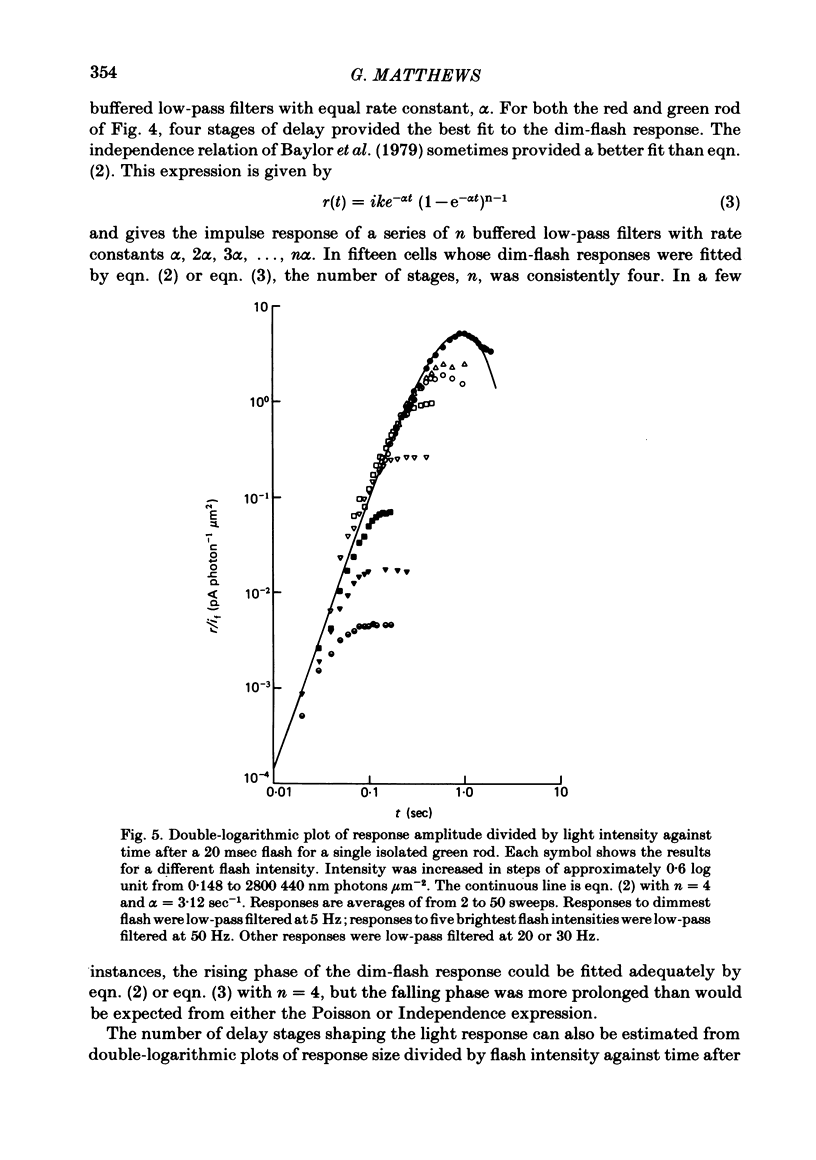
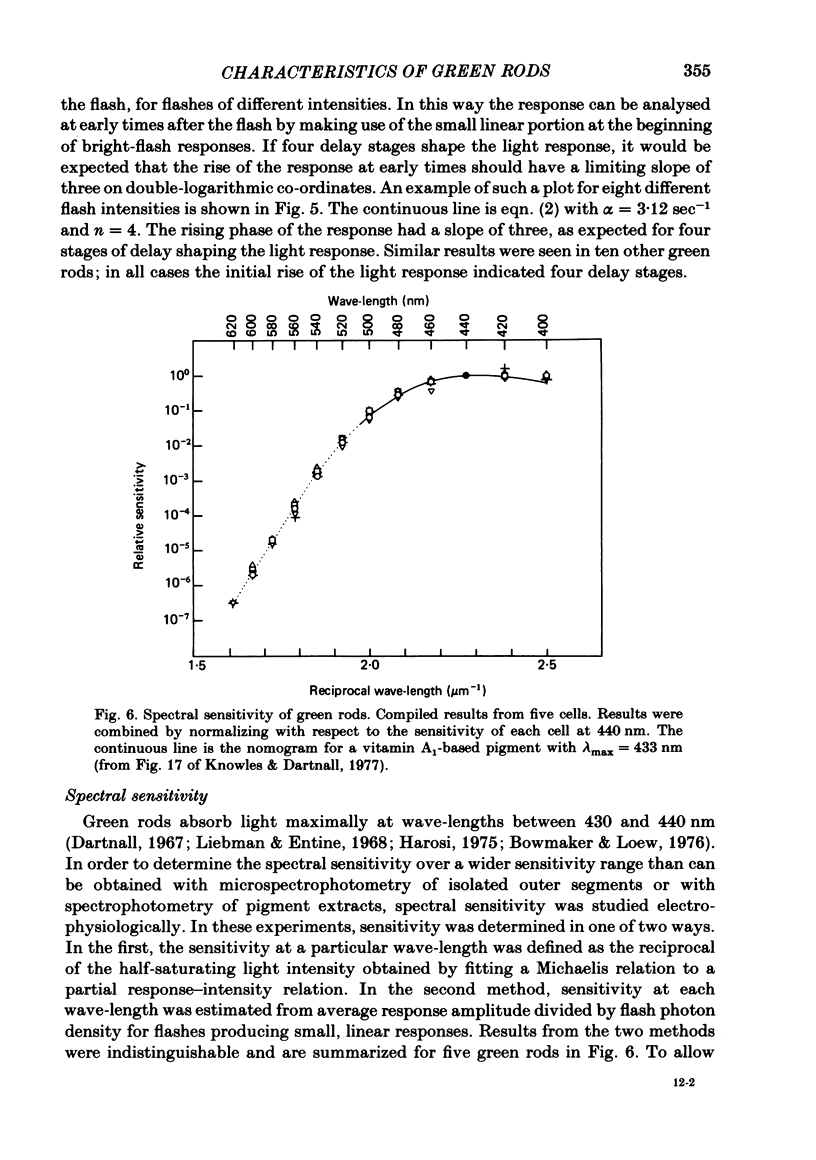
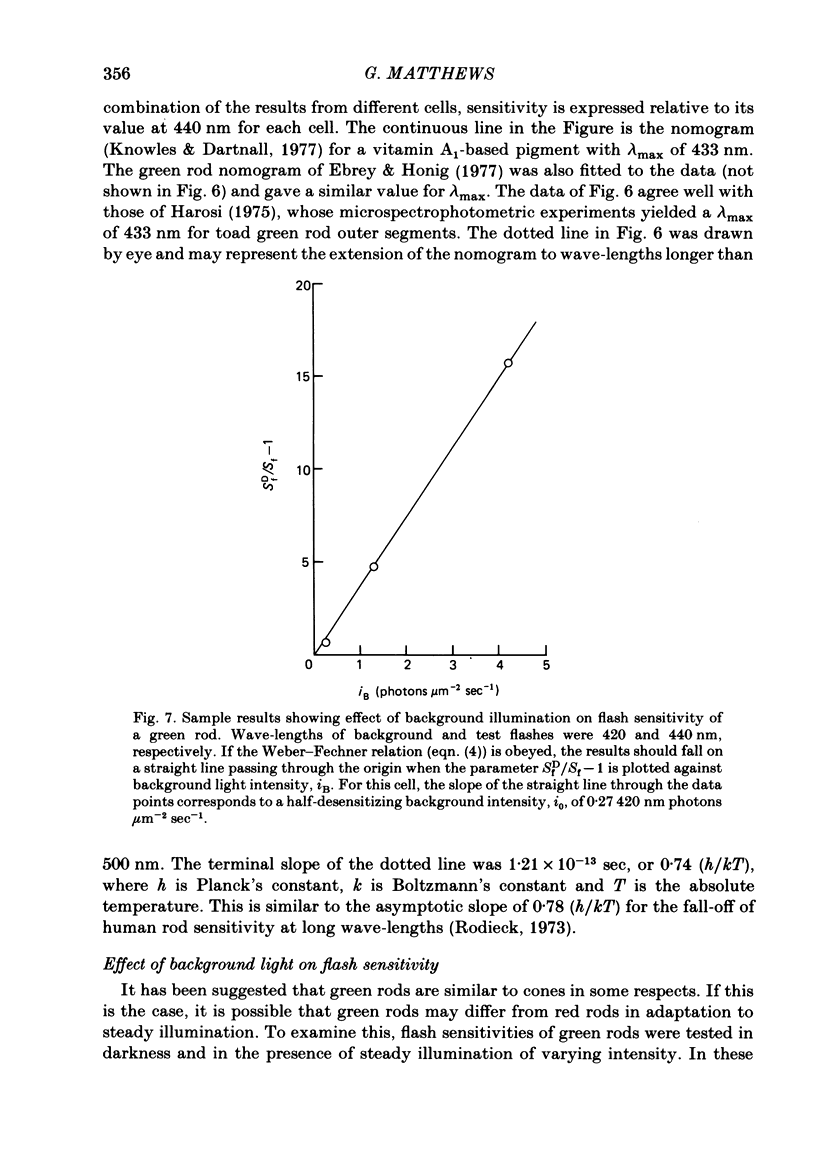
Abstract
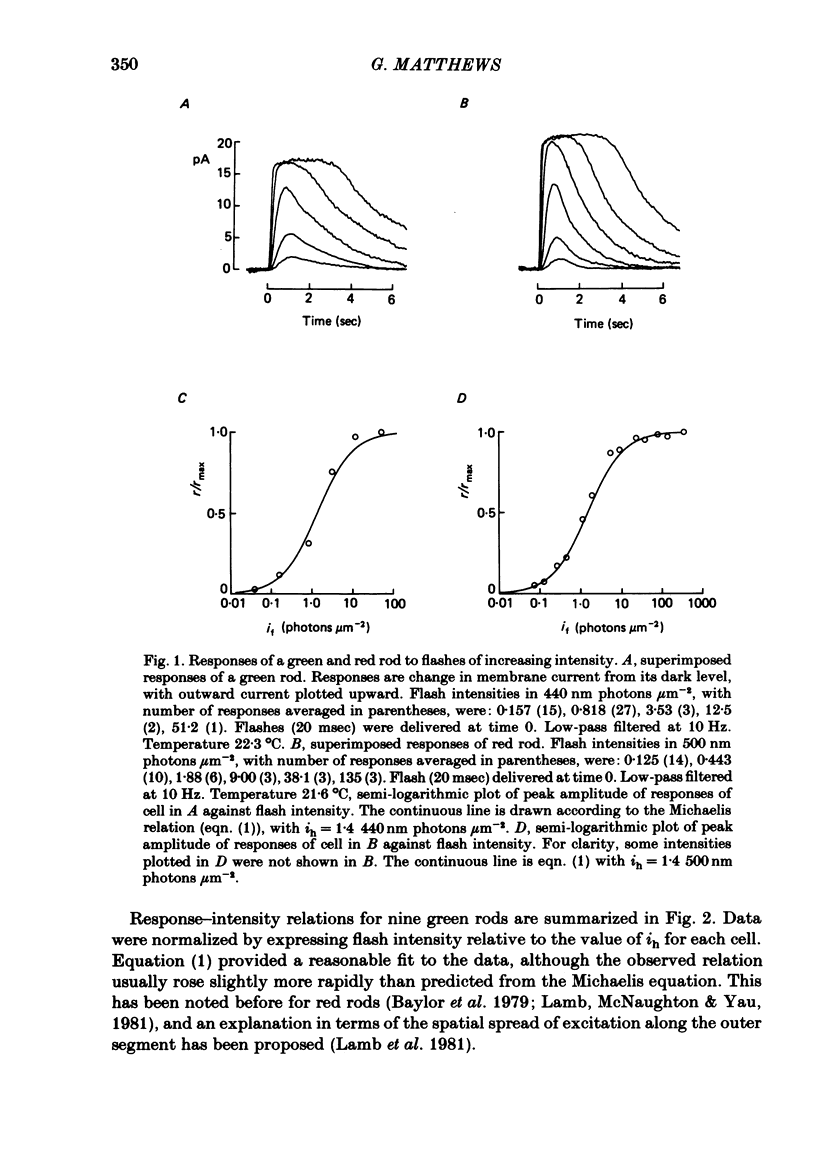
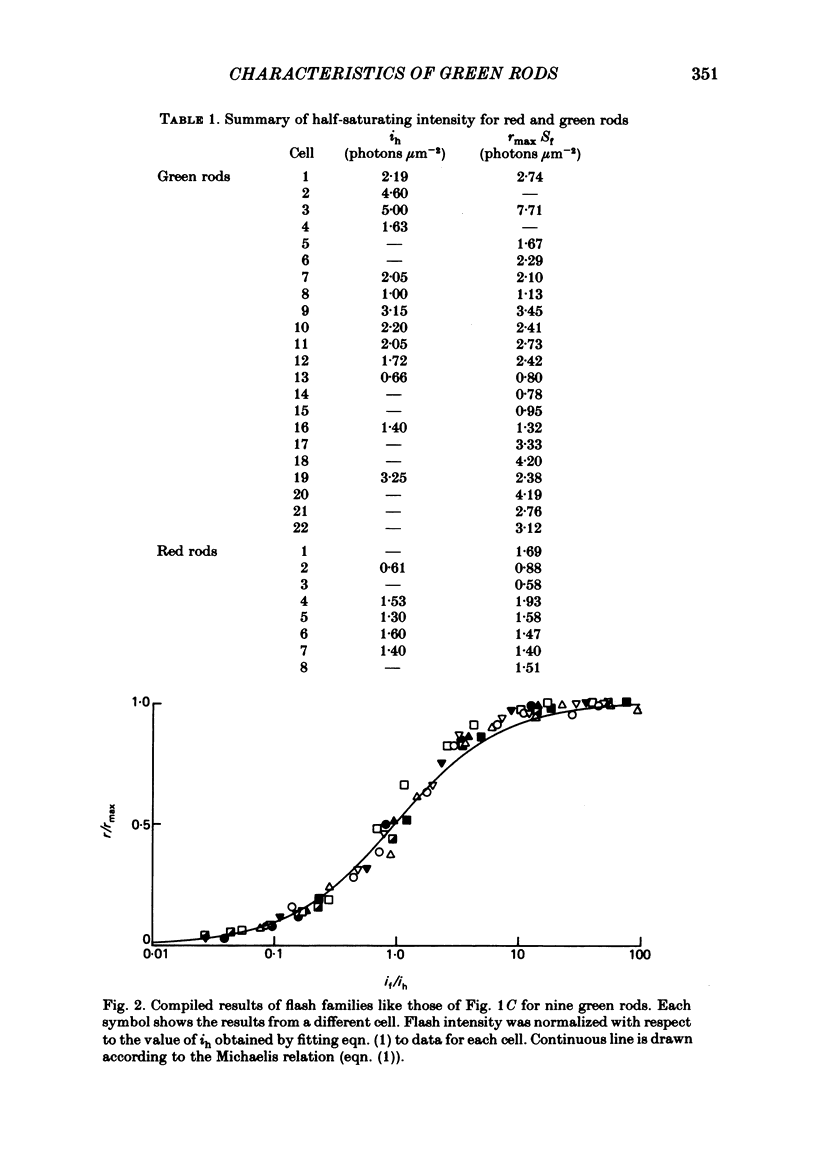
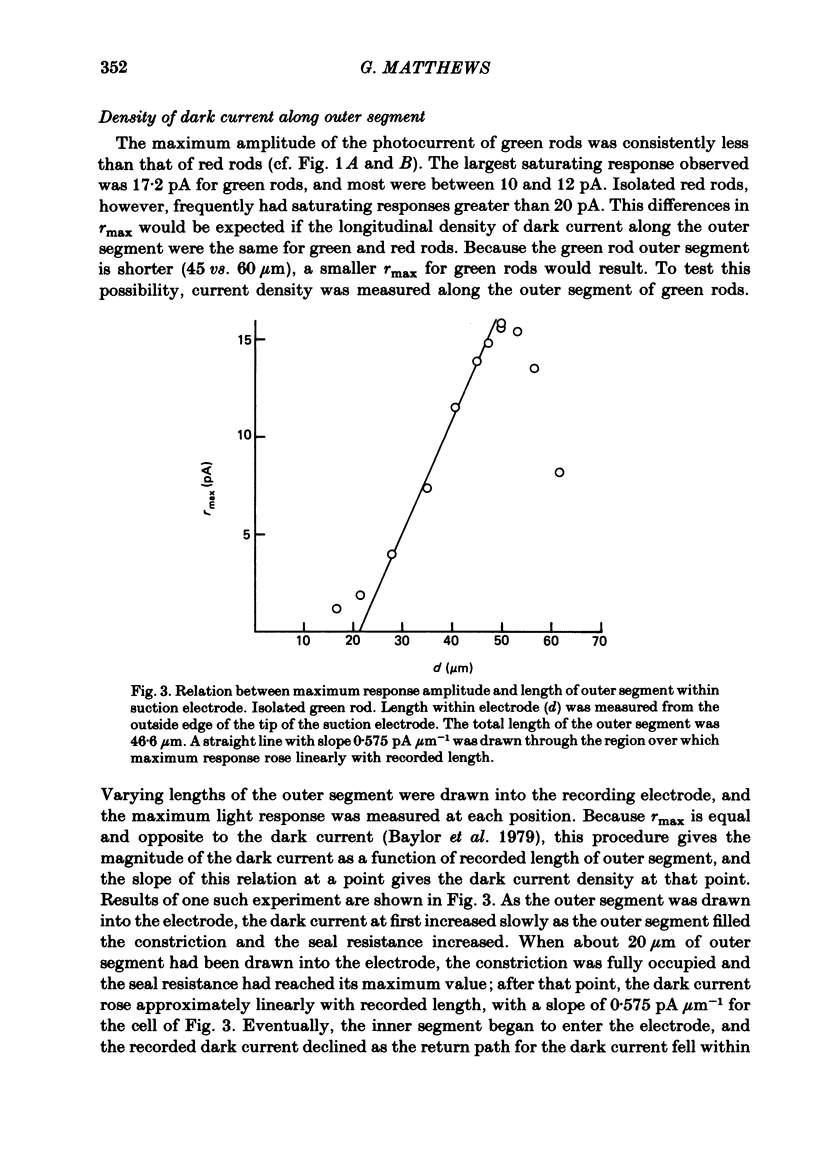
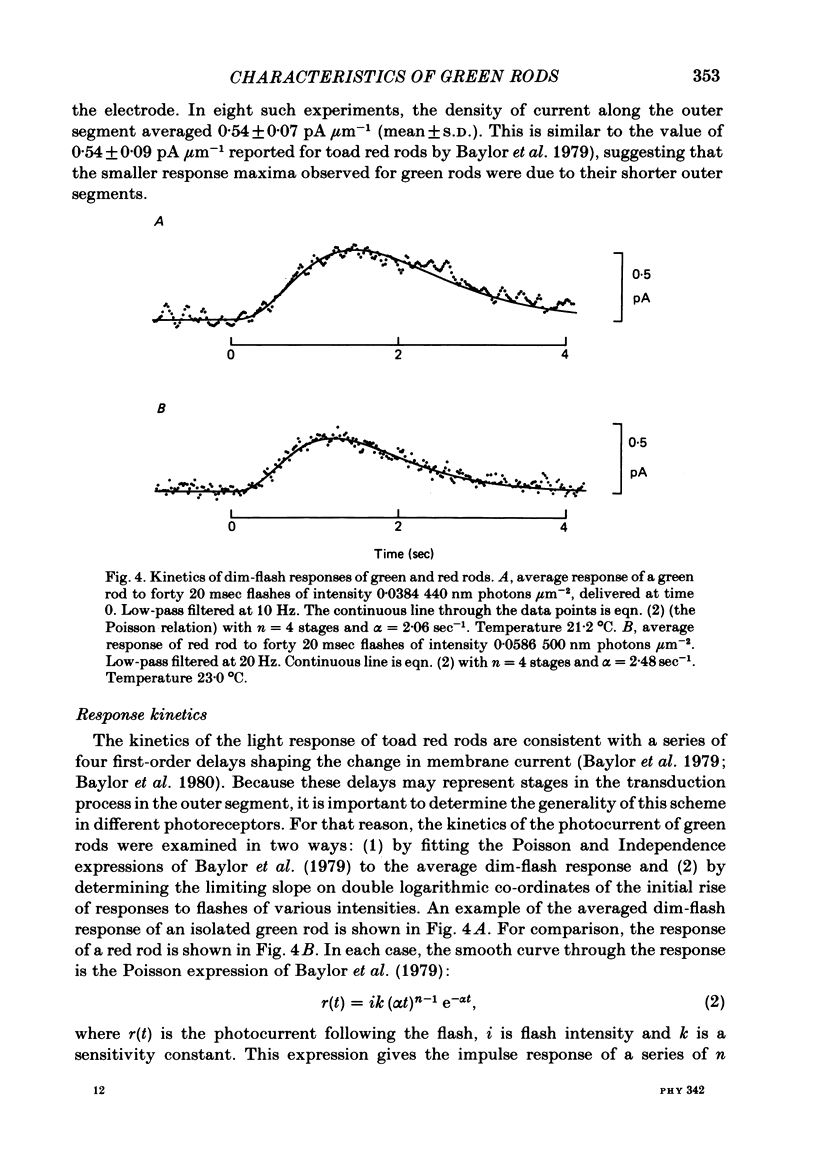
The outer segment membrane current of single isolated green and red rods from toad retina was recorded with a suction electrode, and characteristics of the response to light were examined. The maximum response amplitude of green rods was smaller than that of red rods, but the density of dark current along the green rod outer segment was similar to previously reported values for red rods. Thus, the smaller maximum response is explained by the shorter outer