Abstract
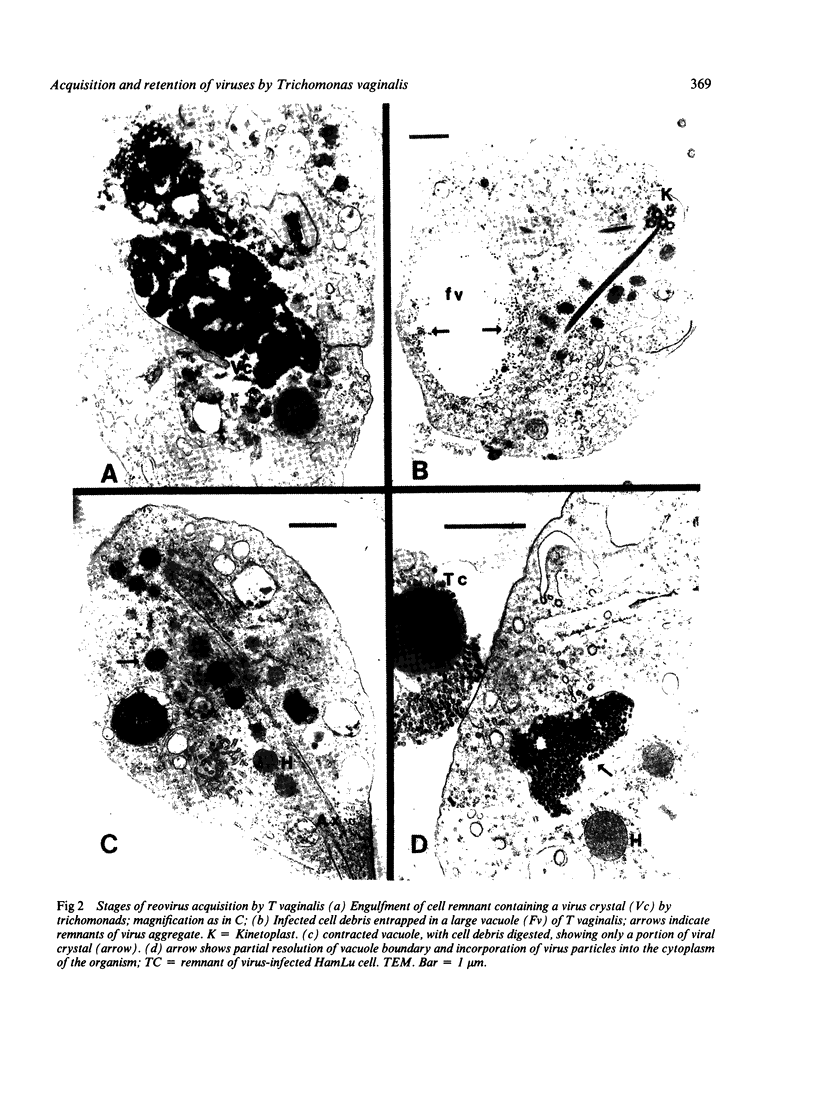
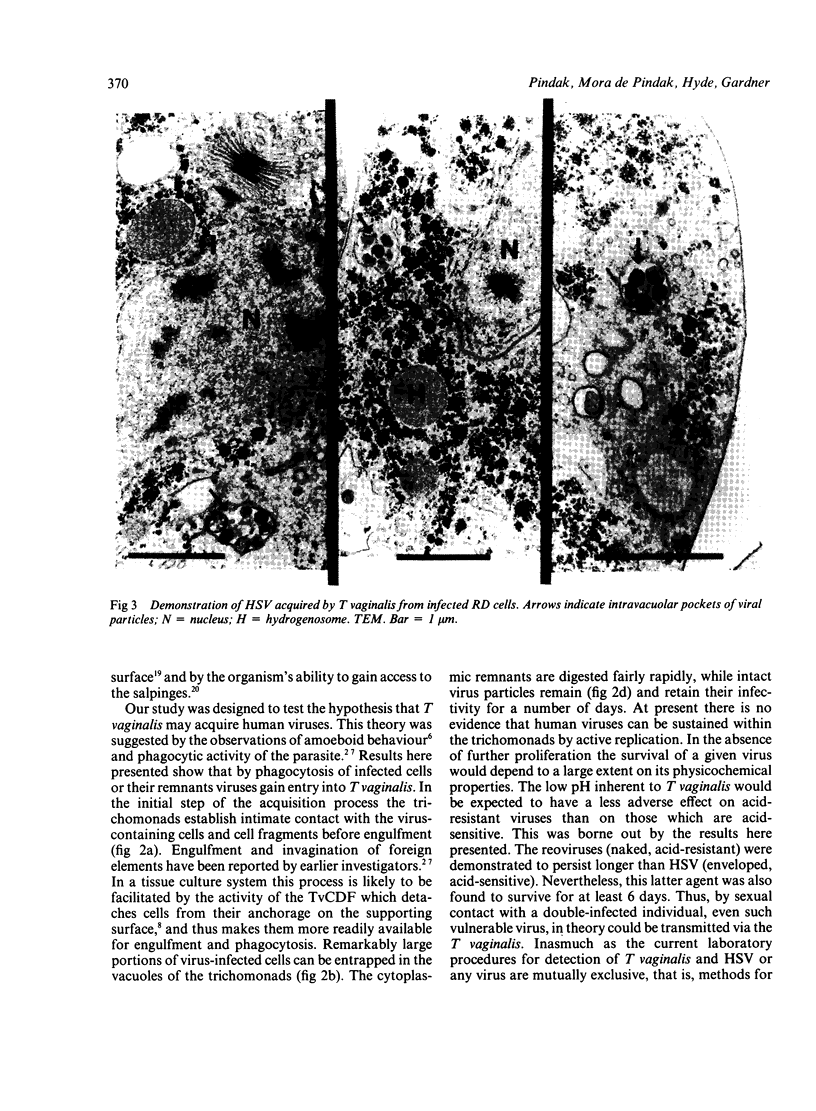
Recently described occurrence of virus-like particles (VLP) in some strains of Trichomonas vaginalis suggests the possibility that the pathogenic significance of this organism may be broadened by its potential for viral transmission. Inasmuch as neither the source nor the host range of the VLP are known, any hazard which they may present for man cannot be estimated. A model has been established for the study of acquisition of known human viruses by T vaginalis. Tissue cultures were infected with two reoviruses and a fresh isolate of genital herpes simplex virus (HSV). A squirrel monkey reovirus was also included in the study. T vaginalis was inoculated into the virus cultures three days later. The progress of virus acquisition by the trichomonads was monitored by transmission electron microscopy and by culture. Virus-containing cell fragments were engulfed by trichomonads and internalised in vacuoles. After digestion of cellular debris only virus particle aggregates were retained. Viable reoviruses were recovered from the trichomonads for nine days, and HSV for six days. The results suggest the possibility of transmission of at least some viruses by T vaginalis.
Full text
PDF

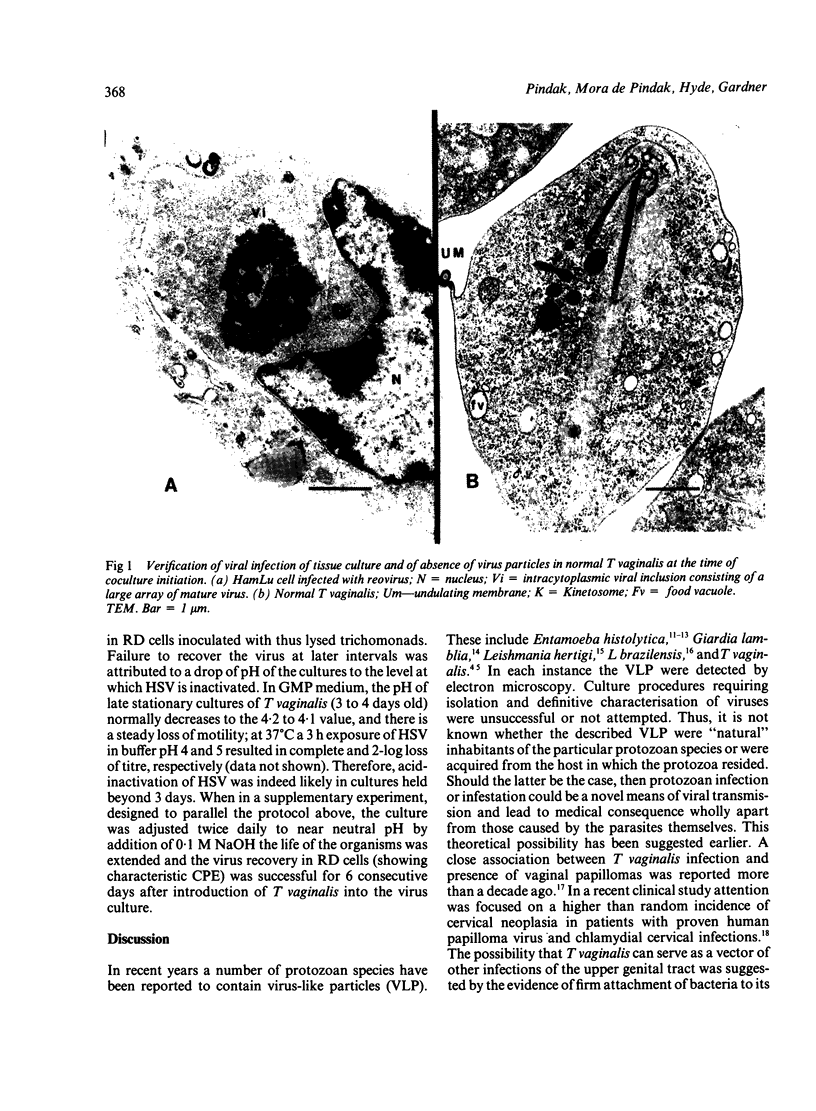

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allerding T. J., Jordan S. W., Boardman R. E. Association of human papillomavirus and Chlamydia infections with incidence cervical neoplasia. Acta Cytol. 1985 Sep-Oct;29(5):653–660. [PubMed] [Google Scholar]
- Bird R. G., McCaul T. F., Knight R. Proceedings: Rhabdo-virus like particles of Entamoeba histolytica. Trans R Soc Trop Med Hyg. 1974;68(1):2–2. doi: 10.1016/0035-9203(74)90214-4. [DOI] [PubMed] [Google Scholar]
