Abstract
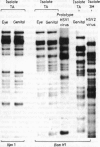
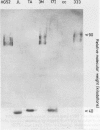
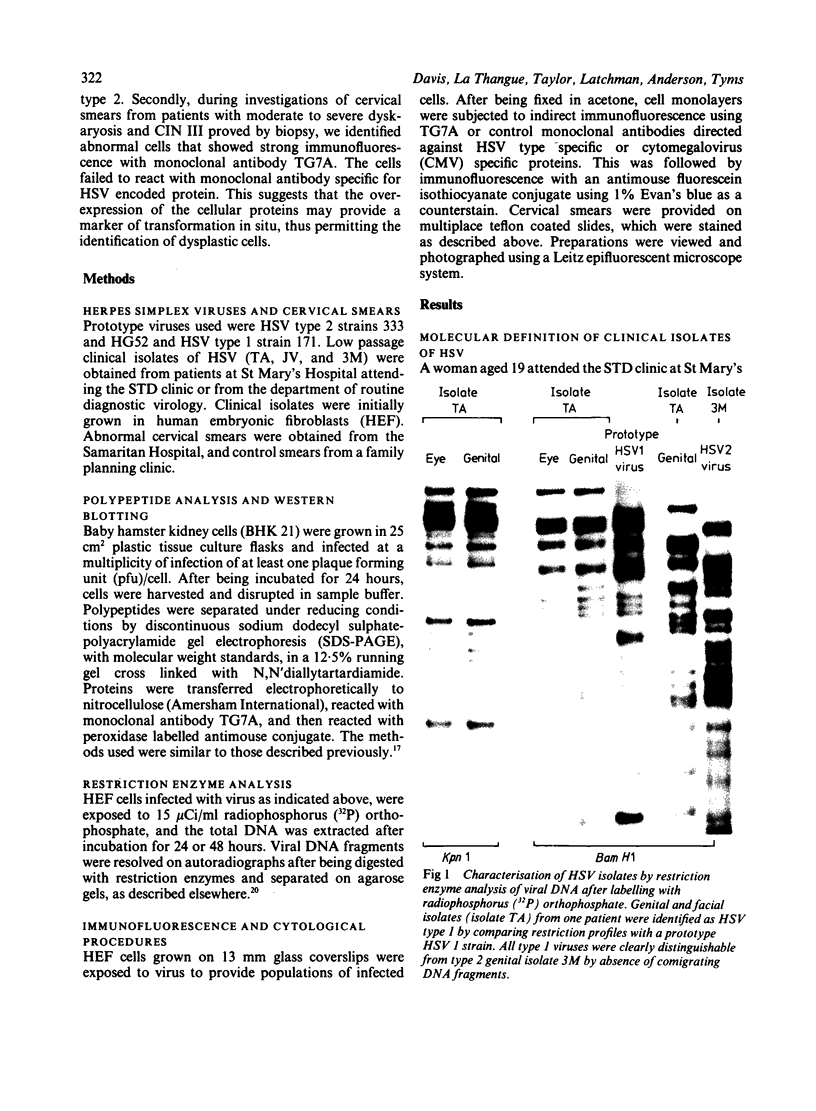
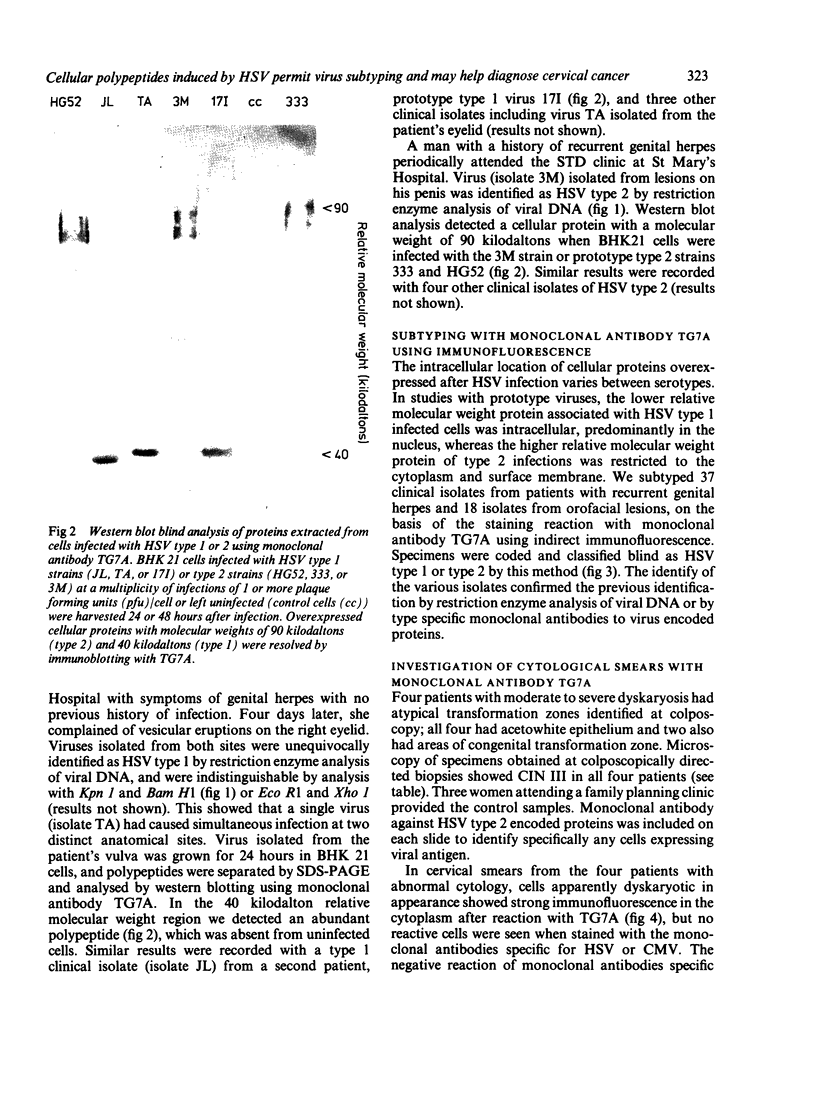
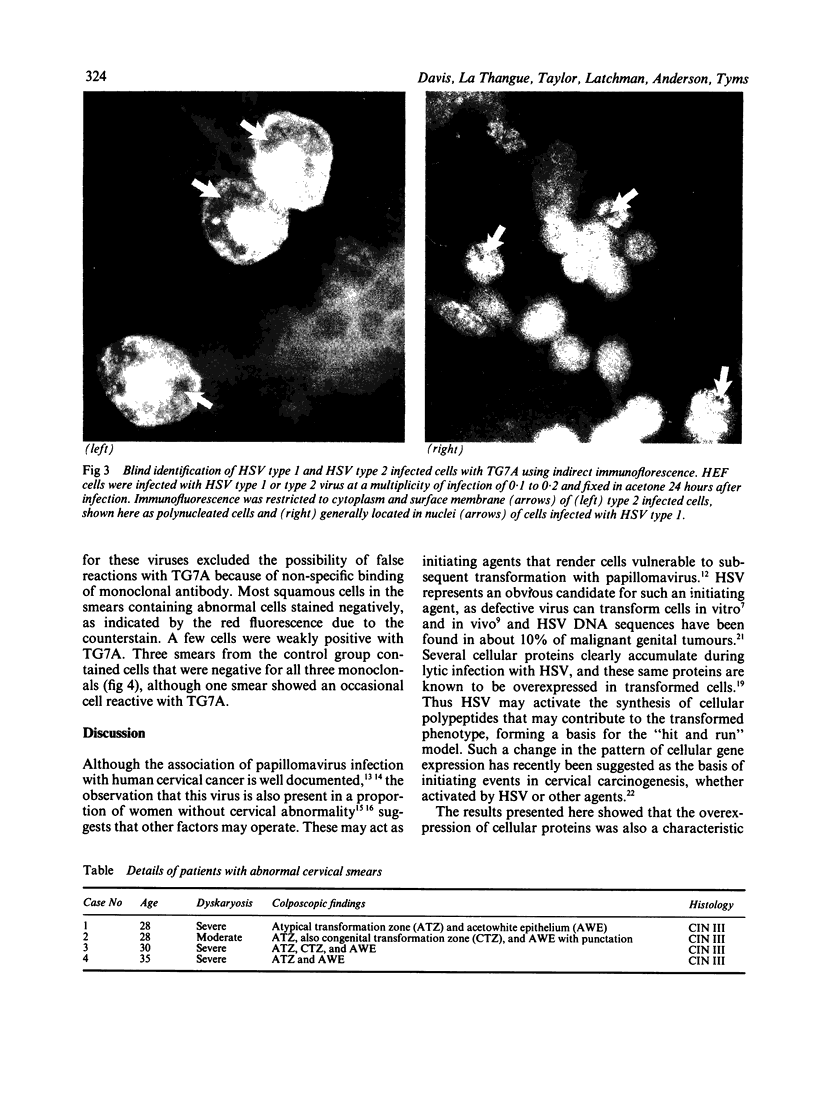
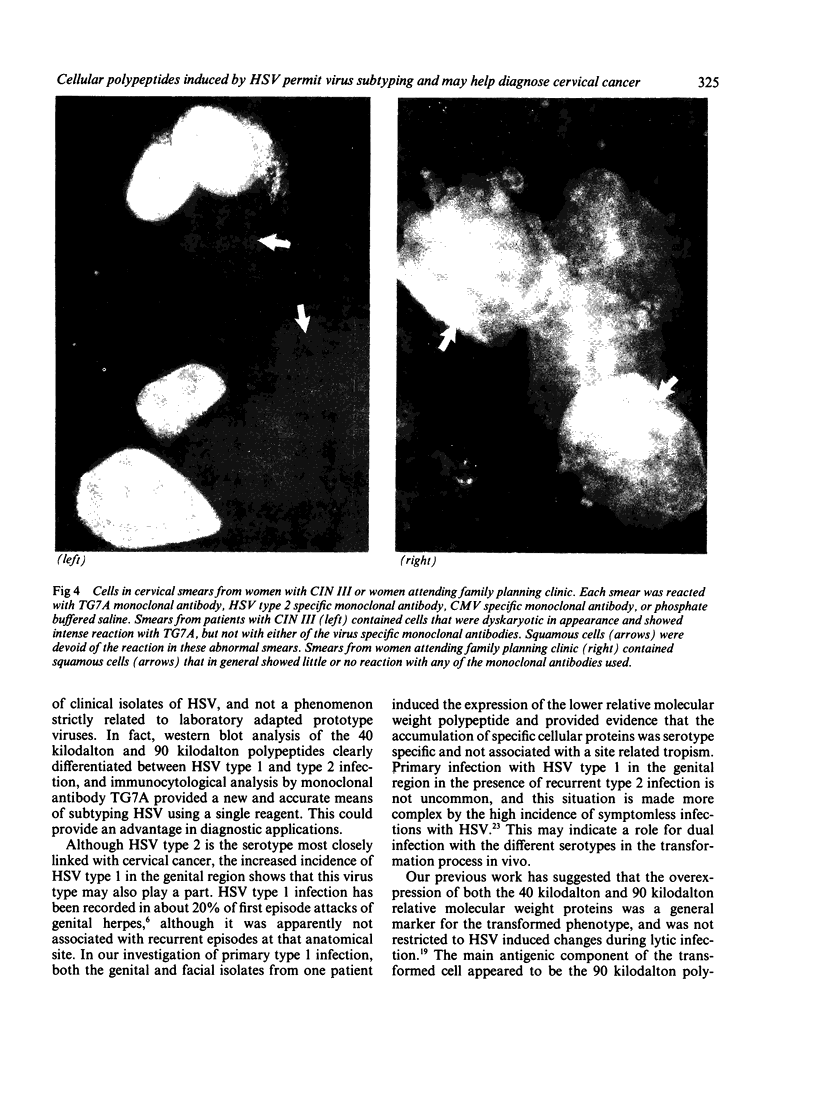
The synthesis of cellular macromolecules is inhibited after infection with herpes simplex viruses (HSV) although certain host proteins accumulate to high concentrations as identified by monoclonal antibody TG7A. By western blotting, a polypeptide with a relative molecular weight of 90 kilodaltons was identified in cells infected with type 2 viruses and a polypeptide of 40 kilodaltons relative molecular weight in type 1 infected cells, and virus typing was confirmed by restriction enzyme analysis of viral DNA. Thirty seven clinical isolates from the genital region were subtyped as HSV type 2 and 18 from the orofacial region as type 1 by the different intracellular location of the 90 kilodalton and 40 kilodalton proteins seen on immunofluorescent staining of cells infected with HSV. Expression of these proteins has been associated with cellular transformation due to gene products of HSV or other viruses. Overexpression of the cellular proteins identified by TG7A reactivity was shown to be a marker for cells in cervical smears from patients with CIN III that appeared to be dyskaryotic. Little or no reaction was observed in squamous epithelial cells found in normal or abnormal smears.