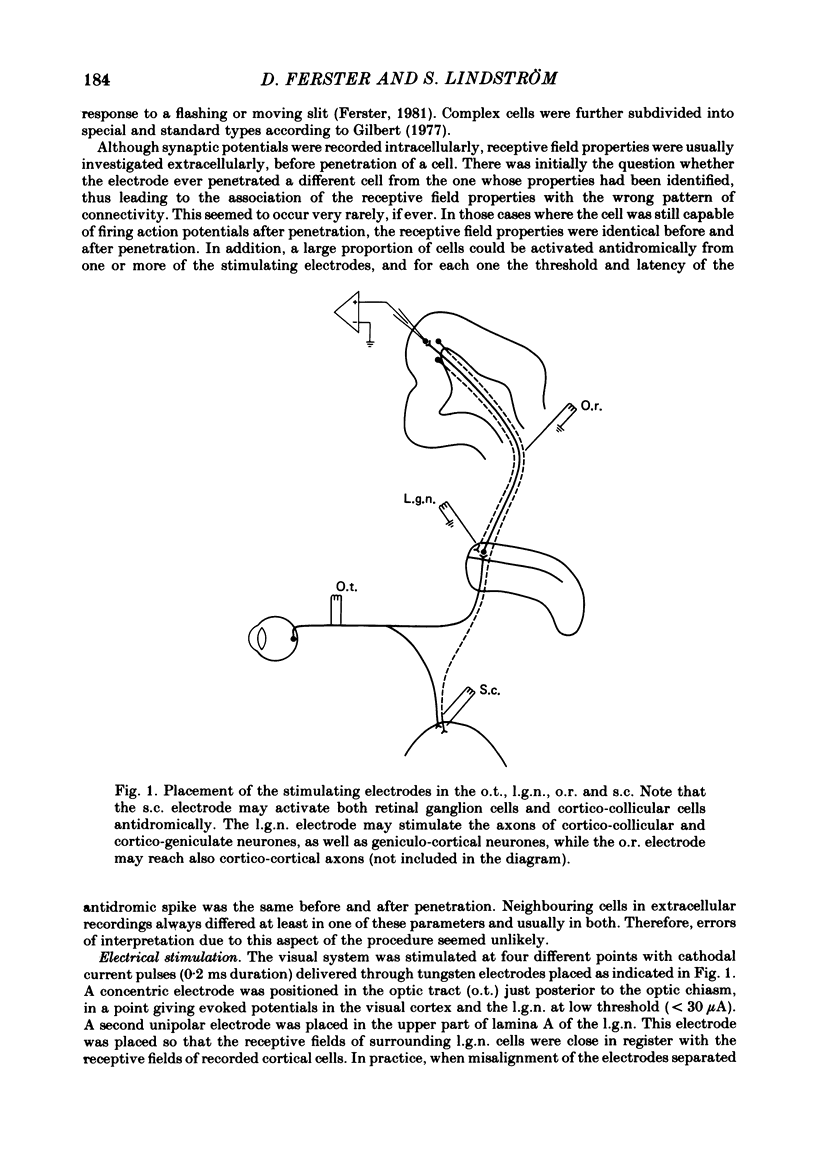
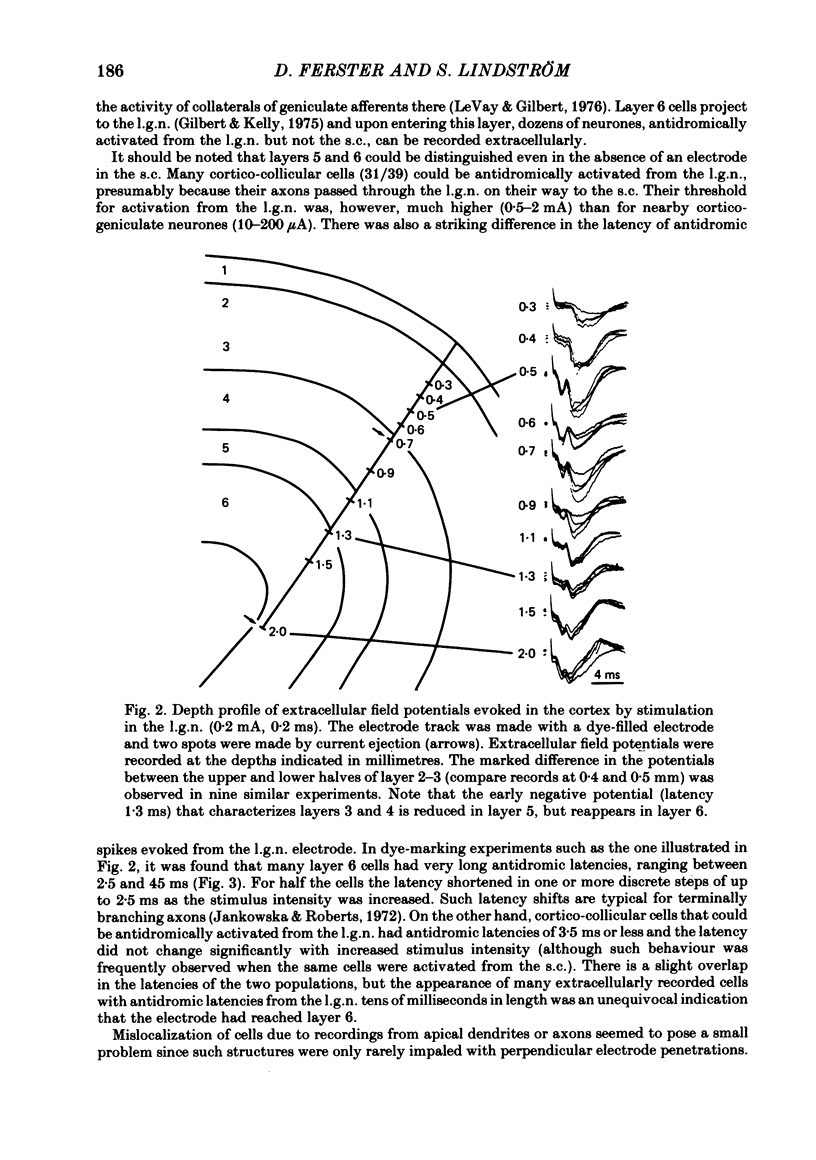
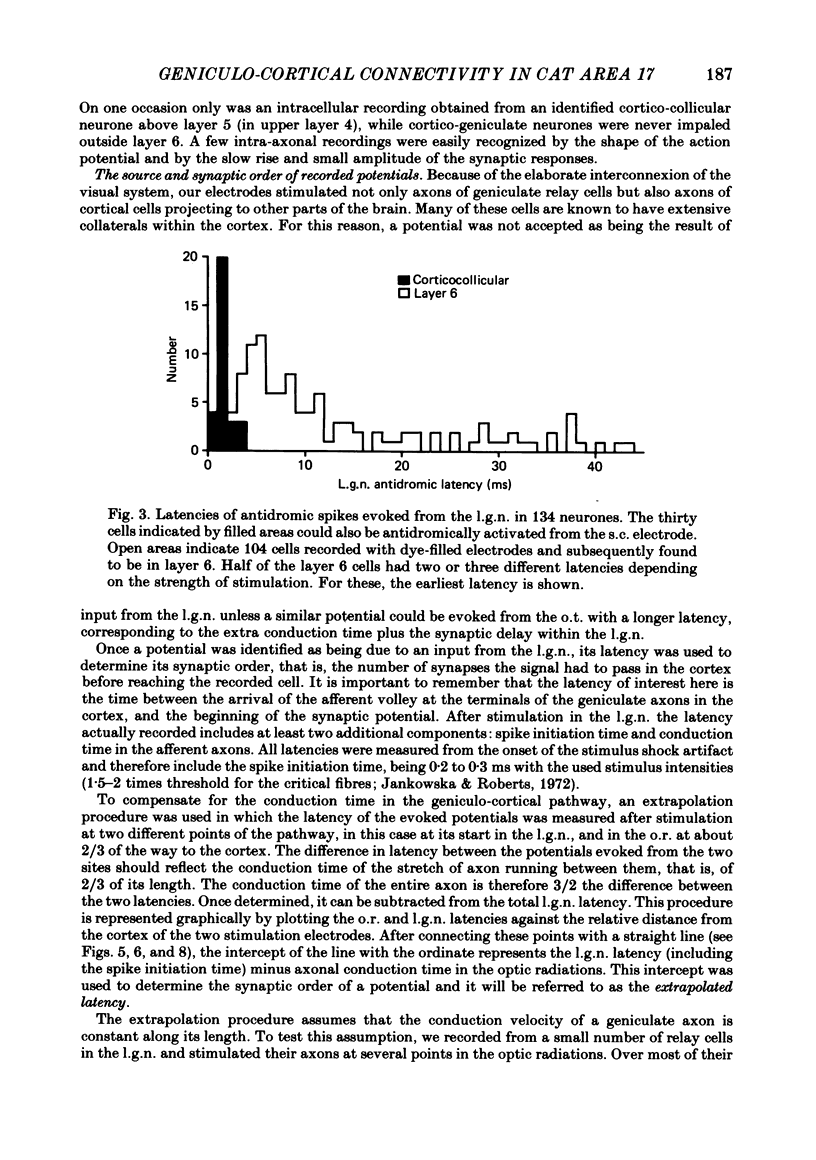
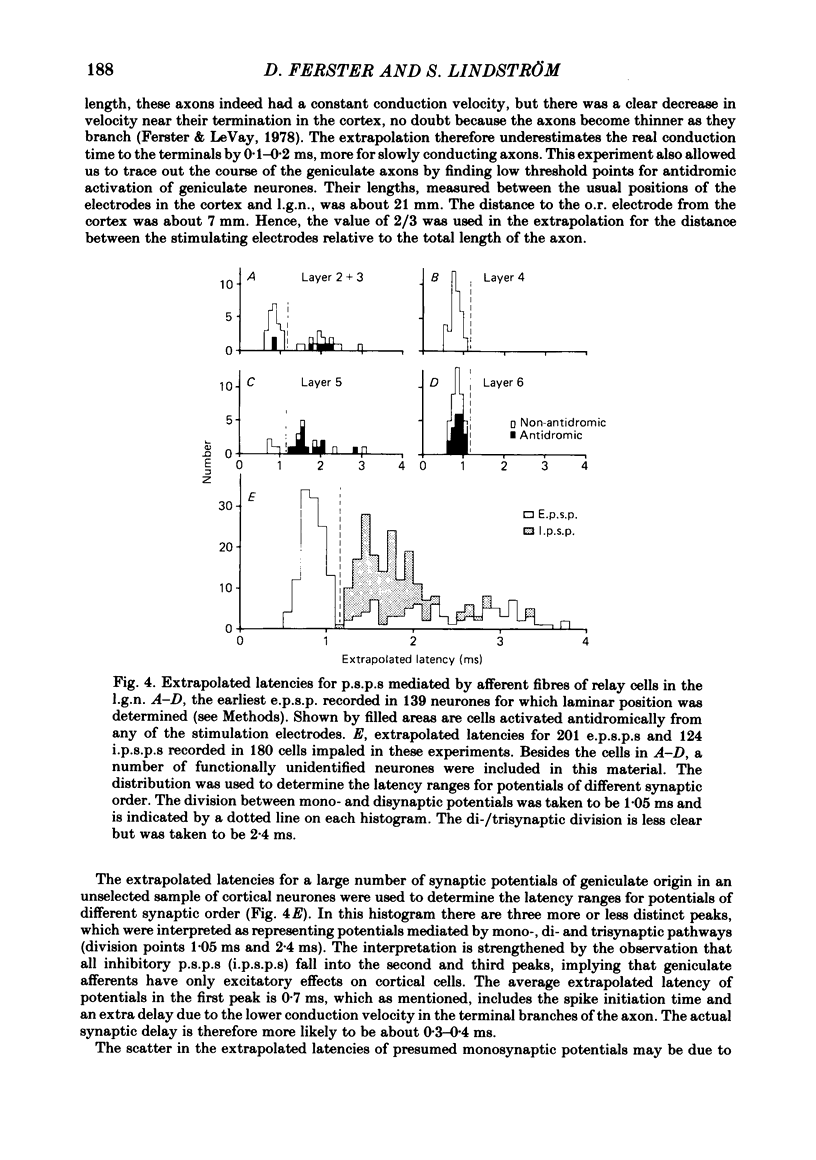
Abstract
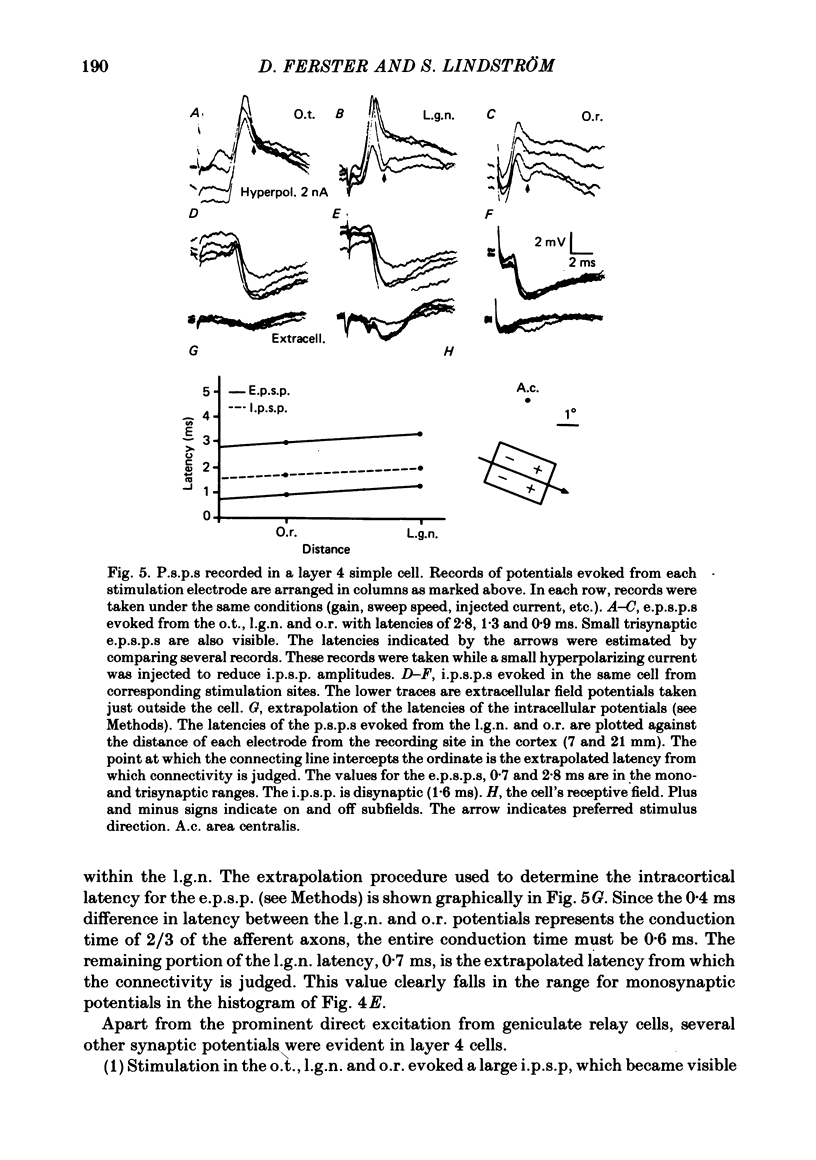
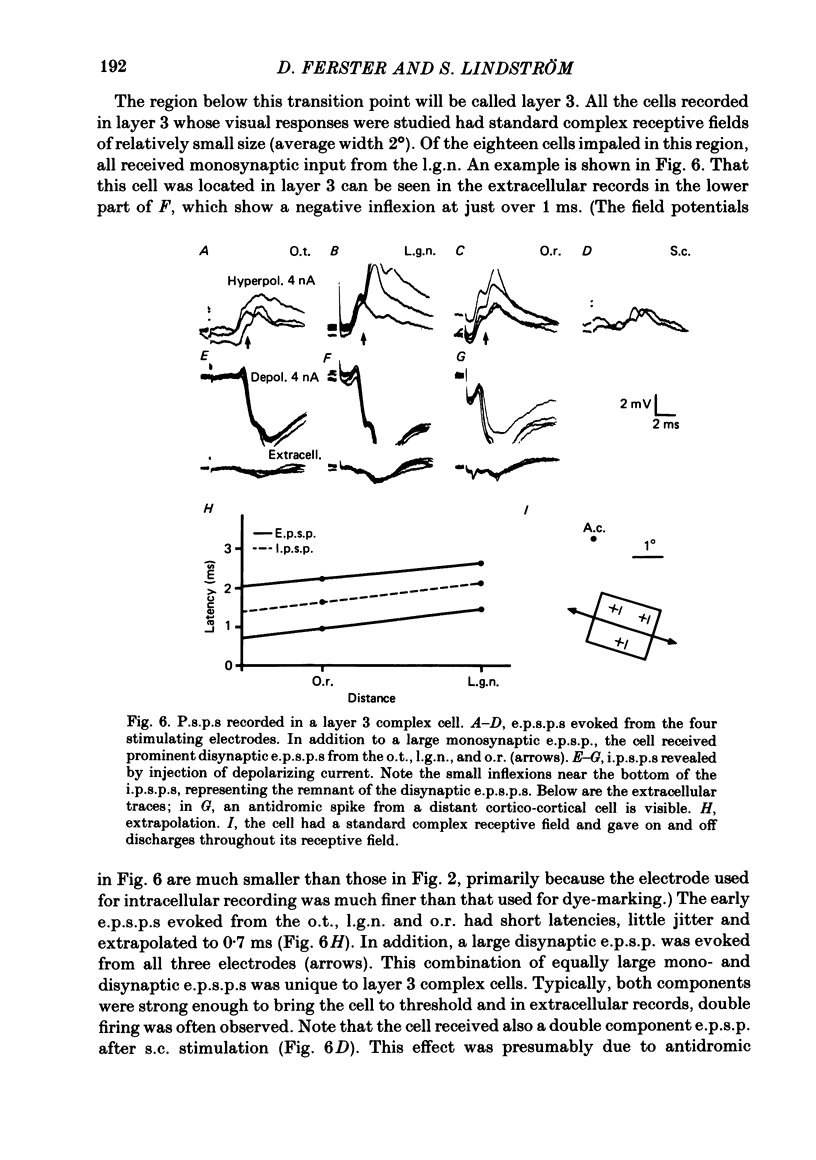
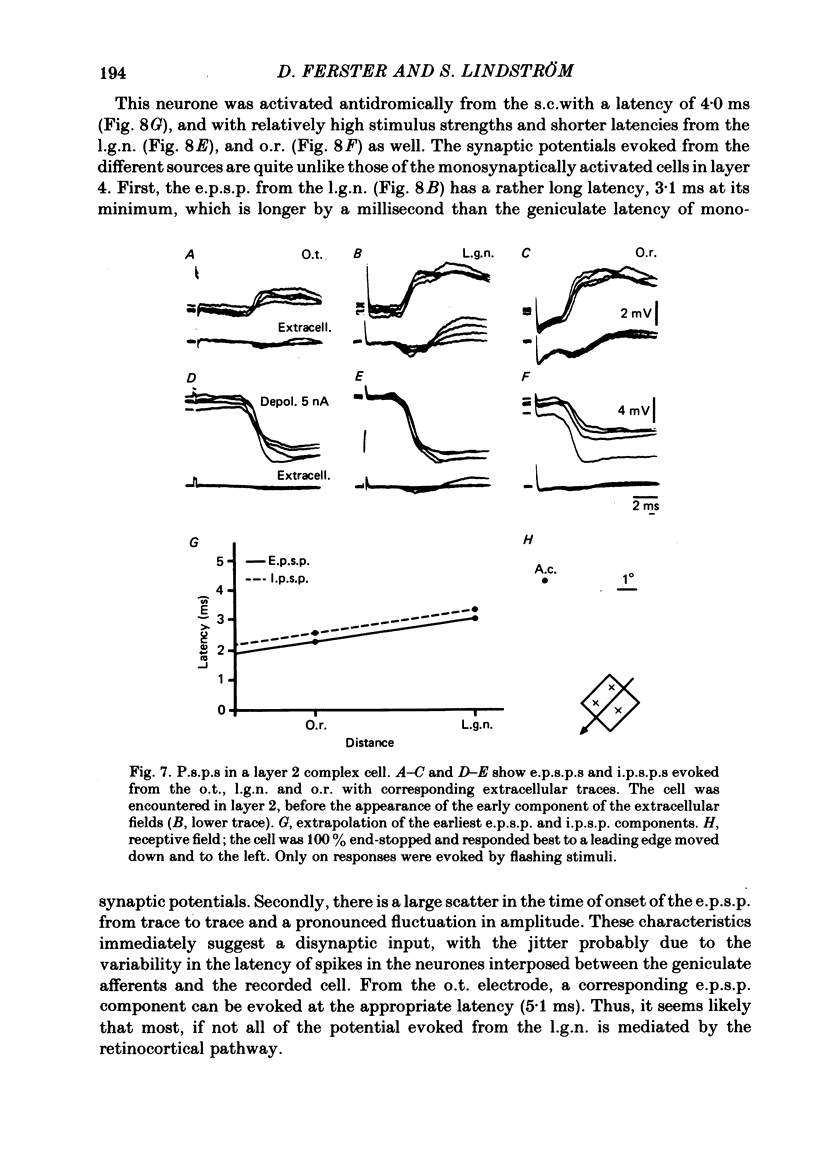
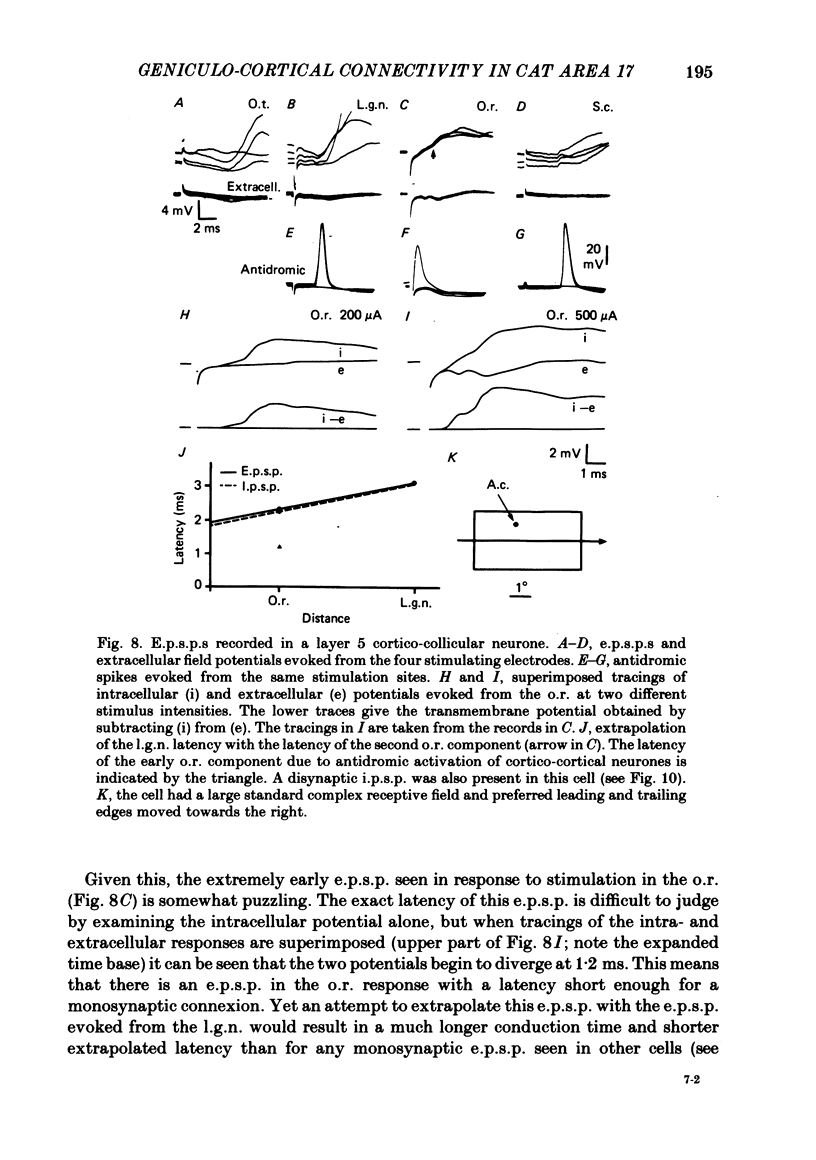
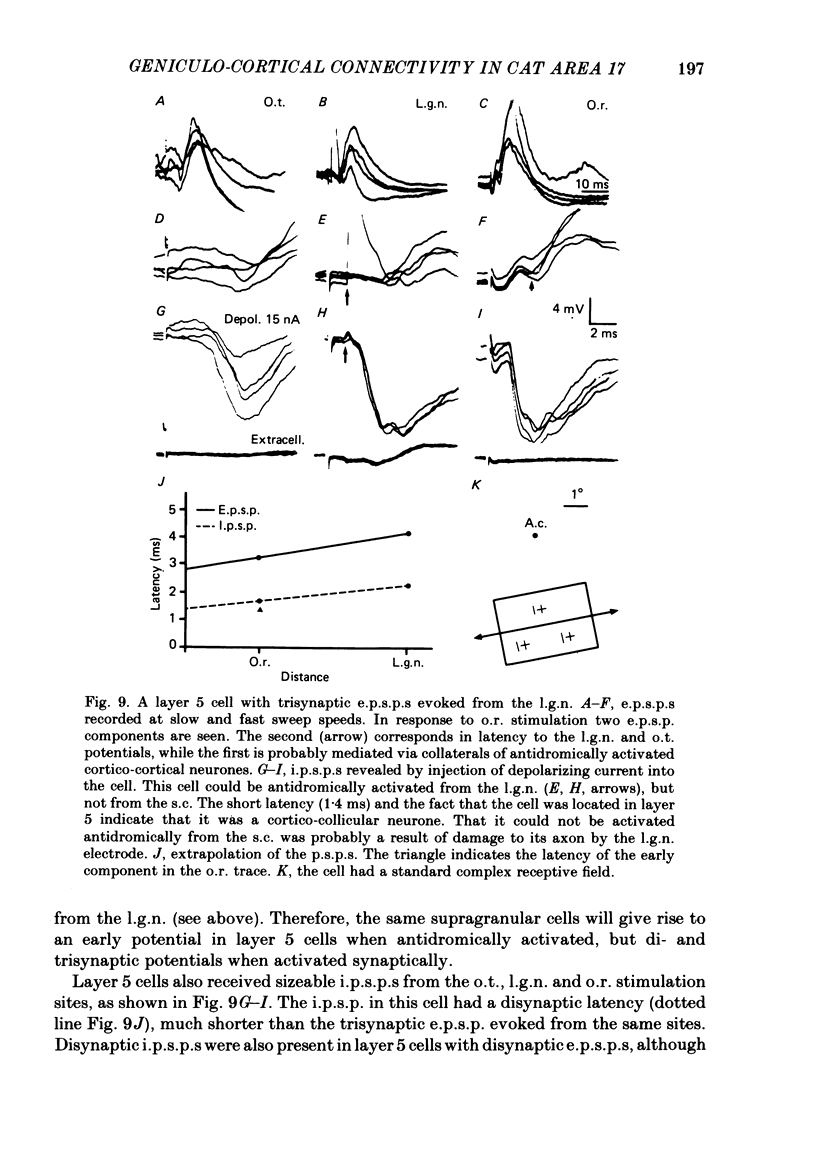
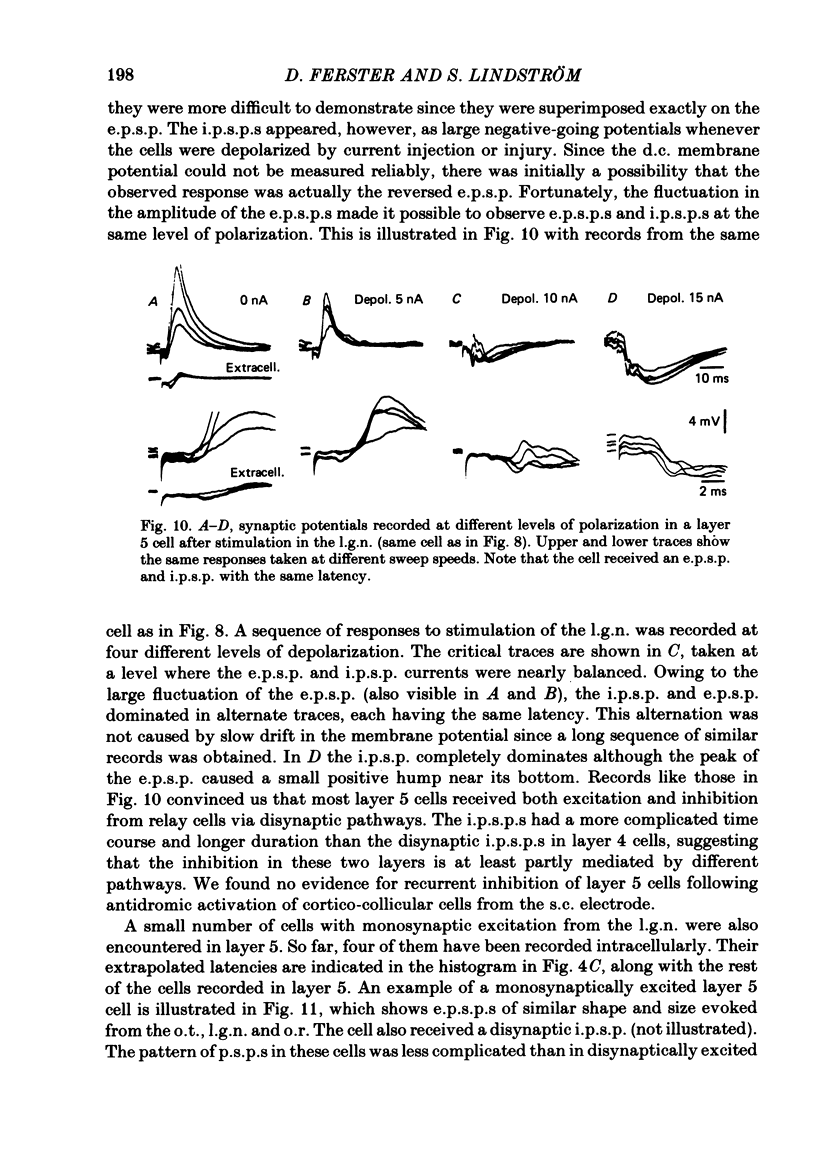
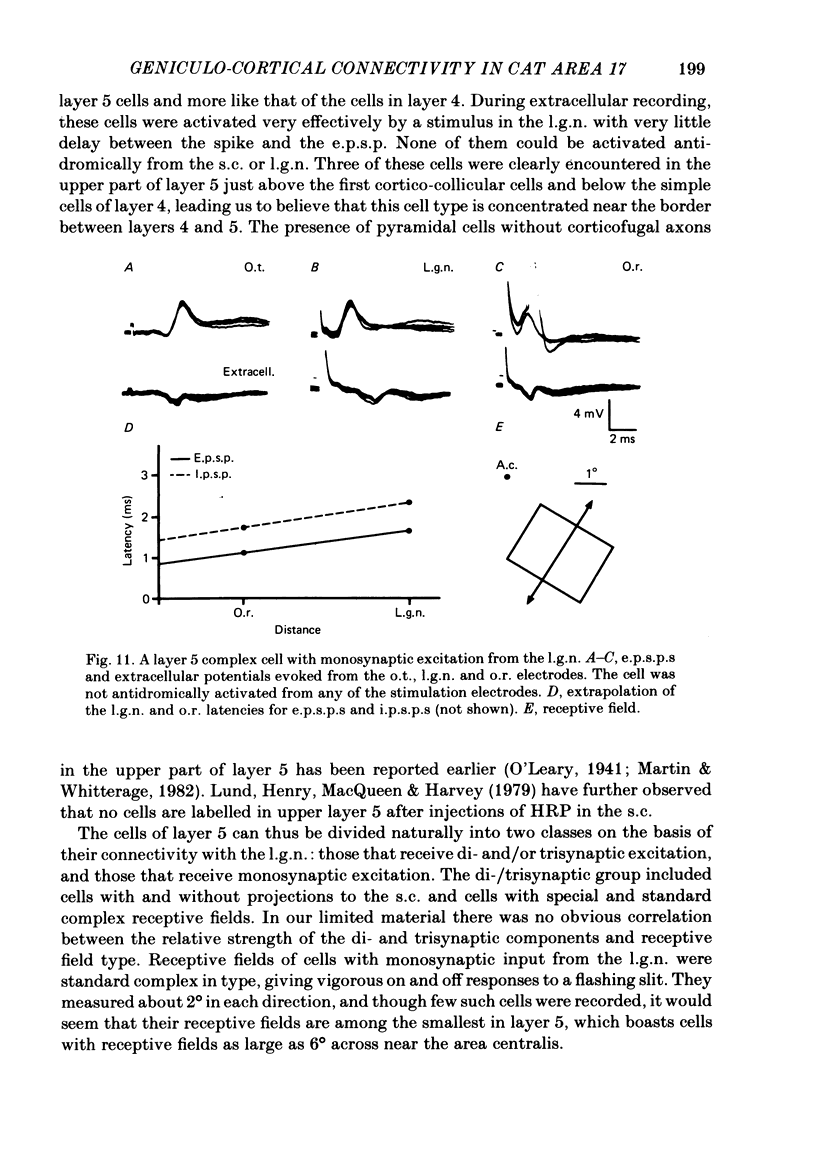
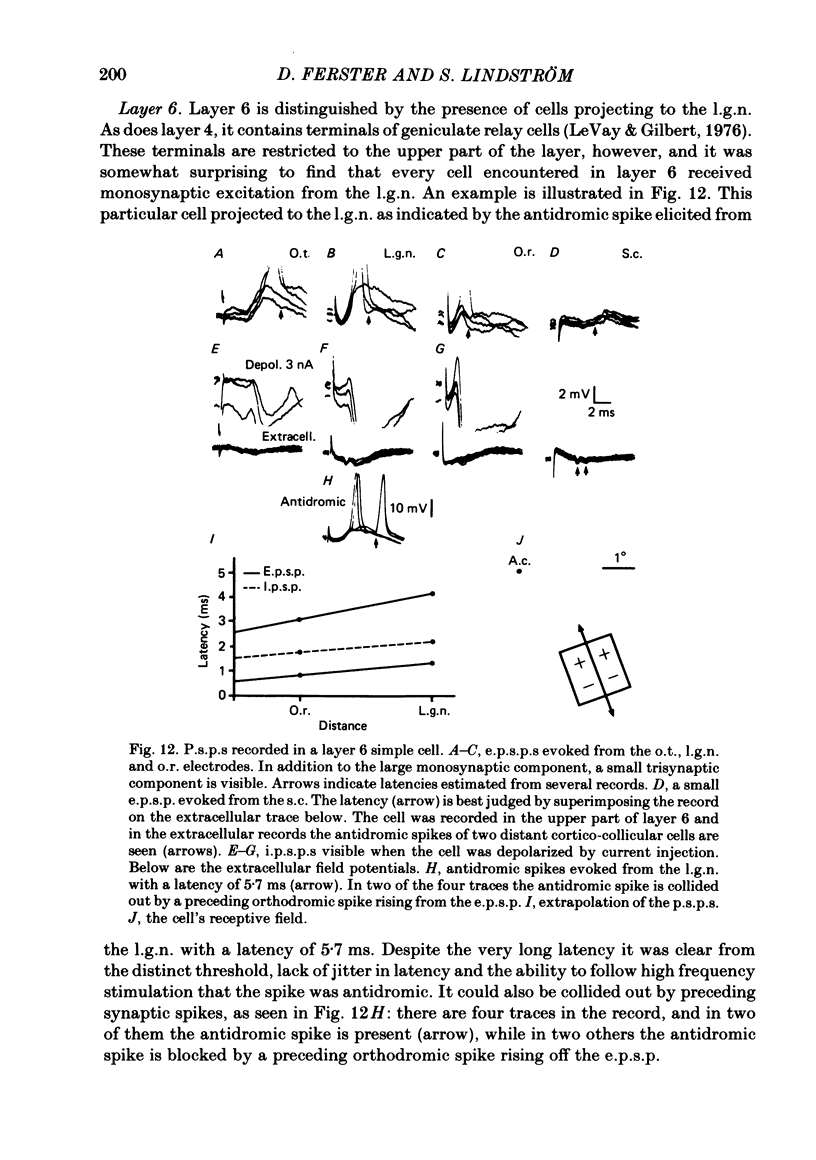
The latencies of excitatory and inhibitory post-synaptic potentials (e.p.s.p.s and i.p.s.p.s) evoked by electrical stimulation of afferents from the lateral geniculate nucleus were recorded in neurones of area 17 of the cat visual cortex. After application of an extrapolation procedure to compensate for the conduction time of the afferent axons, a histogram of latencies formed three distinct peaks. Potentials in each of these were interpreted as being mediated by mono-, di- and trisynaptic pathways. Characteristic laminar differences in the extracellular field potentials evoked from the lateral geniculate nucleus (l.g.n.) and in the antidromic activation of neurones from the l.g.n. and superior colliculus were used to determine the laminar position of recorded neurones. It was found that within a given layer, all cells maintained similar connexions with relay cells in the l.g.n. Cells in layers 3, 4, upper 5 and 6 were monosynaptically excited by geniculate afferents, while cells in layers 2 and lower 5 received only indirect excitation via other cortical neurones. Layer 3 cells were unique in receiving a prominent disynaptic e.p.s.p. in addition to the direct excitation from the l.g.n. Late, trisynaptic