- DIAMOND L. S. The establishment of various trichomonads of animals and man in axenic cultures. J Parasitol. 1957 Aug;43(4):488–490. [PubMed] [Google Scholar]
- Eley S. M., Molyneux D. H., Moore N. F. Investigation of virus-like particles in Leishmania hertigi. Microbios. 1987;51(208-209):145–149. [PubMed] [Google Scholar]
- Feely D. E., Chase D. G., Hardin E. L., Erlandsen S. L. Ultrastructural evidence for the presence of bacteria, viral-like particles, and mycoplasma-like organisms associated with Giardia spp. J Protozool. 1988 Feb;35(1):151–158. doi: 10.1111/j.1550-7408.1988.tb04095.x. [DOI] [PubMed] [Google Scholar]
- Francioli P., Shio H., Roberts R. B., Müller M. Phagocytosis and killing of Neisseria gonorrhoeae by Trichomonas vaginalis. J Infect Dis. 1983 Jan;147(1):87–94. doi: 10.1093/infdis/147.1.87. [DOI] [PubMed] [Google Scholar]
- Heath J. P. Behaviour and pathogenicity of Trichomonas vaginalis in epithelial cell cultures: a study by light and scanning electron microscopy. Br J Vener Dis. 1981 Apr;57(2):106–117. doi: 10.1136/sti.57.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith L. G., Friberg J., Fullan N., Bailey R., Berger G. S. The possible role of Trichomonas vaginalis as a "vector" for the spread of other pathogens. Int J Fertil. 1986 Sep-Oct;31(4):272–277. [PubMed] [Google Scholar]
- Knight R., Bird R. G., McCaul T. F. Fine structural changes at Entamoeba histolytica rabbit kidney cell (RK 13) interface. Ann Trop Med Parasitol. 1975 Jun;69(2):197–202. doi: 10.1080/00034983.1975.11687001. [DOI] [PubMed] [Google Scholar]
