Abstract
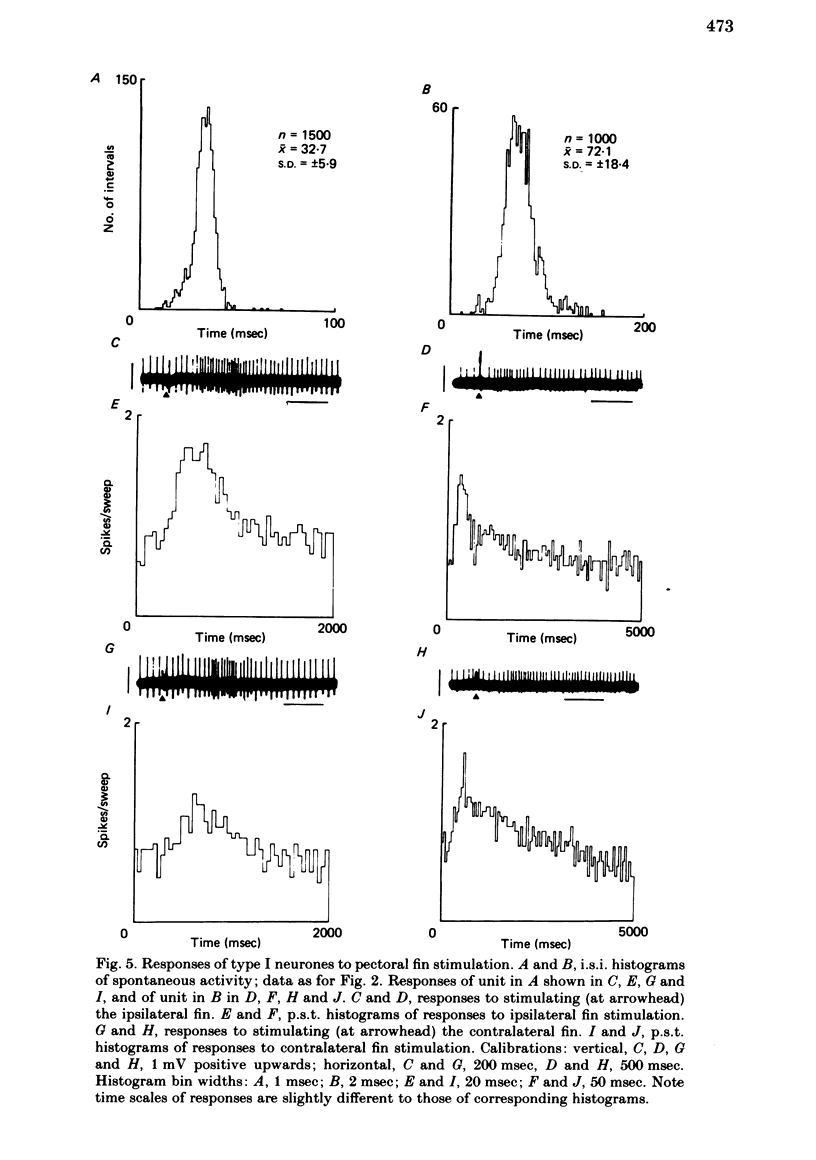
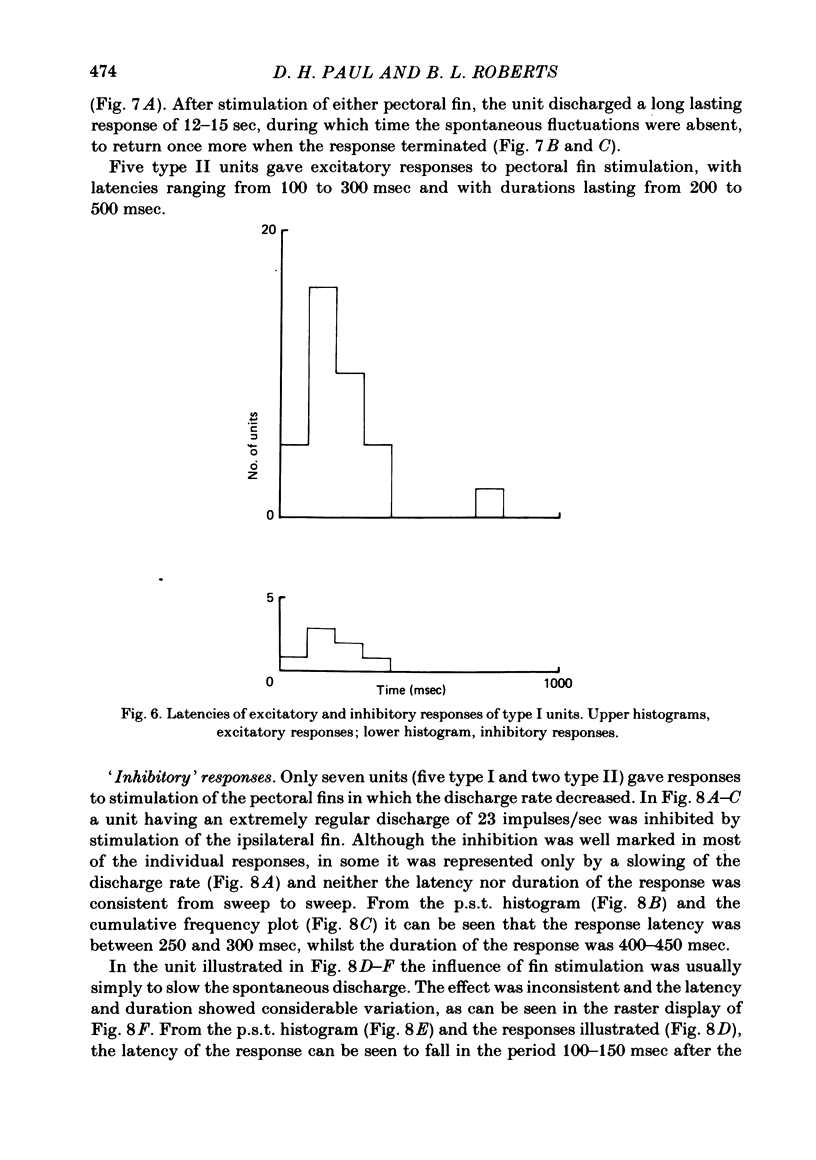
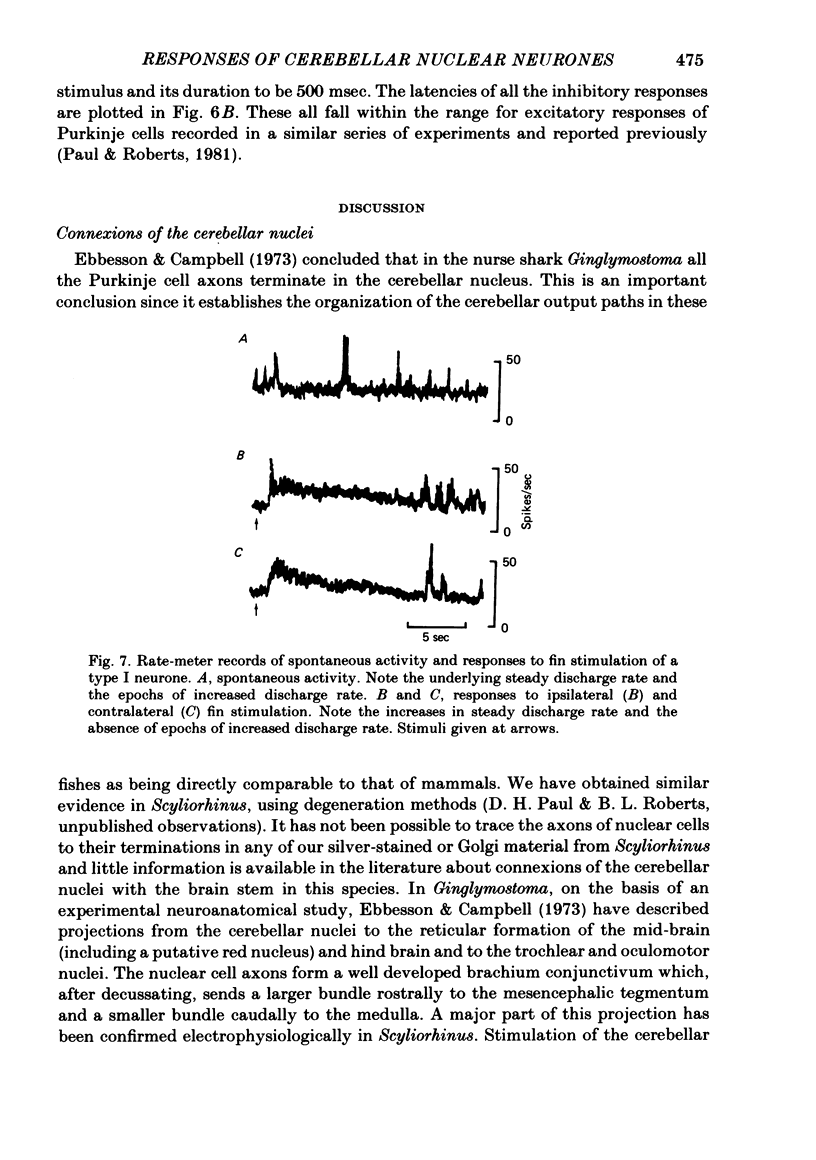
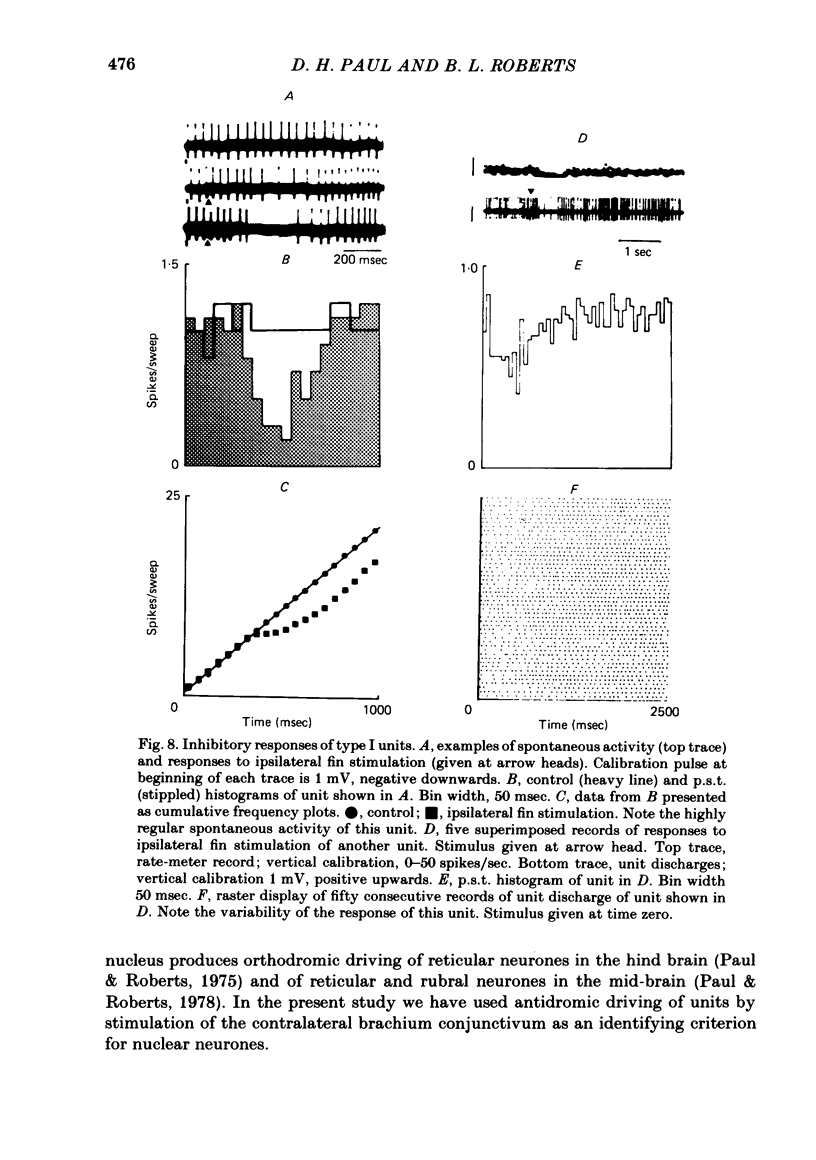
Extracellular single-unit recordings from the cerebellar nucleus were classified into type I and type II units on the basis of their spontaneous discharges. Type I units discharged at a very regular frequency, giving interspike interval histograms with narrow distributions. Type II units had irregular discharges. Type I units were identified as cerebellar nuclear units by their antidromic responses to stimulation of the contralateral brachium conjunctivum (b.c.) in the mid-brain and by their inhibitory responses to stimulation of the cerebellar cortex. Type II units were not driven antidromically by b.c. stimulation but were inhibited by stimulating the cerebellar cortex. Activity of the nuclear neurones was monitored following subcutaneous electrical stimulation of a fin that elicits a reflex elevation. 67% of units responded, the majority with an increased discharge frequency (excitation, 59%) but some with a decreased discharge frequency ('inhibition', 8%). Latencies of both excitatory and inhibitory responses were greater than 50-400 msec. Most excitatory responses lasted for at least 500 msec; several lasted for more than 10 sec. Inhibitory responses lasted for about 500 msec. With units tested by bilateral fin stimulation, the same qualitative response was obtained whichever fin was stimulated. These results are discussed in relation to the known responses of cerebellar Purkinje cells recorded under similar experimental conditions.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amassian V. E., Eberle L., Rudell A. Activity of individual nucleus interpositus projection neurones during maturing forepaw contact placing [proceedings]. J Physiol. 1980 Jan;298:38P–39P. [PubMed] [Google Scholar]
