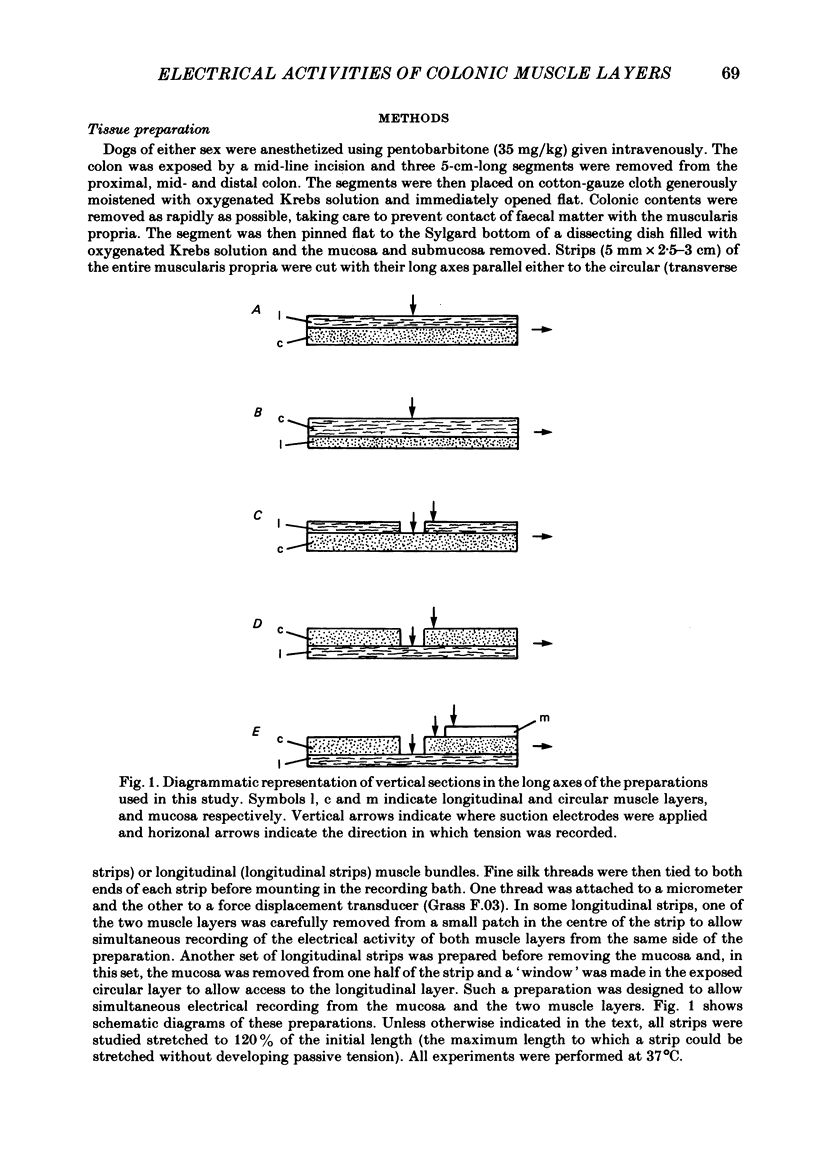
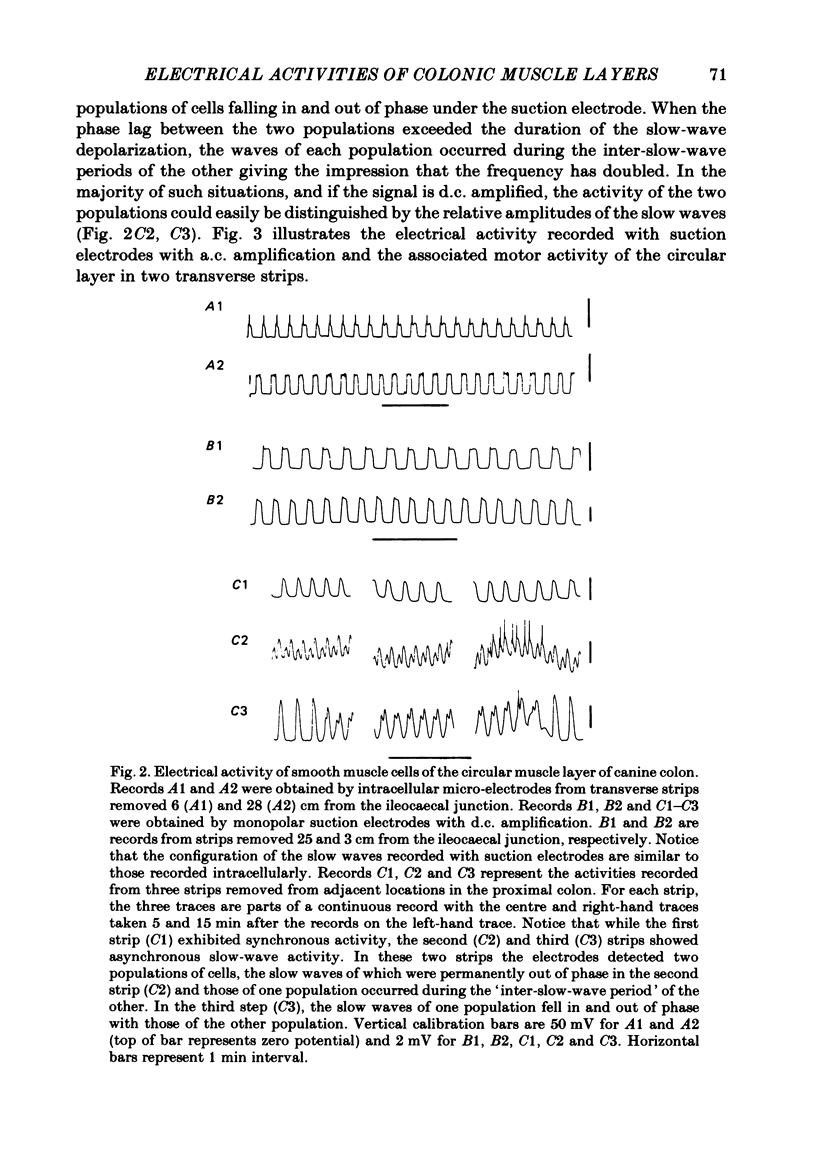
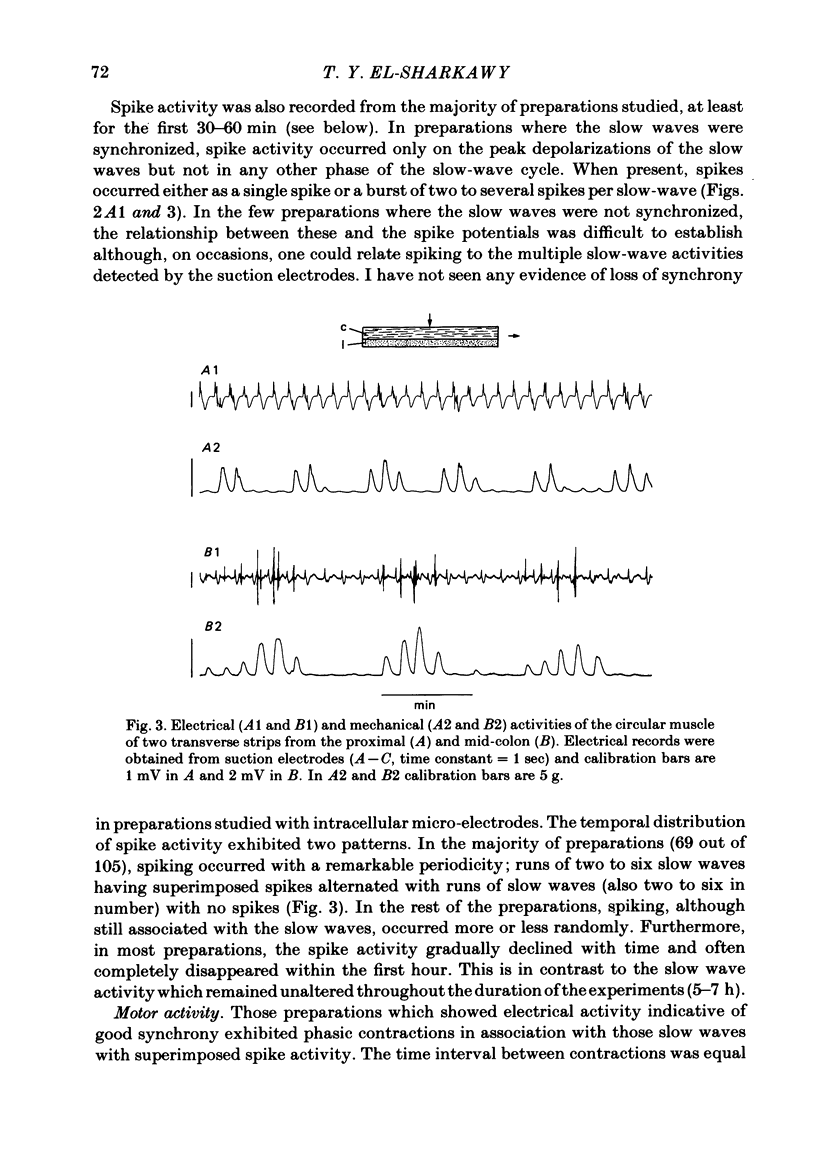
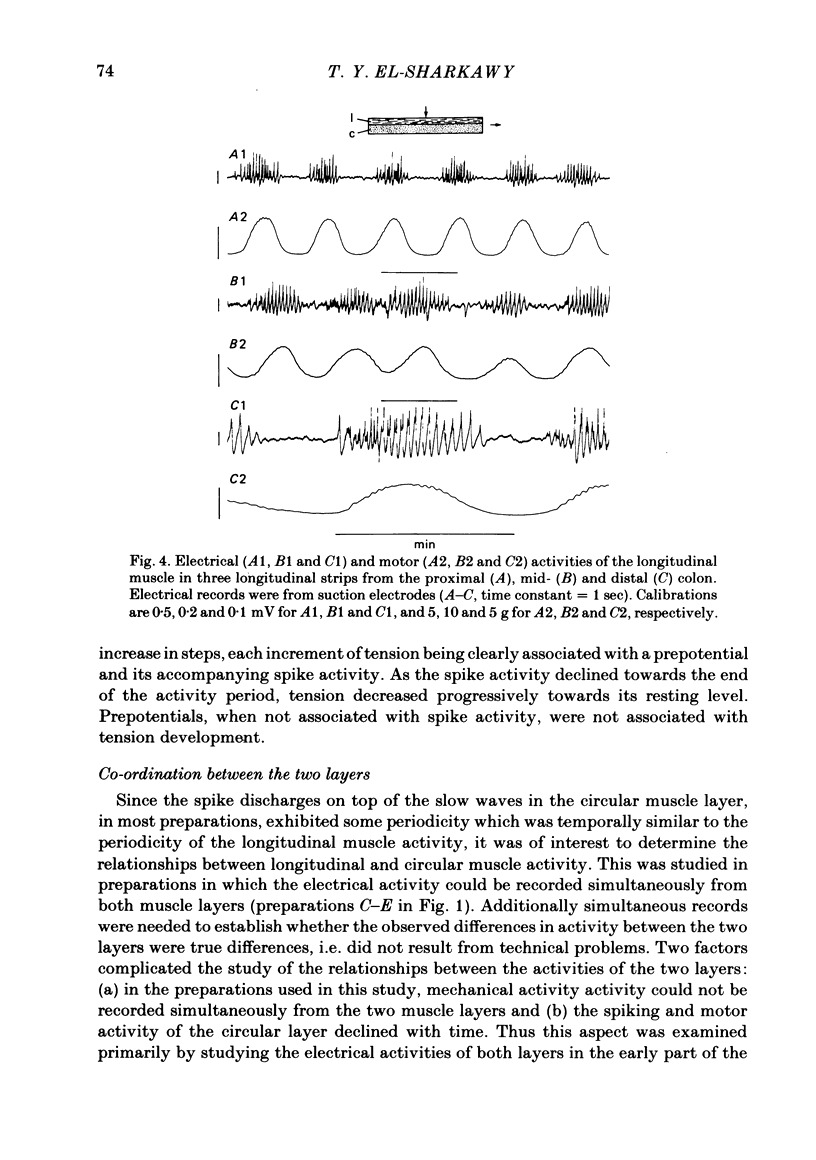
Abstract
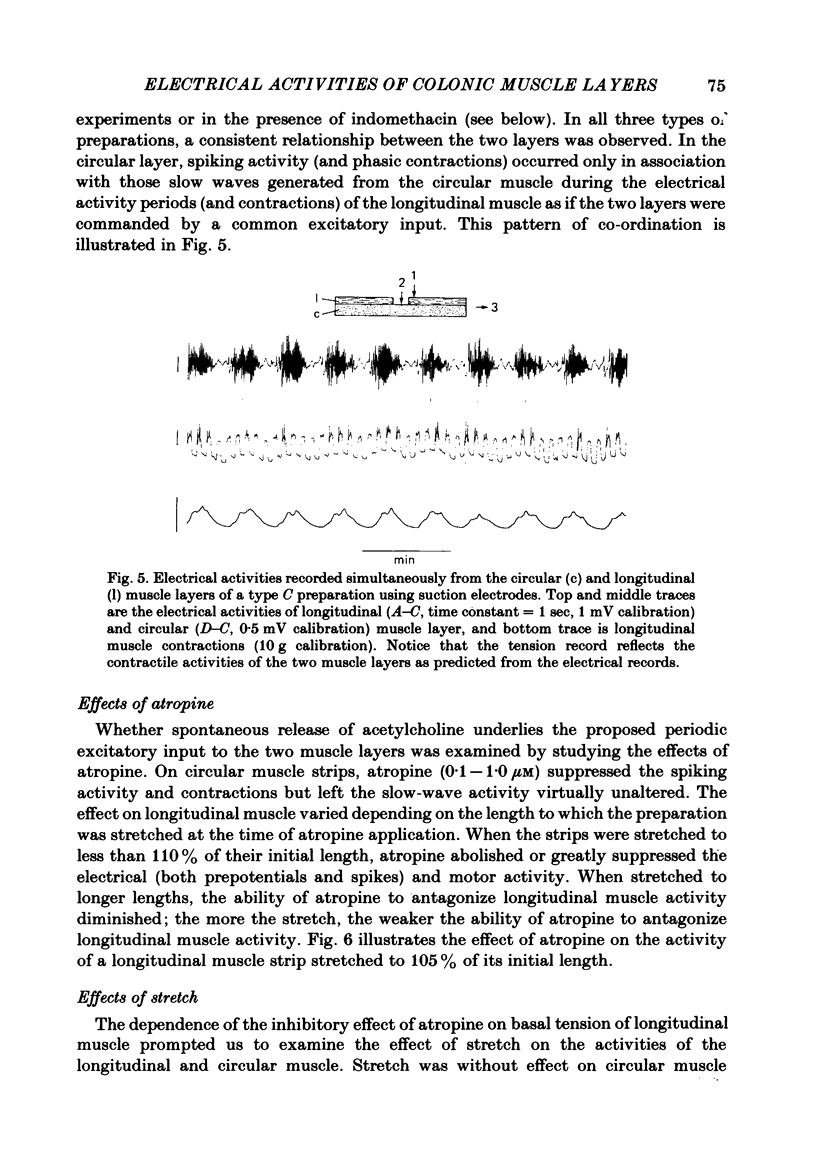
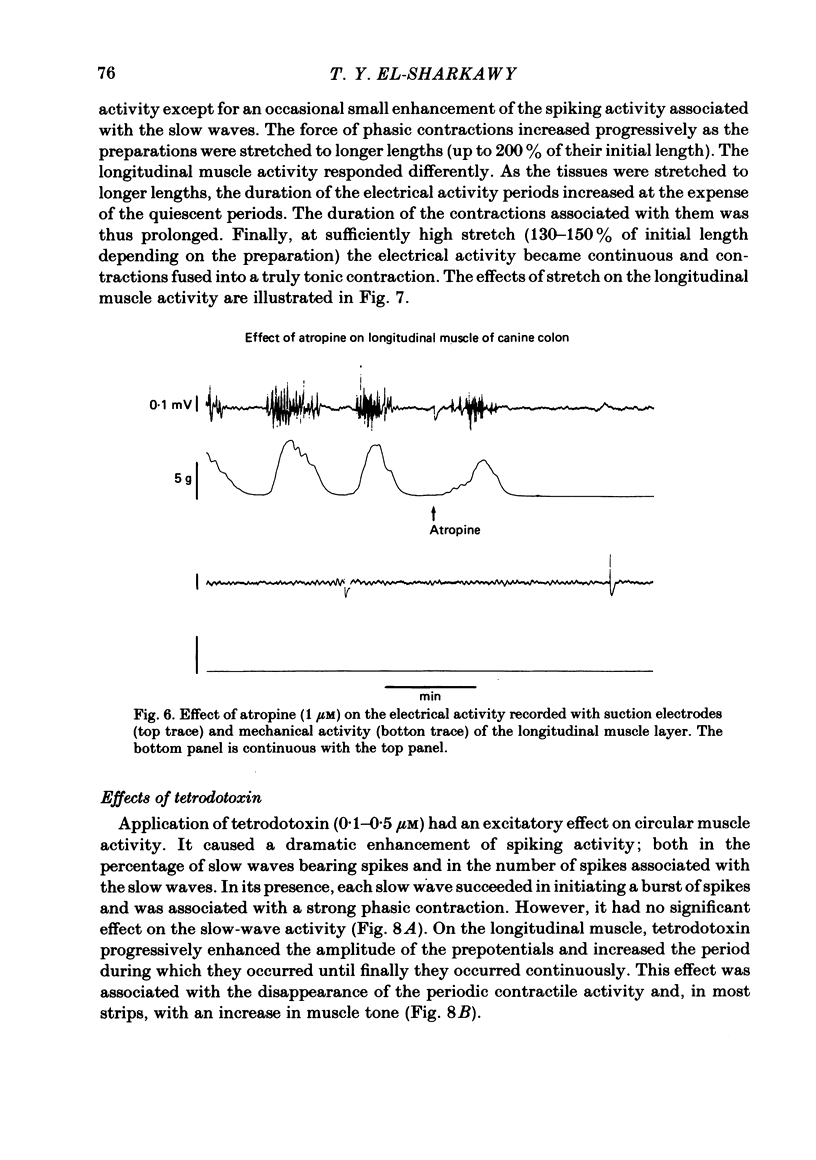
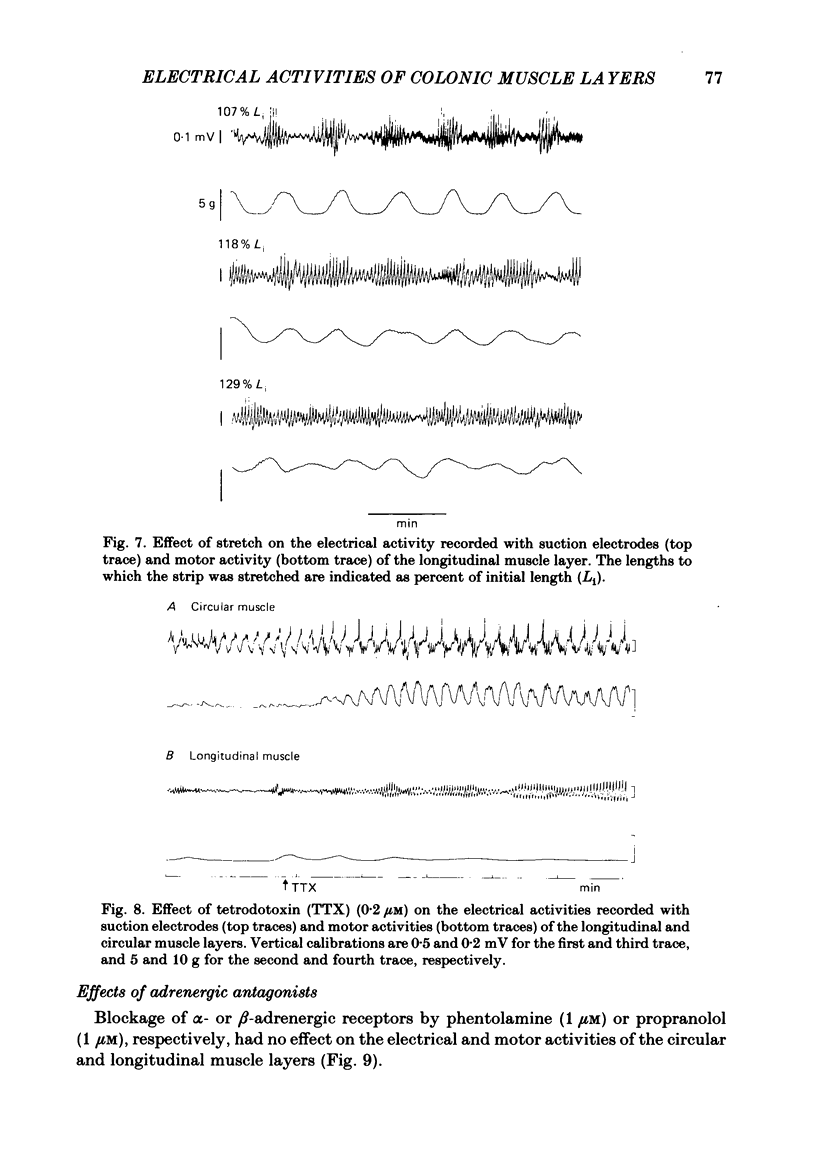
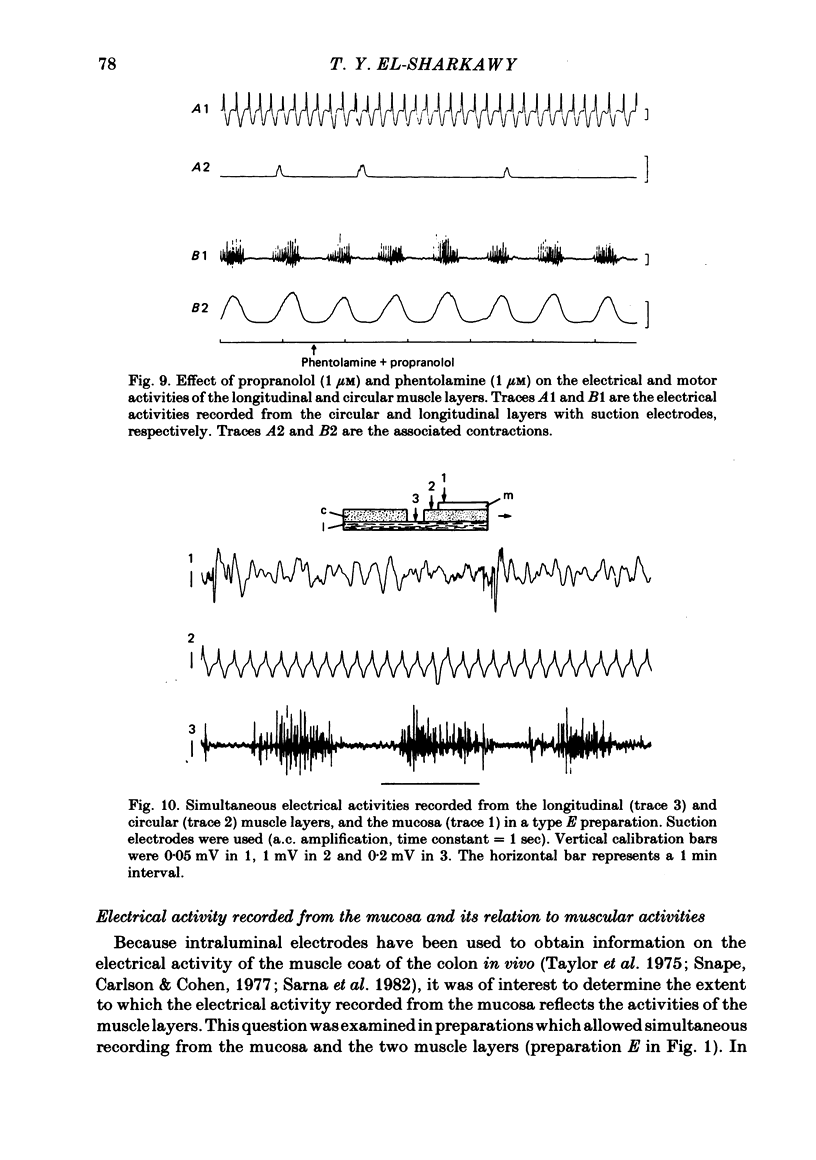
The spontaneous electrical and mechanical activities of the circular and longitudinal muscle layers of the canine colon were studied. The smooth muscle cells of the circular muscle layer exhibited regular, omni-present myogenic slow-wave activity at a frequency ranging from 4 to 7 c/min. With intracellular micro-electrodes, the slow-wave amplitude was 21-38 mV and its duration 3-6 sec. The 'resting' membrane potential was -60 to -76 mV. Some slow waves had superimposed spike bursts on their peak depolarizations and only