- Mattern C. F., Diamond L. S., Daniel W. A. Viruses of Entamoeba histolytica. II. Morphogenesis of the polyhedral particle (ABRM 2 leads to HK-9) leads to HB-301 and the filamentous agent (ABRM) 2 leads to HK-9. J Virol. 1972 Feb;9(2):342–358. doi: 10.1128/jvi.9.2.342-358.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Weström L. Tubal and cervical cultures in acute salpingitis with special reference to Mycoplasma hominis and T-strain mycoplasmas. Br J Vener Dis. 1970 Jun;46(3):179–186. [PMC free article] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V., Turanova E. N., Yashkova G. N. Further studies of Trichomonas Vaginalis with transmission and scanning electron microscopy. Br J Vener Dis. 1975 Dec;51(6):357–375. doi: 10.1136/sti.51.6.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pindak F. F., Gardner W. A., Jr, Mora de Pindak M. Growth and cytopathogenicity of Trichomonas vaginalis in tissue cultures. J Clin Microbiol. 1986 Apr;23(4):672–678. doi: 10.1128/jcm.23.4.672-678.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgerson E. B. Vulvovaginal papillomas and trichomonas vaginalis. Obstet Gynecol. 1972 Sep;40(3):327–333. [PubMed] [Google Scholar]
- Scholtyseck E., Teras J., Kasakova I., Sethi K. K. Electron microscope observations on the interaction of Mycoplasma fermentans with Trichomonas vaginalis. Z Parasitenkd. 1985;71(4):435–442. doi: 10.1007/BF00928346. [DOI] [PubMed] [Google Scholar]
- Tarr P. I., Aline R. F., Jr, Smiley B. L., Scholler J., Keithly J., Stuart K. LR1: a candidate RNA virus of Leishmania. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9572–9575. doi: 10.1073/pnas.85.24.9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7956–7960. doi: 10.1073/pnas.83.20.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Wang C. C., Alderete J. F. Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J Exp Med. 1987 Jul 1;166(1):142–150. doi: 10.1084/jem.166.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]