segment of green rods (45 vs. 60 microns). The intensity-response relation was fitted by a Michaelis equation with half-saturating photon density corresponding to about 55 isomerizations per flash. The form of the green rod light response was similar to that of red rods: in both cases the kinetics were consistent with four first-order delay stages shaping the light response. The time-to-peak of the dim-flash response was usually about 1 sec for both green and red rods in the present experiments. The spectral sensitivity curve of green rods was fitted by the nomogram for a vitamin A1-based pigment with lambda max = 433 nm. The relation between steady light intensity and flash sensitivity of green rods obeyed the Weber-Fechner relation, and the average background intensity necessary to reduce sensitivity to half of its dark level corresponded to about 4 isomerizations sec-1. This is slightly lower than the value of about 8 isomerizations sec-1 reported for toad red rods by Baylor, Matthews & Yau (1980). Green rods were similar to red rods in all respects except spectral sensitivity. Thus, no evidence was found to support the assertion that green rods are 'cone-like'.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastian B. L., Fain G. L. Light adaptation in toad rods: requirement for an internal messenger which is not calcium. J Physiol. 1979 Dec;297(0):493–520. doi: 10.1113/jphysiol.1979.sp013053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Hodgkin A. L. Detection and resolution of visual stimuli by turtle photoreceptors. J Physiol. 