e.p.s.p. components were seen in many layer 5 and 6 cells. The orderly laminar arrangement of the connexions had the consequence that identified cortico-geniculate neurones were monosynaptically excited and cortico-collicular neurones di- and trisynaptically excited by geniculate afferents. Cortico-cortical neurones in layers 2 and 3 received di- or mono- plus disynaptic excitation, depending on laminar position. Post-synaptic inhibitory potentials were evoked in all impaled cells, following stimulation of the geniculo-cortical pathway. Except for a few layer 2 cells, this inhibition was mediated through disynaptic pathways of the feed-forward type. There was a good positive correlation between conduction times for monosynaptic e.p.s.p.s and disynaptic i.p.s.p.s in the same cells, suggesting that cortical neurones receive excitation and inhibition from the same type of geniculate afferents. The stimulating electrodes activated not only geniculo-cortical afferents, but antidromically activated cortical efferent neurones from their extracortical axons. These neurones possess intracortical collaterals, and care must be taken to distinguish the resulting potentials from those mediated by orthodromic activation of geniculate afferents. In doing so, evidence was obtained for excitatory connexions from layers 2 and 3 to layer 5, from layer 5 to layer 6, and from layer 6 to layer 4. Typical recurrent inhibition was not observed.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF



















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlsen G., Grant K., Lindström S. Monosynaptic excitation of principal cells in the lateral geniculate nucleus by corticofugal fibers. Brain Res. 1982 Feb 25;234(2):454–458. doi: 10.1016/0006-8993(82)90886-1. [DOI] [PubMed] [Google Scholar]