- Antziferova L. I., Arshavsky Y. I., Orlovsky G. N., Pavlova G. A. Activity of neurons of cerebellar nuclei during fictitious scratch reflex in cat. I. Fastigial nucleus. Brain Res. 1980 Nov 3;200(2):239–248. doi: 10.1016/0006-8993(80)90916-6. [DOI] [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Responses of neurones in nucleus interpositus of the cerebellum to cutaneous nerve volleys in the awake cat. J Physiol. 1979 Apr;289:403–423. doi: 10.1113/jphysiol.1979.sp012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshavsky Y. I., Orlovsky G. N., Pavlova G. A., Perret C. Activity of neurons of cerebellar nuclei during fictitious scratch reflex in the cat. II. Interpositus and lateral nuclei. Brain Res. 1980 Nov 3;200(2):249–258. doi: 10.1016/0006-8993(80)90917-8. [DOI] [PubMed] [Google Scholar]
- Bentivoglio M., Kuypers H. G. Divergent axon collaterals from rat cerebellar nuclei to diencephalon, mesencephalon, medulla oblongata and cervical cord. A fluorescent double retrograde labeling study. Exp Brain Res. 1982;46(3):339–356. doi: 10.1007/BF00238629. [DOI] [PubMed] [Google Scholar]
- Bharos T. B., Kuypers H. G., Lemon R. N., Muir R. B. Divergent collaterals from deep cerebellar neurons to thalamus and tectum, and to medulla oblongata and spinal cord: retrograde fluorescent and electrophysiological studies. Exp Brain Res. 1981;42(3-4):399–410. doi: 10.1007/BF00237505. [DOI] [PubMed] [Google Scholar]
