Abstract
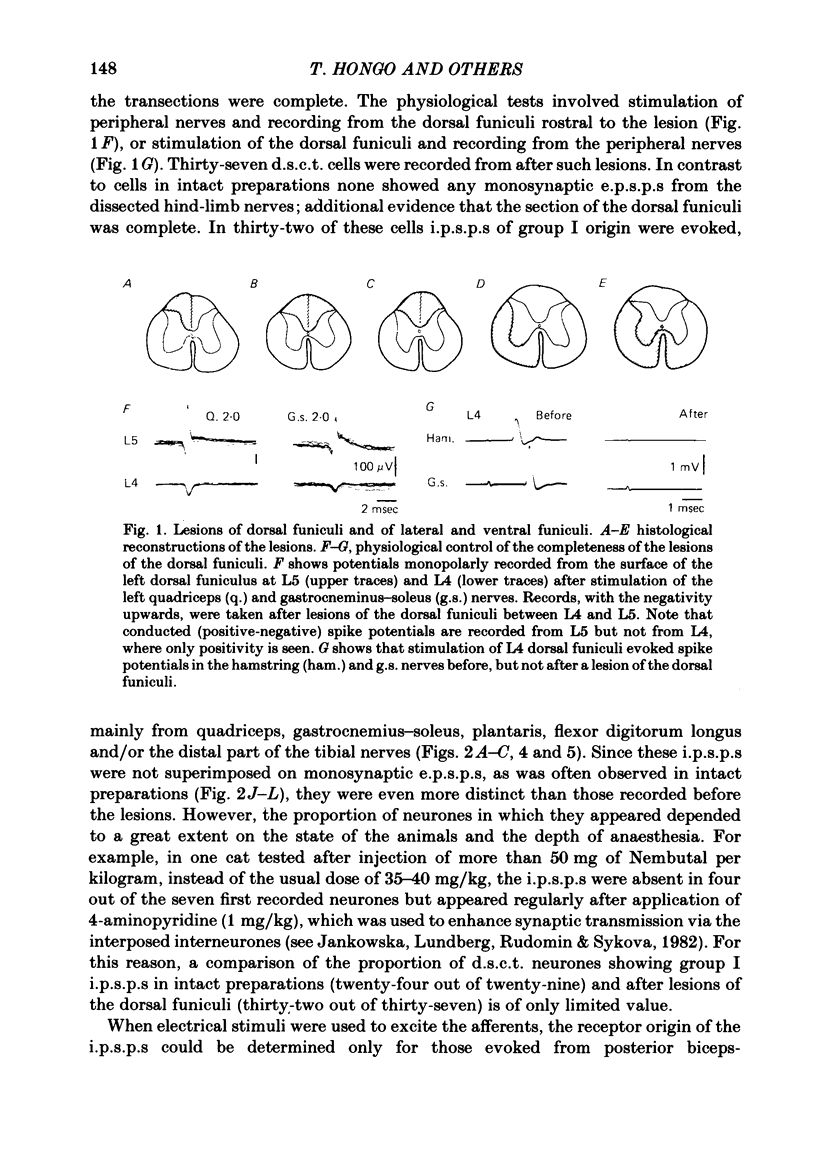
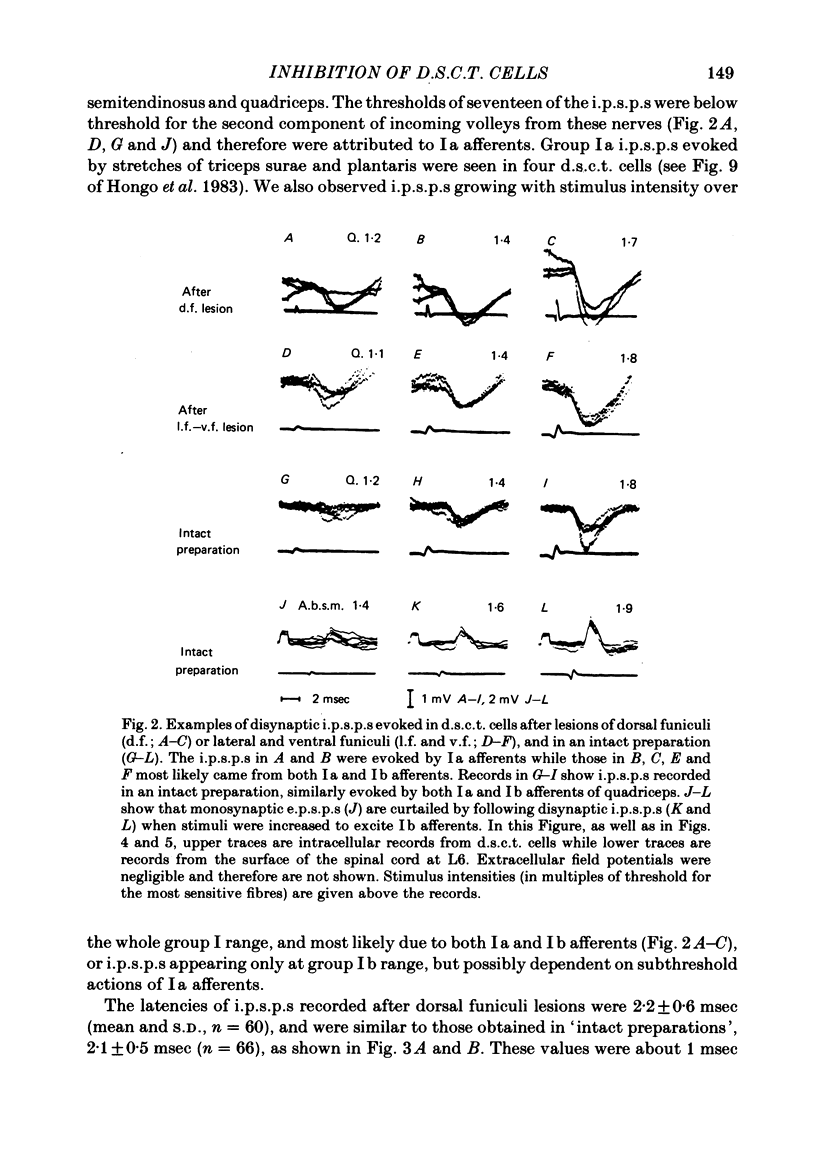
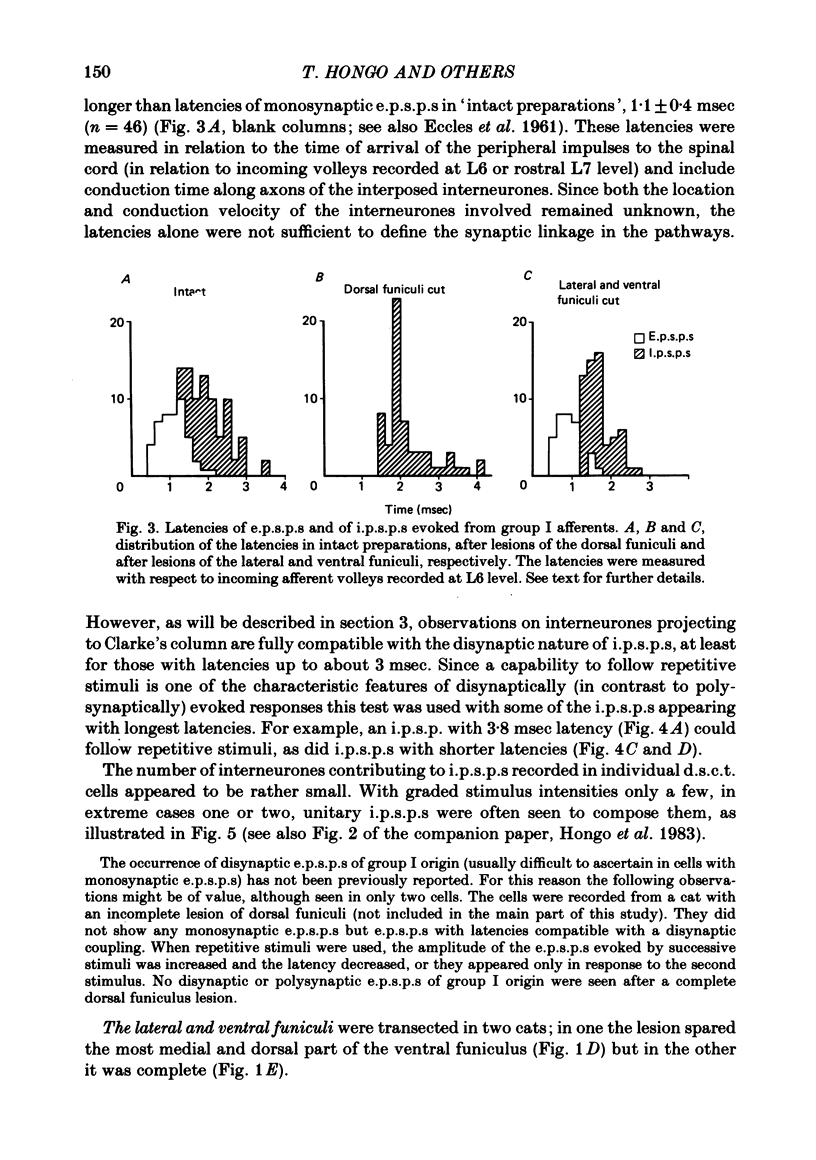
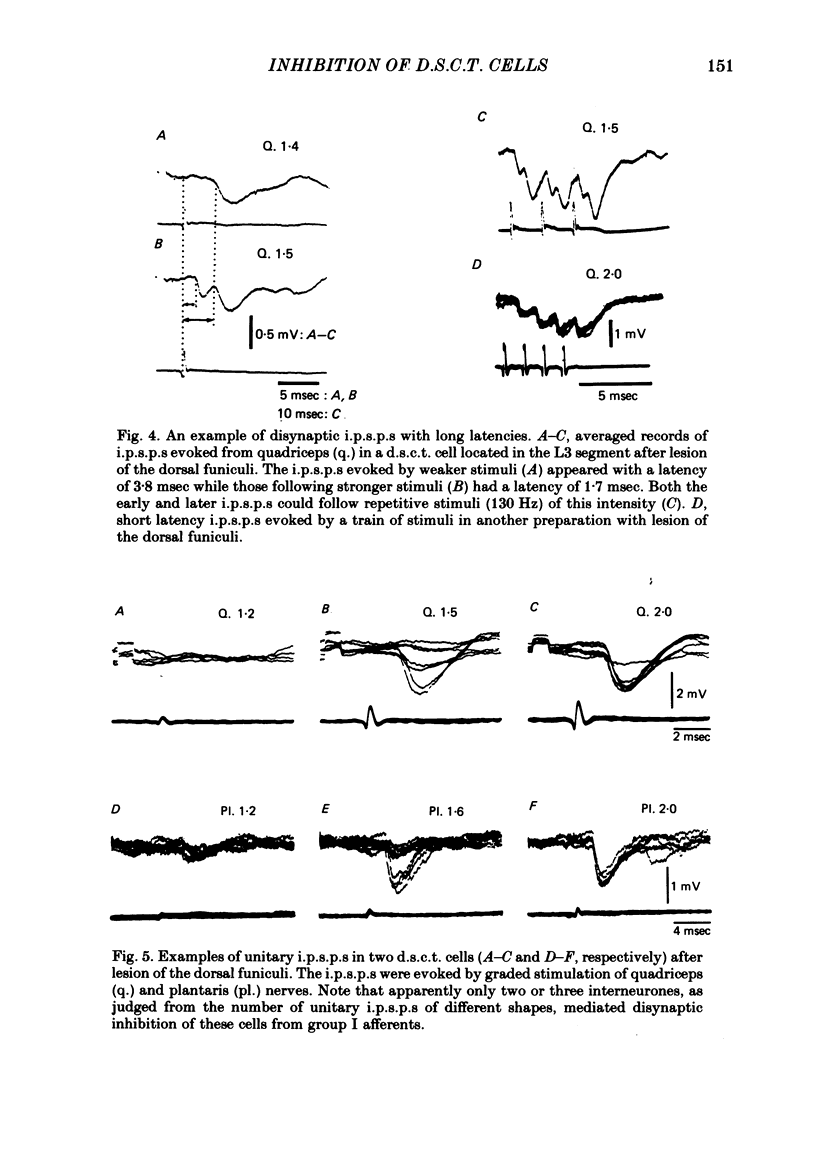
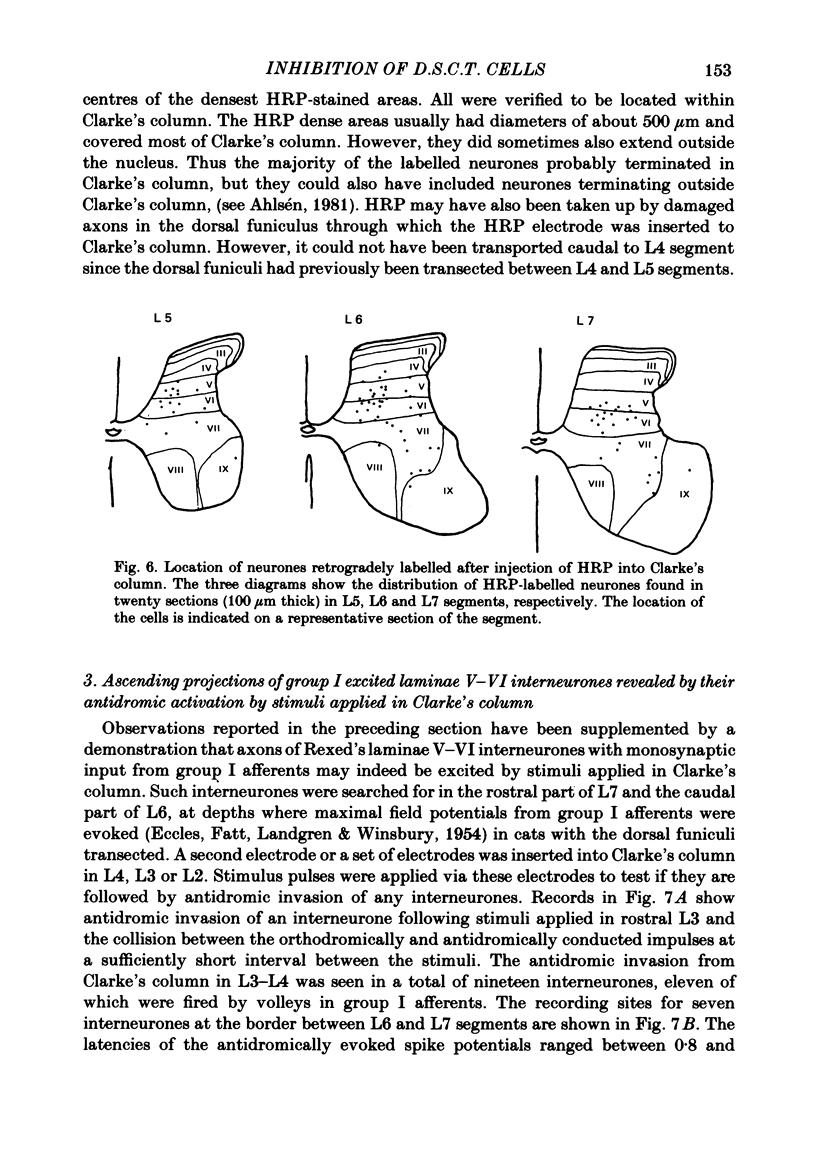
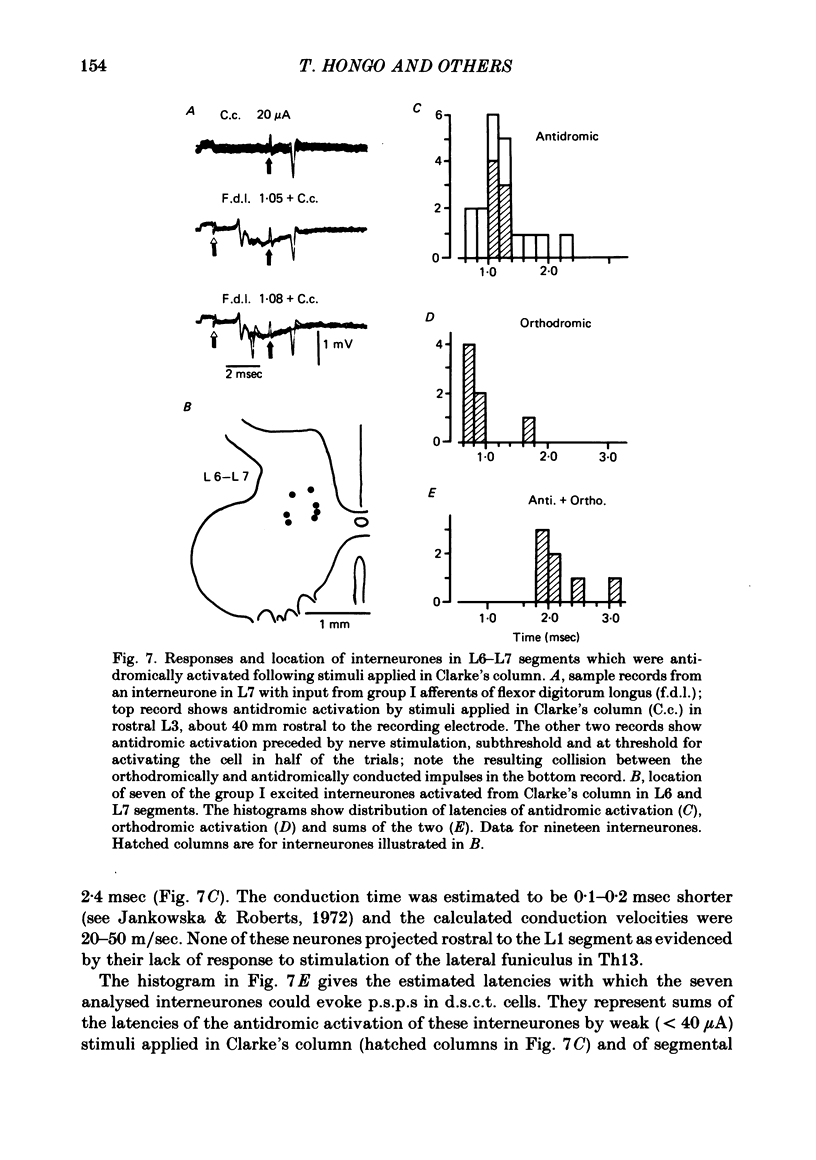
The topographical distribution of interneurones mediating disynaptic inhibition of dorsal spinocerebellar tract (d.s.c.t.) cells from group I muscle afferents in the cat was investigated using both physiological and morphological techniques. Lesions of either the dorsal funiculi or of the lateral and ventral funiculi were made between L4 and L5 segments in two groups of cats. I.p.s.p.s. evoked from group I afferents were seen after both these lesions, showing that the i.p.s.p.s were evoked by interneurones located more caudally as well as by interneurones in the same segments as Clarke's column. Distribution of the caudally located interneurones in the lower lumbar segments was investigated after marking these interneurones with horseradish peroxidase retrogradely transported from Clarke's column. The horseradish peroxidase was injected along L3-L4 segments of Clarke's column in two cats with transected dorsal funiculi. The marked cells were found in L5, L6, L7 and S1 segments, with a highest density in L6 and L7. They were seen in laminae V, VI and VII. A search was made for interneurones which could be antidromically