- Ahlsén G., Lindström S. Excitation of perigeniculate neurones via axon collaterals of principal cells. Brain Res. 1982 Mar 25;236(2):477–481. doi: 10.1016/0006-8993(82)90730-2. [DOI] [PubMed] [Google Scholar]
- Albus K., Donate-Oliver F. Cells of origin of the occipito-pontine projection in the cat: functional properties and intracortical location. Exp Brain Res. 1977 May 23;28(1-2):167–174. doi: 10.1007/BF00237094. [DOI] [PubMed] [Google Scholar]
- Andersen P., Silfvenius H., Sundberg S. H., Sveen O. A comparison of distal and proximal dendritic synapses on CAi pyramids in guinea-pig hippocampal slices in vitro. J Physiol. 1980 Oct;307:273–299. doi: 10.1113/jphysiol.1980.sp013435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Coombs J. S., Henry G. H. Receptive fields of simple cells in the cat striate cortex. J Physiol. 1973 May;231(1):31–60. doi: 10.1113/jphysiol.1973.sp010218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. O., Henry G. H., Smith C. J. Binocular interaction fields of single units in the cat striate cortex. J Physiol. 1971 Jul;216(1):39–68. doi: 10.1113/jphysiol.1971.sp009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Laminar distribution of first-order neurons and afferent terminals in cat striate cortex. J Neurophysiol. 1979 Sep;42(5):1271–1281. doi: 10.1152/jn.1979.42.5.1271. [DOI] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Neural path taken by afferent streams in striate cortex of the cat. J Neurophysiol. 1979 Sep;42(5):1264–1270. doi: 10.1152/jn.1979.42.5.1264. [DOI] [PubMed] [Google Scholar]