- Burton J. E., Onoda N. Dependence of the activity of interpositus and red nucleus neurons on sensory input data generated by movement. Brain Res. 1978 Aug 18;152(1):41–63. doi: 10.1016/0006-8993(78)90133-6. [DOI] [PubMed] [Google Scholar]
- Burton J. E., Onoda N. Interpositus neuron discharge in relation to a voluntary movement. Brain Res. 1977 Jan 31;121(1):167–172. doi: 10.1016/0006-8993(77)90447-4. [DOI] [PubMed] [Google Scholar]
- Ebbesson S. O., Campbell C. B. On the organization of cerebellar efferent pathways in the nurse shark (Ginglymostoma cirratum). J Comp Neurol. 1973 Dec 1;152(3):233–254. doi: 10.1002/cne.901520303. [DOI] [PubMed] [Google Scholar]
- Ebbesson S. O., Hodde K. C. Ascending spinal systems in the nurse shark, Ginglymostoma cirratum. Cell Tissue Res. 1981;216(2):313–331. doi: 10.1007/BF00233622. [DOI] [PubMed] [Google Scholar]
- Grimm R. J., Rushmer D. S. The activity of dentate neurons during an arm movement sequence. Brain Res. 1974 May 17;71(2-3):309–326. doi: 10.1016/0006-8993(74)90974-3. [DOI] [PubMed] [Google Scholar]
- Harvey R. J., Porter R., Rawson J. A. Discharges of intracerebellar nuclear cells in monkeys. J Physiol. 1979 Dec;297(0):559–580. doi: 10.1113/jphysiol.1979.sp013057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayle T. H. A comparative study of spinocerebellar systems in three classes of poikilothermic vertebrates. J Comp Neurol. 1973 Jun 15;149(4):477–496. doi: 10.1002/cne.901490406. [DOI] [PubMed] [Google Scholar]
- Hernandez-Mesa N., Bures J. Skilled forelimb movements and unit activity of cerebellar cortex and dentate nucleus in rats. Physiol Bohemoslov. 1978;27(3):199–208. [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K., Kawai N., Udo M. Inhibitory control of intracerebellar nuclei by the purkinje cell axons. Exp Brain Res. 1970;10(1):64–80. doi: 10.1007/BF00340519. [DOI] [PubMed] [Google Scholar]
