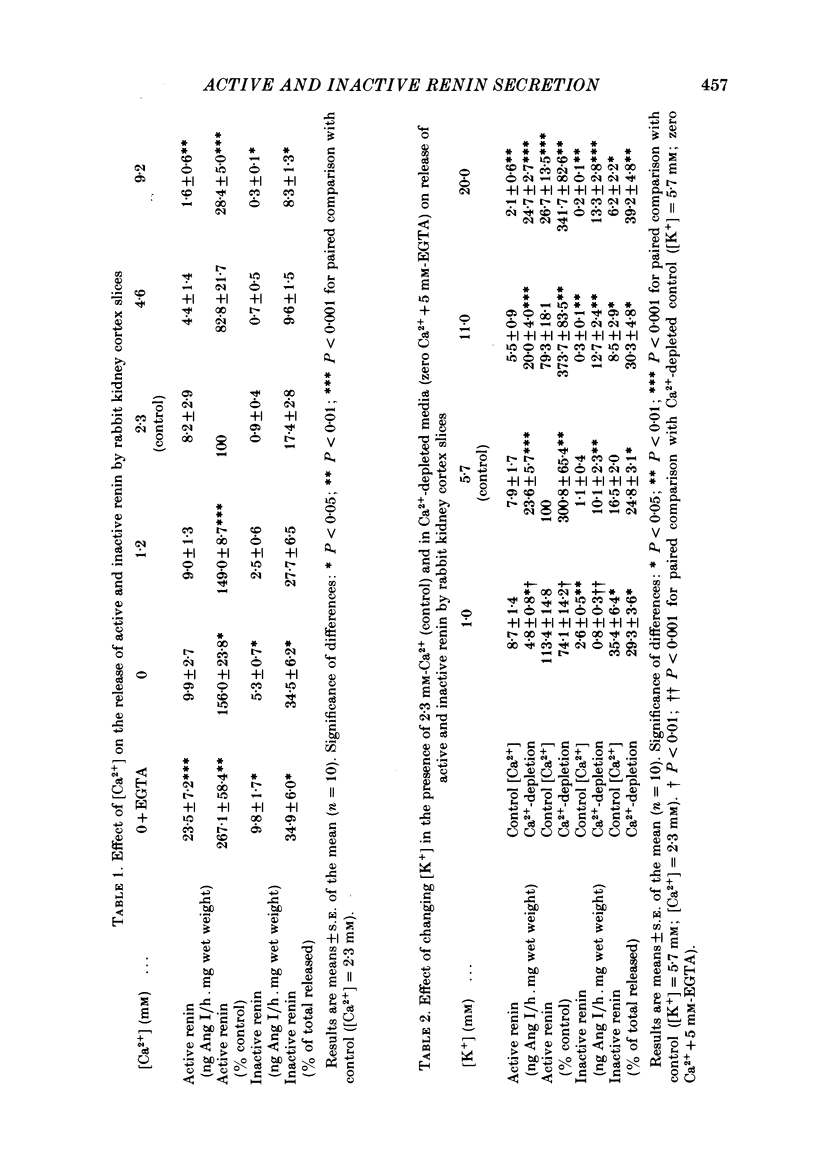
Release of active and inactive renin by rabbit kidney cortex slices was investigated. Inactive renin was estimated as the increase in renin activity after acidification (pH 2.8) of slice supernatant solutions. In Ca2+-free media, release of both active and inactive renin was increased but the changes in inactive renin were more marked. The percentage of total renin released which was inactive ranged from 8.3% ([Ca2+] = 9.2 mM) to 34.5% (zero [Ca2+]) with a linear relationship (r = -0.96) over the range of [Ca2+] studied. Depolarizing media ([K+] = 20 mM) suppressed release of inactive renin more than release of the active form. This effect, for both forms of renin, was lost when Ca2+ ions were omitted from the incubation media. This suggests that influx of Ca2+ ions was responsible for the reduced renin secretion following depolarization of the juxtaglomerular cells. Reducing the [K+] of the incubation buffer from 5.7 mM (control) to 1.0 mM did not alter active renin but increased release of inactive renin. Low [K+] media abolished the stimulatory effect of low [Ca2+] on release of both forms of renin. In incubation media with low [Ca2+] or with low [K+] the mixture of renins released by the kidney cortex slices correlated with that found in extracts of non-incubated kidney: that is, about 35% of the total renin was in the inactive form. Mechanisms controlling the secretion of active and inactive renins by the kidney are at least partially independent. Differential secretion of the two forms, perhaps linked to regulation of the activation of inactive renin before release, appears to be the basis of a control step in the over-all expression of the renin-angiotensin system. This may be coupled in some way to juxtaglomerular cell Na+ ion flux.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett J. D., Eggena P., Sambhi M. P. Activation of rat plasma renin. Endocrinology. 1981 Mar;108(3):778–785. doi: 10.1210/endo-108-3-778. [DOI] [PubMed] [Google Scholar]
- Baumbach L., Leyssac P. P. Studies on the mechanism of renin release from isolated superfused rat glomeruli: effects of calcium, calcium ionophore and lanthanum. J Physiol. 1977 Dec;273(3):745–764. doi: 10.1113/jphysiol.1977.sp012121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach L., Skøtt O. Renin release from isolated rat glomeruli: seasonal variations and effects of D600 on the response to calcium deprivation. J Physiol. 1981 Jan;310:285–292. doi: 10.1113/jphysiol.1981.sp013549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill M. C., Churchill P. C. Separate and combined effects of ouabain and extracellular potassium on renin secretion from rat renal cortical slices. J Physiol. 1980 Mar;300:105–114. doi: 10.1113/jphysiol.1980.sp013154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C. Calcium dependency of the inhibitory effect of antidiuretic hormone on in vitro renin secretion in rats. J Physiol. 1981 Jun;315:21–30. doi: 10.1113/jphysiol.1981.sp013729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C., Churchill M. C. Isoproterenol-stimulated renin secretion in the rat: second messenger roles of Ca and cyclic AMP. Life Sci. 1982 Apr 12;30(15):1313–1319. doi: 10.1016/0024-3205(82)90694-4. [DOI] [PubMed] [Google Scholar]