invaded following stimuli applied in Clarke's column and were monosynaptically excited by group I afferents. Such interneurones were found at locations corresponding to laminae V-VI of Rexed. The latencies of antidromic and orthodromic responses were within ranges allowing them to mediate disynaptic inhibition of d.s.c.t. cells.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2(1):141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Ahlsén G. Retrograde labelling of retinogeniculate neurones in the cat by HRP uptake from the diffuse injection zone. Brain Res. 1981 Nov 2;223(2):374–380. doi: 10.1016/0006-8993(81)91150-1. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system: I. Existence of long ascending postsynaptic fibres in the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):457–470. doi: 10.1007/BF00237348. [DOI] [PubMed] [Google Scholar]
- Angaut-Petit D. The dorsal column system: II. Functional properties and bulbar relay of the postsynaptic fibres of the cat's fasciculus gracilis. Exp Brain Res. 1975 May 22;22(5):471–493. doi: 10.1007/BF00237349. [DOI] [PubMed] [Google Scholar]
- Bodian D. Electron microscopy: two major synaptic types on spinal motoneurons. Science. 1966 Mar 4;151(3714):1093–1094. doi: 10.1126/science.151.3714.1093. [DOI] [PubMed] [Google Scholar]
- Boehme C. C. The neural structure of Clarke's nucleus of the spinal cord. J Comp Neurol. 1968 Mar;132(3):445–461. doi: 10.1002/cne.901320306. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Form and function of dorsal horn neurones with axons ascending the dorsal columns in cat. J Physiol. 1981 Dec;321:31–47. doi: 10.1113/jphysiol.1981.sp013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ib afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1979 Nov;296:215–226. doi: 10.1113/jphysiol.1979.sp013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., ECCLES J. C., LUNDBERG A. Intracellular recording from cells in Clarke's column. Acta Physiol Scand. 1958 Oct 8;43(3-4):303–314. doi: 10.1111/j.1748-1716.1958.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Czarkowska J., Jankowska E., Sybirska E. Diameter and internodal length of axons of spinal interneurones excited by group I afferents in the cat. Brain Res. 1976 Dec 10;118(1):119–122. doi: 10.1016/0006-8993(76)90845-3. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., FATT P., LANDGREN S., WINSBURY G. J. Spinal cord potentials generated by volleys in the large muscle afferents. J Physiol. 1954 Sep 28;125(3):590–606. doi: 10.1113/jphysiol.1954.sp005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., OSCARSSON O., WILLIS W. D. Synaptic action of group I and II afferent fibres of muscle on the cells of the dorsal spinocerebellar tract. J Physiol. 