Abstract
TGF-β is a ubiquitously expressed cytokine that signals through the Smad proteins to regulate many diverse cellular processes. SnoN is an important negative regulator of Smad signaling. It has been described as a nuclear protein, based on studies of ectopically expressed SnoN and endogenous SnoN in cancer cell lines. In the nucleus, SnoN binds to Smad2, Smad3, and Smad4 and represses their ability to activate transcription of TGF-β target genes through multiple mechanisms. Here, we show that, whereas SnoN is localized exclusively in the nucleus in cancer tissues or cells, in normal tissues and nontumorigenic or primary epithelial cells, SnoN is predominantly cytoplasmic. Upon morphological differentiation or cell-cycle arrest, SnoN translocates into the nucleus. In contrast to nuclear SnoN that represses the transcriptional activity of the Smad complexes, cytoplasmic SnoN antagonizes TGF-β signaling by sequestering the Smad proteins in the cytoplasm. Interestingly, cytoplasmic SnoN is resistant to TGF-β-induced degradation and therefore is more potent than nuclear SnoN in repressing TGF-β signaling. Thus, we have identified a mechanism of regulation of TGF-β signaling via differential subcellular localization of SnoN that is likely to produce different patterns of downstream TGF-β responses and may influence the proliferation or differentiation states of epithelial cells.
Keywords: intracellular localization, signal transduction, differentiation, mammary epithelial cells
TGF-β regulates a wide array of cellular activities through the Smad proteins (1, 2). Upon phosphorylation by the TGF-β receptor kinases, Smad2 and Smad3 oligomerize with Smad4, translocate into the nucleus, and regulate expression of TGF-β-responsive genes (3–5). The activity of the Smad complexes can be negatively regulated by SnoN, which is a member of the Ski family of protooncoproteins that are involved in regulation of cellular transformation and differentiation (6, 7). In the nucleus, SnoN binds to Smad2, Smad3, and Smad4 on TGF-β-responsive promoters; also, it represses their ability to activate expression of TGF-β target genes by disrupting active heteromeric complexes of Smad2 or Smad3 with Smad4, by recruiting a transcriptional repressor complex containing N-CoR/SMRT, Sin3A, and HDAC-1 and by blocking the binding of transcriptional coactivators (8–11). The ability of SnoN and Ski to antagonize TGF-β-induced growth arrest is thought to be important for their transforming activity (12).
The expression level of SnoN is subject to regulation by TGF-β. Immediately after TGF-β stimulation, SnoN is polyubiquitinated and degraded in a Smad- and proteasome-dependent manner, allowing the activation of TGF-β target genes (10, 13–16). After 2 h, TGF-β elicits a marked increase in the levels of SnoN through transcriptional activation (10). In malignant cells, SnoN expression is often elevated because of increased transcription, gene amplification, and/or protein stability (17–19). This elevated SnoN expression may be responsible for the resistance of malignant cancer cells to TGF-β-induced growth arrest. Thus, cellular levels of SnoN are intricately regulated, which may have a critical role in the appropriate and accurate control of TGF-β signaling.
Regulation of SnoN activity by intracellular localization has not been studied. SnoN has always been considered a nuclear protein, based on examination of ectopically expressed proteins in chicken embryo fibroblasts and tissue culture cell lines, as well as endogenous SnoN in tumor cell lines (12, 20, 21) (data not shown). Only two studies (22, 23) have reported that the localization of SnoN and the related Ski can change during malignant progression of specific types of cancer cells. However, the localization of these proteins has not been characterized carefully in normal tissues and nontumorigenic cells. The physiological significance of intracellular localization with respect to the function of SnoN also is not clear.
In this study, we examined the intracellular localization of endogenous SnoN in normal and malignant tissues and carried out mechanistic studies on the function of cytoplasmic SnoN. We show that SnoN exhibits predominantly cytoplasmic localization in nonmalignant tissues and cells but is exclusively nuclear in malignant tissues and cell lines. Also, cytoplasmic SnoN can repress TGF-β signaling more potently than nuclear SnoN. Because TGF-β signaling has important roles in both cellular differentiation and malignant transformation, understanding the role of SnoN intracellular localization in the regulation of TGF-β signaling may provide insight into the ability of TGF-β to influence these processes in vivo.