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boshart M., Gissmann L., Ikenberg H., Kleinheinz A., Scheurlen W., zur Hausen H. A new type of papillomavirus DNA, its presence in genital cancer biopsies and in cell lines derived from cervical cancer. EMBO J. 1984 May;3(5):1151–1157. doi: 10.1002/j.1460-2075.1984.tb01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman T. G., Simpson T., Nosal C., Roizman B., Nahmias A. J. The structure of herpes simplex virus DNA and its application to molecular epidemiology. Ann N Y Acad Sci. 1980;354:279–290. doi: 10.1111/j.1749-6632.1980.tb27972.x. [DOI] [PubMed] [Google Scholar]
- Burns J. C., Murray B. K. Conversion of herpetic lesions to malignancy by ultraviolet exposure and promoter application. J Gen Virol. 1981 Aug;55(Pt 2):305–313. doi: 10.1099/0022-1317-55-2-305. [DOI] [PubMed] [Google Scholar]
- Chaney S. M., Warren K. G., Kettyls J., Zbitnue A., Subak-Sharpe J. H. A comparative analysis of restriction enzyme digests of the DNA of herpes simplex virus isolated from genital and facial lesions. J Gen Virol. 1983 Feb;64(Pt 2):357–371. doi: 10.1099/0022-1317-64-2-357. [DOI] [PubMed] [Google Scholar]
- Cox M. F., Meanwell C. A., Maitland N. J., Blackledge G., Scully C., Jordan J. A. Human papillomavirus type-16 homologous DNA in normal human ectocervix. Lancet. 1986 Jul 19;2(8499):157–158. doi: 10.1016/s0140-6736(86)91965-3. [DOI] [PubMed] [Google Scholar]