- Bullier J., Henry G. H. Ordinal position of neurons in cat striate cortex. J Neurophysiol. 1979 Sep;42(5):1251–1263. doi: 10.1152/jn.1979.42.5.1251. [DOI] [PubMed] [Google Scholar]
- Davis T. L., Sterling P. Microcircuitry of cat visual cortex: classification of neurons in layer IV of area 17, and identification of the patterns of lateral geniculate input. J Comp Neurol. 1979 Dec 15;188(4):599–627. doi: 10.1002/cne.901880407. [DOI] [PubMed] [Google Scholar]
- Dreher B., Cottee L. J. Visual receptive-field properties of cells in area 18 of cat's cerebral cortex before and after acute lesions in area 17. J Neurophysiol. 1975 Jul;38(4):735–750. doi: 10.1152/jn.1975.38.4.735. [DOI] [PubMed] [Google Scholar]
- Dubin M. W., Cleland B. G. Organization of visual inputs to interneurons of lateral geniculate nucleus of the cat. J Neurophysiol. 1977 Mar;40(2):410–427. doi: 10.1152/jn.1977.40.2.410. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. A comparison of binocular depth mechanisms in areas 17 and 18 of the cat visual cortex. J Physiol. 1981 Feb;311:623–655. doi: 10.1113/jphysiol.1981.sp013608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D., LeVay S. The axonal arborizations of lateral geniculate neurons in the striate cortex of the cat. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):923–944. doi: 10.1002/cne.901820510. [DOI] [PubMed] [Google Scholar]
- Finlay B. L., Schiller P. H., Volman S. F. Quantitative studies of single-cell properties in monkey striate cortex. IV. Corticotectal cells. J Neurophysiol. 1976 Nov;39(6):1352–1361. doi: 10.1152/jn.1976.39.6.1352. [DOI] [PubMed] [Google Scholar]