these were associated with phasic contractions. It is concluded that they serve a pace-maker function similar to their counterpart in the small intestine. The longitudinal muscle layer exhibited periods of electrical activity alternating with periods of electrical quiescence. During the activity periods electrical oscillations occurred at a frequency of 13-35 c/min with spikes on top of them. Each electrical activity period was associated with a prolonged 'tonic' contraction. The duration of these periods was 30-120 sec and their frequency 0.4-1.1 period/min. This activity is similar to that recorded from the longitudinal muscle of the guinea-pig caecum despite the anatomical differences. The electrical activity periods of the longitudinal muscle appeared to require an excitatory input (stretch and/or acetylcholine release). Provided the strips were not excessively stretched, atropine abolished all electrical and motor activity. Stretching prolonged the electrical activity periods until they eventually fused together and the muscle developed maintained tone. Simultaneously recording from both layers showed that, although electrotonic spread between the two layers is probably insignificant, the activity of the two layers was co-ordinated. Only those slow waves of the circular layer that occurred during the electrical activity periods of the longitudinal layer had superimposed spikes. It is suggested that this co-ordination may indicate that the two muscle layers may be commanded by a common input from periodically active, cholinergic intramural neurones. It is proposed that the complex patterns of colonic electrical and motor activities may be explained as consisting of two major components: one