- Churchill P. C. Effect of D-600 on inhibition of in vitro renin release in the rat by high extracellular potassium and angiotensin II. J Physiol. 1980 Jul;304:449–458. doi: 10.1113/jphysiol.1980.sp013335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill P. C., McDonald F. D., Churchill M. C. Effect of diltiazem, a calcium antagonist, on renin secretion from rat kidney slices. Life Sci. 1981 Jul 27;29(4):383–389. doi: 10.1016/0024-3205(81)90331-3. [DOI] [PubMed] [Google Scholar]
- Churchill P. C. Possible mechanism of the inhibitory effect of ouabain on renin secretion from rat renal cortical slices. J Physiol. 1979 Sep;294:123–134. doi: 10.1113/jphysiol.1979.sp012919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettienne E. M., Fray J. C. Influence of potassium, sodium, calcium, perfusion pressure, and isoprenaline on renin release induced by high concentrations of magnesium. J Physiol. 1979 Jul;292:373–380. doi: 10.1113/jphysiol.1979.sp012857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. C. Membrane potential of juxtaglomerular cells. Nature. 1976 Apr 8;260(5551):542–544. doi: 10.1038/260542a0. [DOI] [PubMed] [Google Scholar]
- Fray J. C., Park C. S. Influence of potassium, sodium, perfusion pressure, and isoprenaline on renin release induced by acute calcium deprivation. J Physiol. 1979 Jul;292:363–372. doi: 10.1113/jphysiol.1979.sp012856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray J. C. Stimulus-secretion coupling of renin. Role of hemodynamic and other factors. Circ Res. 1980 Oct;47(4):485–492. doi: 10.1161/01.res.47.4.485. [DOI] [PubMed] [Google Scholar]
- Fray J. C. Stretch receptor control of renin release in perfused rat kidney: effect of high perfusate potassium. J Physiol. 1978 Sep;282:207–217. doi: 10.1113/jphysiol.1978.sp012458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fray J. S. Stimulation of renin release in perfused kidney by low calcium and high magnesium. Am J Physiol. 1977 Apr;232(4):F377–F382. doi: 10.1152/ajprenal.1977.232.4.F377. [DOI] [PubMed] [Google Scholar]
- Fynn M., Onomakpome N., Peart W. S. The effects of ionophores (A23187 and RO2-2985) on renin secretion and renal vasoconstriction. Proc R Soc Lond B Biol Sci. 1977 Nov 14;199(1135):199–212. doi: 10.1098/rspb.1977.0135. [DOI] [PubMed] [Google Scholar]
- Gallagher J. F., Laragh J. H., Atlas S. A., Sealey J. E. Effect of trypsin or acid treatment on dog plasma renin activity measurements. Endocrinology. 1980 Jul;107(1):147–154. doi: 10.1210/endo-107-1-147. [DOI] [PubMed] [Google Scholar]