1961 Oct;158:517–543. doi: 10.1113/jphysiol.1961.sp006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E., Fedina L., Jansen J., Lundberg A., Vyklický L. Properties of Clarke's column neurones. Acta Physiol Scand. 1969 Sep-Oct;77(1):125–144. doi: 10.1111/j.1748-1716.1969.tb04558.x. [DOI] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMQVIST B., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. V. Further experiments on convergence of excitatory and inhibitory actions. Acta Physiol Scand. 1956 Dec 29;38(1):76–90. doi: 10.1111/j.1748-1716.1957.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Ohno T., Sasaki S., Yamashita M., Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol. 1983 Sep;342:161–180. doi: 10.1113/jphysiol.1983.sp014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T., Okada Y. Cortically evoked pre- and postsynaptic inhibition of impulse transmission to the dorsal spinocerebellar tract. Exp Brain Res. 1967;3(2):163–177. doi: 10.1007/BF00233260. [DOI] [PubMed] [Google Scholar]
- Ishizuka N., Mannen H., Hongo T., Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979 Jul 15;186(2):189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Johannisson T., Lipski J. Common interneurones in reflex pathways from group 1a and 1b afferents of ankle extensors in the cat. J Physiol. 1981 Jan;310:381–402. doi: 10.1113/jphysiol.1981.sp013556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Lundberg A., Rudomin P., Sykova E. Effects of 4-aminopyridine on synaptic transmission in the cat spinal cord. Brain Res. 1982 May 20;240(1):117–129. doi: 10.1016/0006-8993(82)90649-7. [DOI] [PubMed] [Google Scholar]
- Jankowska E., McCrea D., Mackel R. Pattern of 'non-reciprocal' inhibition of motoneurones by impulses in group Ia muscle spindle afferents in the cat. J Physiol. 1981 Jul;316:393–409. doi: 10.1113/jphysiol.1981.sp013796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal columns. J Physiol. 1979 May;290(2):185–200. doi: 10.1113/jphysiol.1979.sp012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol. 1972 May;222(3):597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen J. K., Nicolaysen K., Walloe L. On the inhibition of transmission to the dorsal spinocerebellar tract by stretch of various ankle muscles of the cat. Acta Physiol Scand. 1967 Jul-Aug;70(3):362–368. doi: 10.1111/j.1748-1716.1967.tb03635.x. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Nicolaysen K., Walloe L. The firing pattern of dorsal spinocerebellar tract neurones during inhibition. Acta Physiol Scand. 1969 Sep-Oct;77(1):68–84. doi: 10.1111/j.1748-1716.1969.tb04554.x. [DOI] [PubMed] [Google Scholar]