arising from the longitudinal (long spike bursts, high-frequency oscillations and tonic contractions) and the other from the circular layer (slow waves, short spike and phasic contractions). Simultaneous electrical records from the two muscle layers and the mucosa failed to show a consistent relationship between the mucosal record and the activity of either layer. Caution should be exercised in the interpretation of intraluminally derived electrical recordings.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen J., Anuras S., Hauser R. L. Migrating spike bursts and electrical slow waves in the cat colon: effect of sectioning. Gastroenterology. 1974 Feb;66(2):240–247. [PubMed] [Google Scholar]
- Christensen J., Caprilli R., Lund G. F. Electric slow waves in circular muscle of cat colon. Am J Physiol. 1969 Sep;217(3):771–776. doi: 10.1152/ajplegacy.1969.217.3.771. [DOI] [PubMed] [Google Scholar]
- Christensen J., Hauser R. L. Circumferential coupling of electric slow waves in circular muscle of cat colon. Am J Physiol. 1971 Oct;221(4):1033–1037. doi: 10.1152/ajplegacy.1971.221.4.1033. [DOI] [PubMed] [Google Scholar]
- Duthie H. L., Kirk D. Electrical activity of human colonic smooth muscle in vitro. J Physiol. 1978 Oct;283:319–330. doi: 10.1113/jphysiol.1978.sp012502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sharkawy T. Y., Daniel E. E. Electrical activity of small intestinal smooth muscle and its temperature dependence. Am J Physiol. 1975 Nov;229(5):1268–1276. doi: 10.1152/ajplegacy.1975.229.5.1268. [DOI] [PubMed] [Google Scholar]
- Fioramonti J., Bueno L. Motor activity in the large intestine of the pig related to dietary fibre and retention time. Br J Nutr. 1980 Jan;43(1):155–162. doi: 10.1079/bjn19800074. [DOI] [PubMed] [Google Scholar]