- Ito M., Yoshida M., Obata K. Monosynaptic inhibition of the intracerebellar nuclei induced rom the cerebellar cortex. Experientia. 1964 Oct 15;20(10):575–576. doi: 10.1007/BF02150304. [DOI] [PubMed] [Google Scholar]
- Matsushita M., Iwahori N. Structural organization of the fastigial nucleus. I. Dendrites and axonal pathways. Brain Res. 1971 Feb 5;25(3):597–610. doi: 10.1016/0006-8993(71)90463-x. [DOI] [PubMed] [Google Scholar]
- Mortimer J. A. Cerebellar responses to teleceptive stimuli in alert monkeys. Brain Res. 1975 Jan 17;83(3):369–390. doi: 10.1016/0006-8993(75)90831-8. [DOI] [PubMed] [Google Scholar]
- Orlovskii G. N. Rabota neironov mozzhechkovykh iader pri lokomotsii. Biofizika. 1972 Nov-Dec;17(6):1119–1126. [PubMed] [Google Scholar]
- Paul D. H., Roberts B. L. An acute preparation for the study of cerebellar activity during movement in dogfish [proceedings]. J Physiol. 1979 Feb;287:3P–3P. [PubMed] [Google Scholar]
- Paul D. H., Roberts B. L. Organization of the reticular formation in the dogfish Scyliorhinus canicula [proceedings]. J Physiol. 1978 Jul;280:71P–72P. [PubMed] [Google Scholar]
- Paul D. H., Roberts B. L. Proceedings: Connexions between the cerebellum and the reticular formation in the dogfish Scyliorhinus canicula. J Physiol. 1975 Jul;249(1):62P–63P. [PubMed] [Google Scholar]
- Paul D. H., Roberts B. L. Proceedings: Responses of cerebellar nuclear neurones in the elasmobranch fish Scyliorhinus canicula. J Physiol. 1976 May;257(1):43P–44P. [PubMed] [Google Scholar]
- Paul D. H., Roberts B. L. Studies on a primitive cerebellar cortex. I. The anatomy of the lateral-line lobes of the dogfish, Scyliorhinus canicula. Proc R Soc Lond B Biol Sci. 1977 Feb 11;195(1121):453–466. doi: 10.1098/rspb.1977.0020. [DOI] [PubMed] [Google Scholar]