- Fukuda Y., Stone J. Retinal distribution and central projections of Y-, X-, and W-cells of the cat's retina. J Neurophysiol. 1974 Jul;37(4):749–772. doi: 10.1152/jn.1974.37.4.749. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D., Kelly J. P. The projections of cells in different layers of the cat's visual cortex. J Comp Neurol. 1975 Sep;163(1):81–105. doi: 10.1002/cne.901630106. [DOI] [PubMed] [Google Scholar]
- Gilbert C. D. Laminar differences in receptive field properties of cells in cat primary visual cortex. J Physiol. 1977 Jun;268(2):391–421. doi: 10.1113/jphysiol.1977.sp011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert C. D., Wiesel T. N. Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature. 1979 Jul 12;280(5718):120–125. doi: 10.1038/280120a0. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. RECEPTIVE FIELDS AND FUNCTIONAL ARCHITECTURE IN TWO NONSTRIATE VISUAL AREAS (18 AND 19) OF THE CAT. J Neurophysiol. 1965 Mar;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. R. A physiological analysis of subcortical and commissural projections of areas 17 and 18 of the cat. J Physiol. 1980 May;302:507–534. doi: 10.1113/jphysiol.1980.sp013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. R. The afferent connexions and laminar distribution of cells in area 18 of the cat. J Physiol. 1980 May;302:483–505. doi: 10.1113/jphysiol.1980.sp013257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heggelund P. Receptive field organization of simple cells in cat striate cortex. Exp Brain Res. 1981;42(1):89–98. doi: 10.1007/BF00235733. [DOI] [PubMed] [Google Scholar]
- Henry G. H., Harvey A. R., Lund J. S. The afferent connections and laminar distribution of cells in the cat striate cortex. J Comp Neurol. 1979 Oct 15;187(4):725–744. doi: 10.1002/cne.901870406. [DOI] [PubMed] [Google Scholar]