- Fioramonti J., Garcia-Villar R., Bueno L., Ruckebusch Y. Colonic myoelectrical activity and propulsion in the dog. Dig Dis Sci. 1980 Sep;25(9):641–646. doi: 10.1007/BF01308321. [DOI] [PubMed] [Google Scholar]
- Golenhoffen K., von Loh D. Elektrophysiologische Untersuchungen zur normalen Spontanaktivität der isolierten Taenia coli des Meerschweinchens. Pflugers Arch. 1970;314(4):312–328. doi: 10.1007/BF00592289. [DOI] [PubMed] [Google Scholar]
- Kocylowski M., Bowes K. L., Kingma Y. J. Electrical and mechanical activity in the ex vivo perfused total canine colon. Gastroenterology. 1979 Nov;77(5):1021–1026. [PubMed] [Google Scholar]
- Sarna S. K., Bardakjian B. L., Waterfall W. E., Lind J. F. Human colonic electrical control activity (ECA). Gastroenterology. 1980 Jun;78(6):1526–1536. [PubMed] [Google Scholar]
- Sarna S. K., Waterfall W. E., Bardakjian B. L., Lind J. F. Types of human colonic electrical activities recorded postoperatively. Gastroenterology. 1981 Jul;81(1):61–70. [PubMed] [Google Scholar]
- Sarna S., Latimer P., Campbell D., Waterfall W. E. Electrical and contractile activities of the human rectosigmoid. Gut. 1982 Aug;23(8):698–705. doi: 10.1136/gut.23.8.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snape W. J., Jr, Carlson G. M., Cohen S. Human colonic myoelectric activity in response to prostigmin and the gastrointestinal hormones. Am J Dig Dis. 1977 Oct;22(10):881–887. doi: 10.1007/BF01076164. [DOI] [PubMed] [Google Scholar]
- Taylor I., Duthie H. L., Smallwood R., Linkens D. Large bowel myoelectrical activity in man. Gut. 1975 Oct;16(10):808–814. doi: 10.1136/gut.16.10.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienbeck M., Christensen J., Weisbrodt N. W. Electromyography of the colon in the unanesthetized cat. Am J Dig Dis. 1972 Apr;17(4):356–362. doi: 10.1007/BF02231738. [DOI] [PubMed] [Google Scholar]
- Wood J. D. Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Am J Dig Dis. 1973 Jun;18(6):477–488. doi: 10.1007/BF01076598. [DOI] [PubMed] [Google Scholar]
- el-Sharkawy T. Y., Morgan K. G., Szurszewski J. H. Intracellular electrical activity of canine and human gastric smooth muscle. J Physiol. 1978 Jun;279:291–307. doi: 10.1113/jphysiol.1978.sp012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Sharkawy T. Y., Szurszewski J. H. Modulation of canine antral circular smooth muscle by acetylcholine, noradrenaline and pentagastrin. J Physiol. 1978 Jun;279:309–320. doi: 10.1113/jphysiol.1978.sp012346. [DOI] [PMC free article] [PubMed] [Google Scholar]