- Davis J. M., Jeffries D. J., Tyms A. S., Walker D. Molecular biology in viral diagnosis: restriction enzyme analysis of viruses from recurrent genital herpes infections. Analyst. 1985 Jun;110(6):605–609. doi: 10.1039/an9851000605. [DOI] [PubMed] [Google Scholar]
- Duff R., Rapp F. Oncogenic transformation of hamster cells after exposure to herpes simplex virus type 2. Nat New Biol. 1971 Sep 8;233(36):48–50. doi: 10.1038/newbio233048a0. [DOI] [PubMed] [Google Scholar]
- Dundarov S., Andonov P., Bakalov B. Characterization of herpes simplex virus strains isolated from patients with various diseases. Arch Virol. 1980;63(2):115–121. doi: 10.1007/BF01320768. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Thangue N. B., Latchman D. S. A cellular protein related to heat-shock protein 90 accumulates during herpes simplex virus infection and is overexpressed in transformed cells. Exp Cell Res. 1988 Sep;178(1):169–179. doi: 10.1016/0014-4827(88)90388-6. [DOI] [PubMed] [Google Scholar]
- LaThangue N. B., Shriver K., Dawson C., Chan W. L. Herpes simplex virus infection causes the accumulation of a heat-shock protein. EMBO J. 1984 Feb;3(2):267–277. doi: 10.1002/j.1460-2075.1984.tb01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNab J. C. Tumour production by HSV-2 transformed lines in rats and the varying response to immunosuppression. J Gen Virol. 1979 Apr;43(1):39–56. doi: 10.1099/0022-1317-43-1-39. [DOI] [PubMed] [Google Scholar]