1973 Oct;234(1):163–198. doi: 10.1113/jphysiol.1973.sp010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowmaker J. K., Loew E. R. The action of hydroxylamine on visual pigments in the intact retina of the frog (Rana temporaria). Vision Res. 1976;16(8):811–818. doi: 10.1016/0042-6989(76)90140-1. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K. Long-lived photoproducts of the green-rod pigment of the frog, Rana temporaria. Vision Res. 1977;17(1):17–23. doi: 10.1016/0042-6989(77)90195-x. [DOI] [PubMed] [Google Scholar]
- DENTON E. J., WYLLIE J. H. Study of the photosensitive pigments in the pink and green rods of the frog. J Physiol. 1955 Jan 28;127(1):81–89. doi: 10.1113/jphysiol.1955.sp005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall H. J. The visual pigment of the green rods. Vision Res. 1967 Jan;7(1):1–16. doi: 10.1016/0042-6989(67)90022-3. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Honig B. New wavelength dependent visual pigment nomograms. Vision Res. 1977;17(1):147–151. doi: 10.1016/0042-6989(77)90213-9. [DOI] [PubMed] [Google Scholar]
- Frank R. N. Electroretinographic response from the green rods of the isolated, perfused frog retina. Vision Res. 1970 Nov;10(11):1101–1107. doi: 10.1016/0042-6989(70)90027-1. [DOI] [PubMed] [Google Scholar]
- Gold G. H. Photoreceptor coupling in retina of the toad, Bufo marinus. II. Physiology. J Neurophysiol. 1979 Jan;42(1 Pt 1):311–328. doi: 10.1152/jn.1979.42.1.311. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I. Absorption spectra and linear dichroism of some amphibian photoreceptors. J Gen Physiol. 1975 Sep;66(3):357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., McNaughton P. A., Yau K. W. Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol. 1981;319:463–496. doi: 10.1113/jphysiol.1981.sp013921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Entine G. Visual pigments of frog and tadpole (Rana pipiens). Vision Res. 1968 Jul;8(7):761–775. doi: 10.1016/0042-6989(68)90128-4. [DOI] [PubMed] [Google Scholar]
- MUNTZ W. R. The development of phototaxis in the frog (Rana temporaria). J Exp Biol. 1963 Jun;40:371–379. doi: 10.1242/jeb.40.2.371. [DOI] [PubMed] [Google Scholar]
- NILSSON S. E. RECEPTOR CELL OUTER SEGMENT DEVELOPMENT AND ULTRASTRUCTURE OF THE DISK MEMBRANES IN THE RETINA OF THE TADPOLE (RANA PIPIENS). J Ultrastruct Res. 1964 Dec;11:581–602. doi: 10.1016/s0022-5320(64)80084-8. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkovsky P., Levine J. S., Engbretson G. A., Hassin G., MacNichol E. F., Jr A microspectrophotometric study of normal and artificial visual pigments in the photoreceptors of Xenopus laevis. Vision Res. 1981;21(6):867–873. doi: 10.1016/0042-6989(81)90187-5. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Yang C. Y., Ripps H. Properties of a blue-sensitive rod in the Xenopus retina. Vision Res. 1981;21(6):875–883. doi: 10.1016/0042-6989(81)90188-7. [DOI] [PubMed] [Google Scholar]