- Jansen J. K., Rudjord T. Dorsal spinocerebellar tract: response pattern of nerve fibers to muscle stretch. Science. 1965 Sep 3;149(3688):1109–1111. doi: 10.1126/science.149.3688.1109. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LUNDBERG A. Functional organization of the dorsal spino-cerebellar tract in the cat. III. Single fibre recording in Flechsig's fasciculus on adequate stimulation of primary afferent neurons. Acta Physiol Scand. 1956 Mar 24;36(1-2):204–218. doi: 10.1111/j.1748-1716.1956.tb01318.x. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. II. Single fibre recording in Flechsig's fasciculus on electrical stimulation of various peripheral nerves. Acta Physiol Scand. 1956 Mar 24;36(1-2):188–203. doi: 10.1111/j.1748-1716.1956.tb01317.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., NORRSELL U., VOORHOEVE P. EFFECTS FROM THE SENSORIMOTOR CORTEX ON ASCENDING SPINAL PATHWAYS. Acta Physiol Scand. 1963 Dec;59:462–473. doi: 10.1111/j.1748-1716.1963.tb02762.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. VII. Identification of units by antidromic activation from the cerebellar cortex with recognition of five functional subdivisions. Acta Physiol Scand. 1960 Dec 30;50:356–374. doi: 10.1111/j.1748-1716.1960.tb00189.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spinocerebellar tract in the cat. IV. Synaptic connections of afferents from Golgi tendon organs and muscle spindles. Acta Physiol Scand. 1956 Dec 29;38(1):53–75. doi: 10.1111/j.1748-1716.1957.tb00173.x. [DOI] [PubMed] [Google Scholar]