- Macnab J. C. Herpes simplex virus and human cytomegalovirus: their role in morphological transformation and genital cancers. J Gen Virol. 1987 Oct;68(Pt 10):2525–2550. doi: 10.1099/0022-1317-68-10-2525. [DOI] [PubMed] [Google Scholar]
- Macnab J. C., Orr A., La Thangue N. B. Cellular proteins expressed in herpes simplex virus transformed cells also accumulate on herpes simplex virus infection. EMBO J. 1985 Dec 1;4(12):3223–3228. doi: 10.1002/j.1460-2075.1985.tb04069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meanwell C. A., Cox M. F., Blackledge G., Maitland N. J. HPV 16 DNA in normal and malignant cervical epithelium: implications for the aetiology and behaviour of cervical neoplasia. Lancet. 1987 Mar 28;1(8535):703–707. doi: 10.1016/s0140-6736(87)90353-9. [DOI] [PubMed] [Google Scholar]
- Mertz G. J., Schmidt O., Jourden J. L., Guinan M. E., Remington M. L., Fahnlander A., Winter C., Holmes K. K., Corey L. Frequency of acquisition of first-episode genital infection with herpes simplex virus from symptomatic and asymptomatic source contacts. Sex Transm Dis. 1985 Jan-Mar;12(1):33–39. doi: 10.1097/00007435-198501000-00007. [DOI] [PubMed] [Google Scholar]
- Park M., Kitchener H. C., Macnab J. C. Detection of herpes simplex virus type-2 DNA restriction fragments in human cervical carcinoma tissue. EMBO J. 1983;2(7):1029–1034. doi: 10.1002/j.1460-2075.1983.tb01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer G., Goodheart C. R., Miyagi M., Skinner G. R., Thouless M. E., Wildy P. Herpes simplex viruses: discrimination of types and correlation between different characteristics. Virology. 1974 Jul;60(1):206–216. doi: 10.1016/0042-6822(74)90378-x. [DOI] [PubMed] [Google Scholar]
- Schmidt O. W., Fife K. H., Corey L. Reinfection is an uncommon occurrence in patients with symptomatic recurrent genital herpes. J Infect Dis. 1984 Apr;149(4):645–646. doi: 10.1093/infdis/149.4.645. [DOI] [PubMed] [Google Scholar]
- Warren K. G., Koprowski H., Lonsdale D. M., Brown S. M., Subak-Sharpe J. H. The polypeptide and the DNA restriction enzyme profiles of spontaneous isolates of herpes simplex virus type 1 from explants of human trigeminal, superior cervical and vagus ganglia. J Gen Virol. 1979 Apr;43(1):151–171. doi: 10.1099/0022-1317-43-1-151. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Human genital cancer: synergism between two virus infections or synergism between a virus infection and initiating events? Lancet. 1982 Dec 18;2(8312):1370–1372. doi: 10.1016/s0140-6736(82)91273-9. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Intracellular surveillance of persisting viral infections. Human genital cancer results from deficient cellular control of papillomavirus gene expression. Lancet. 1986 Aug 30;2(8505):489–491. doi: 10.1016/s0140-6736(86)90360-0. [DOI] [PubMed] [Google Scholar]