- Paul D. H., Roberts B. L. Studies on a primitive cerebellar cortex. II. The projection of the posterior lateral-line nerve to the lateral-line lobes of the dogfish brain. Proc R Soc Lond B Biol Sci. 1977 Feb 11;195(1121):467–478. doi: 10.1098/rspb.1977.0021. [DOI] [PubMed] [Google Scholar]
- Paul D. H., Roberts B. L. The activity of cerebellar neurones of an elasmobranch fish (Scyliorhinus canicula) during a reflex movement of a fin. J Physiol. 1981 Dec;321:369–383. doi: 10.1113/jphysiol.1981.sp013990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson L. T., Grimm R. J. Responses of primate dentate neurons to different trajectories of the limb. Exp Brain Res. 1975 Nov 14;23(5):447–462. doi: 10.1007/BF00234914. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Correlation of neural discharge with pattern and force of muscular activity, joint position, and direction of intended next movement in motor cortex and cerebellum. J Neurophysiol. 1978 May;41(3):654–676. doi: 10.1152/jn.1978.41.3.654. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of Purkinje and cerebellar nuclear neurons during rapidly alternating arm movements in the monkey. J Neurophysiol. 1968 Sep;31(5):785–797. doi: 10.1152/jn.1968.31.5.785. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol. 1970 Jul;33(4):527–536. doi: 10.1152/jn.1970.33.4.527. [DOI] [PubMed] [Google Scholar]
- Thach W. T. Timing of activity in cerebellar dentate nucleus and cerebral motor cortex during prompt volitional movement. Brain Res. 1975 May 2;88(2):233–241. doi: 10.1016/0006-8993(75)90387-x. [DOI] [PubMed] [Google Scholar]