- NATHAN P. W., SMITH M. C. Fasciculi proprii of the spinal cord in man. Brain. 1959 Dec;82:610–668. doi: 10.1093/brain/82.4.610. [DOI] [PubMed] [Google Scholar]
- REXED B. A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol. 1954 Apr;100(2):297–379. doi: 10.1002/cne.901000205. [DOI] [PubMed] [Google Scholar]
- Randić M., Miletić V., Loewy A. D. A morphological study of cat dorsal spinocerebellar tract neurons after intracellular injection of horseradish peroxidase. J Comp Neurol. 1981 May 20;198(3):453–466. doi: 10.1002/cne.901980306. [DOI] [PubMed] [Google Scholar]
- Réthelyi M. The Golgi architecture of Clarke's column. Acta Morphol Acad Sci Hung. 1968;16(3):311–330. [PubMed] [Google Scholar]
- Réthelyi M. Ultrastructural synaptology of Clarke's column. Exp Brain Res. 1970;11(2):159–174. doi: 10.1007/BF00234320. [DOI] [PubMed] [Google Scholar]
- SZENTAGOTHAI J., ALBERT A. The synaptology of Clarke's column. Acta Morphol Acad Sci Hung. 1955;5(1-2):43–51. [PubMed] [Google Scholar]
- Uchizono K. Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature. 1965 Aug 7;207(997):642–643. doi: 10.1038/207642a0. [DOI] [PubMed] [Google Scholar]
- Uddenberg N. Functional organization of long, second-order afferents in the dorsal funiculus. Exp Brain Res. 1968;4(4):377–382. doi: 10.1007/BF00235702. [DOI] [PubMed] [Google Scholar]