- Hoffman K. P., Stone J. Conduction velocity of afferents to cat visual cortex: a correlation with cortical receptive field properties. Brain Res. 1971 Sep 24;32(2):460–466. doi: 10.1016/0006-8993(71)90340-4. [DOI] [PubMed] [Google Scholar]
- Hornung J. P., Garey L. J. The thalamic projection to cat visual cortex: ultrastructure of neurons identified by Golgi impregnation or retrograde horseradish peroxidase transport. Neuroscience. 1981;6(6):1053–1068. doi: 10.1016/0306-4522(81)90070-1. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Roberts W. J. An electrophysiological demonstration of the axonal projections of single spinal interneurones in the cat. J Physiol. 1972 May;222(3):597–622. doi: 10.1113/jphysiol.1972.sp009817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A., Hashimoto H. Recording site of the single cone response determined by an electrode marking technique. Vision Res. 1967 Nov;7(11):847–851. doi: 10.1016/0042-6989(67)90005-3. [DOI] [PubMed] [Google Scholar]
- LeVay S., Gilbert C. D. Laminar patterns of geniculocortical projection in the cat. Brain Res. 1976 Aug 20;113(1):1–19. doi: 10.1016/0006-8993(76)90002-0. [DOI] [PubMed] [Google Scholar]
- LeVay S., Sherk H. The visual claustrum of the cat. I. Structure and connections. J Neurosci. 1981 Sep;1(9):956–980. doi: 10.1523/JNEUROSCI.01-09-00956.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVay S. Synaptic patterns in the visual cortex of the cat and monkey. Electron microscopy of Golgi preparations. J Comp Neurol. 1973 Jul 1;150(1):53–85. doi: 10.1002/cne.901500104. [DOI] [PubMed] [Google Scholar]
- Lennie P. Parallel visual pathways: a review. Vision Res. 1980;20(7):561–594. doi: 10.1016/0042-6989(80)90115-7. [DOI] [PubMed] [Google Scholar]