- Gillies A., Morgan T., Fitzgibbon W. Changes in "active" and "inactive" renin in the juxtaglomerular apparatuses of rat nephrons and plasma induced by different salt intake. Pflugers Arch. 1982 Jun;393(4):308–312. doi: 10.1007/BF00581415. [DOI] [PubMed] [Google Scholar]
- Grace S. A., Munday K. A., Noble A. R., Richards H. K. Plasma active and inactive renin in the rabbit: effect of dietary sodium depletion and repletion. J Physiol. 1979 Jul;292:421–428. doi: 10.1113/jphysiol.1979.sp012861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckie B. J. Inactive renin: an attempt at a perspective. Clin Sci (Lond) 1981 Feb;60(2):119–130. doi: 10.1042/cs0600119. [DOI] [PubMed] [Google Scholar]
- Munday K. A., Noble A. R., Richards H. K. Active and inactive renin release from rabbit kidney cortex slices: effect of sodium concentration and of furosemide. J Physiol. 1982 Jul;328:421–430. doi: 10.1113/jphysiol.1982.sp014274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane H., Nakane Y., Corvol P., Menard J. Sodium balance and renin regulation in rats: role of intrinsic renal mechanisms. Kidney Int. 1980 May;17(5):607–614. doi: 10.1038/ki.1980.71. [DOI] [PubMed] [Google Scholar]
- Park C. S., Malvin R. L. Calcium in the control of renin release. Am J Physiol. 1978 Jul;235(1):F22–F25. doi: 10.1152/ajprenal.1978.235.1.F22. [DOI] [PubMed] [Google Scholar]
- Richards H. K., Grace S. A., Noble A. R., Munday K. A. Inactive renin in rabbit plasma: effect of haemorrhage. Clin Sci (Lond) 1979 Feb;56(2):105–108. doi: 10.1042/cs0560105. [DOI] [PubMed] [Google Scholar]
- Richards H. K., Lush D. J., Noble A. R., Munday K. A. Inactive renin in rabbit plasma: effect of frusemide. Clin Sci (Lond) 1981 Apr;60(4):393–398. doi: 10.1042/cs0600393. [DOI] [PubMed] [Google Scholar]
- Richards H. K., Noble A. R., Munday K. A. Isoprenaline-induced secretion of active and inactive renin in anaesthetized rabbits and by kidney cortex slices. Clin Sci (Lond) 1981 Dec;61(6):679–684. doi: 10.1042/cs0610679. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H. Prorenin and other large molecular weight forms of renin. Endocr Rev. 1980 Fall;1(4):365–391. doi: 10.1210/edrv-1-4-365. [DOI] [PubMed] [Google Scholar]
- Skinner S. L. Improved assay methods for renin "concentration" and "activity" in human plasma. Methods using selective denaturation of renin substrate. Circ Res. 1967 Apr;20(4):391–402. doi: 10.1161/01.res.20.4.391. [DOI] [PubMed] [Google Scholar]
- Van Dongen R., Peart W. S. Calcium dependence of the inhibitory effect of angiotensin on renin secretion in the isolated perfused kidney of the rat. Br J Pharmacol. 1974 Jan;50(1):125–129. doi: 10.1111/j.1476-5381.1974.tb09599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Keijzer M. H., Provoost A. P., Derkx F. H. Absence of activation in vitro of renin in rat plasma. Clin Sci (Lond) 1982 Apr;62(4):435–437. [PubMed] [Google Scholar]