- Lund J. S., Henry G. H., MacQueen C. L., Harvey A. R. Anatomical organization of the primary visual cortex (area 17) of the cat. A comparison with area 17 of the macaque monkey. J Comp Neurol. 1979 Apr 15;184(4):599–618. doi: 10.1002/cne.901840402. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Thompson I. D., Tolhurst D. J. Spatial and temporal contrast sensitivity of neurones in areas 17 and 18 of the cat's visual cortex. J Physiol. 1978 Oct;283:101–120. doi: 10.1113/jphysiol.1978.sp012490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer L. A., Davis T. L. Receptive-field structure in cat striate cortex. J Neurophysiol. 1981 Aug;46(2):260–276. doi: 10.1152/jn.1981.46.2.260. [DOI] [PubMed] [Google Scholar]
- Palmer L. A., Rosenquist A. C. Visual receptive fields of single striate corical units projecting to the superior colliculus in the cat. Brain Res. 1974 Feb 15;67(1):27–42. doi: 10.1016/0006-8993(74)90295-9. [DOI] [PubMed] [Google Scholar]
- Peters A., Proskauer C. C., Feldman M. L., Kimerer L. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. V. Degenerating axon terminals synapsing with Golgi impregnated neurons. J Neurocytol. 1979 Jun;8(3):331–357. doi: 10.1007/BF01236125. [DOI] [PubMed] [Google Scholar]
- Ribak C. E. Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. J Neurocytol. 1978 Aug;7(4):461–478. doi: 10.1007/BF01173991. [DOI] [PubMed] [Google Scholar]
- Schiller P. H. Central connections of the retinal ON and OFF pathways. Nature. 1982 Jun 17;297(5867):580–583. doi: 10.1038/297580a0. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W., Tretter F., Cynader M. Organization of cat striate cortex: a correlation of receptive-field properties with afferent and efferent connections. J Neurophysiol. 1975 Sep;38(5):1080–1098. doi: 10.1152/jn.1975.38.5.1080. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B., Leventhal A. Hierarchical and parallel mechanisms in the organization of visual cortex. Brain Res. 1979 Dec;180(3):345–394. doi: 10.1016/0165-0173(79)90010-9. [DOI] [PubMed] [Google Scholar]
- Stone J., Dreher B. Projection of X- and Y-cells of the cat's lateral geniculate nucleus to areas 17 and 18 of visual cortex. J Neurophysiol. 1973 May;36(3):551–567. doi: 10.1152/jn.1973.36.3.551. [DOI] [PubMed] [Google Scholar]
- Stone J. Morphology and physiology of the geniculocortical synapse in the cat: the question of parallel input to the striate cortex. Invest Ophthalmol. 1972 May;11(5):338–346. [PubMed] [Google Scholar]
- Toyama K., Maekawa K., Takeda T. Convergence of retinal inputs into visual cortical cells: I. A study of the cells monosynaptically excited from the lateral geniculate body. Brain Res. 1977 Dec 2;137(2):207–220. doi: 10.1016/0006-8993(77)90334-1. [DOI] [PubMed] [Google Scholar]
- Toyama K., Matsunami K., Ono T., Tokashiki S. An intracellular study of neuronal organization in the visual cortex. Exp Brain Res. 1974;21(1):45–66. doi: 10.1007/BF00234257. [DOI] [PubMed] [Google Scholar]
- Tsumoto T., Suda K. Three groups of cortico-geniculate neurons and their distribution in binocular and monocular segments of cat striate cortex. J Comp Neurol. 1980 Sep 1;193(1):223–236. doi: 10.1002/cne.901930115. [DOI] [PubMed] [Google Scholar]
- Wickelgren B. G., Sterling P. Influence of visual cortex on receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):16–23. doi: 10.1152/jn.1969.32.1.16. [DOI] [PubMed] [Google Scholar]