Methods
Cell Culture and Reagents. We maintained 293T and Phoenix-Eco cells in DMEM containing 10% FBS. The Hep3B human hepatoma cell line (American Type Culture Collection) was cultured in MEM with 5% FBS. Ba/F3 pro-B cells were grown in RPMI medium 1640 with 10% FBS and 10% WEHI-conditioned medium as a source of IL-3. HMT-3522 S1 human mammary epithelial cells were propagated in chemically defined medium, as described (24). For 3D cultures, HMT-3522 S1 cells were embedded in Matrigel, as described (25). Adult human epidermal keratinocytes (HEKa) were obtained from Cascade Biologics (Portland, OR) and cultured in EpiLife serum-free medium containing EpiLife defined-growth supplement (Cascade Biologics). MDA-MB-231 (American Type Culture Collection) breast cancer cells were maintained in DMEM containing 5% FBS. A375 (American Type Culture Collection) malignant melanoma cells were cultured in DMEM containing 10% FBS. Mammary and skin tissue sections were generously provided by the University of California (San Francisco) Tissue Core and the Department of Plastic Surgery at the Hôpital Saint-Louis (Paris), respectively.
Antisera against Smad3 (FL-425) and Smad4 (H552) were purchased from Santa Cruz Biotechnology. Monoclonal anti-Smad2 antibodies were purchased from BD Transduction Laboratories. Anti-Flag and anti-hemagglutinin (HA) antibodies were purchased from Sigma. Anti-phospho-Smad2 and anti-phospho-Smad3 antisera were a generous gift from Aristidis Moustakas (Ludwig Institute for Cancer Research, Uppsala, Sweden). Polyclonal antisera against SnoN are described in ref. 10. Anti-Ki67 antibody was obtained from Sigma. Alexa-fluorophore-conjugated secondary antibodies were purchased from Molecular Probes.
SnoN deletions and point mutations were generated by PCR and cloned into pCMV5b, pRK, or pBABE vectors for mammalian expression.
Transfection and Retroviral Infections. Transient transfections were performed by using Lipofectamine Plus reagents (Invitrogen) according to the manufacturer's protocol. Ba/F3 cells stably expressing WT and mutant SnoN proteins were generated by transfecting Phoenix-Eco packaging cells with SnoN in the pMX-IRES-GFP vector. At 48 h after transfection, the viral supernatant was harvested and used to infect Ba/F3 cells with centrifugation for 2 h. The infected cells were then cultured for 48 h in complete medium before sorting for GFP-positive cells.
Immunofluorescence. Frozen tissue sections and tissue culture cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked in PBS containing 10% newborn calf serum, 1% BSA, and 0.02% Triton X-100. Transfected Flag- and HA-tagged proteins were detected by using antisera against the Flag or HA tags. Endogenous SnoN and Smad proteins were visualized with the antisera described above. For competition with the peptide antigen, anti-SnoN antibody was preincubated with the C-terminal peptide for 2 h at room temperature before being used for immunofluorescent staining. Immunofluorescence was observed with an Axiophot epifluorescence microscope or a confocal LSM 510 microscope (Zeiss).
Luciferase Assay. Hep3B cells were cotransfected with WT or mutant SnoN K30,31N and 0.5 μg of p3TP-lux. At 24 h after transfection, cells were serum-starved for 8 h and stimulated with 50 pM for 16 h, as described (10).
Growth-Inhibition Assays. We cultured 5 × 103 Ba/F3 cells with various concentrations of TGF-β1 for 4 days. Relative cell growth was determined by counting cells and expressing the number of TGF-β-treated cells relative to the number of unstimulated cells.
Pulse–Chase Assays. Transfected 293T cells were starved with methionine- and cysteine-free media for 30 min, pulse-labeled with 35S-Express (0.25 mCi/ml; 1 Ci = 37 GBq) for 30 min, and then chased with cold medium for various times before lysis. SnoN was purified by immunoprecipitation and resolved on SDS/PAGE.
Immunoprecipitation and Immunoblotting. Flag-tagged SnoN and Smad proteins were isolated from transfected 293T cells and infected Ba/F3 cell lines by immunoprecipitation with anti-Flag antibody, followed by elution with Flag peptide, as described (10). Expression of Flag-tagged SnoN or Smad proteins was detected by Western blotting with anti-Flag antibody. HA-SnoN bound to Smad proteins was visualized by immunoblotting with anti-HA antibody. HA-tagged Smad proteins that associated with Flag-SnoN were detected by immunoblotting with anti-HA antibody. Phosphorylation of Smad2 was detected by immunoblotting with anti-phospho-Smad2 antibody.
Results
SnoN Is Predominantly Cytoplasmic in Normal Human Tissues and Nontumorigenic Cell Lines. To examine the expression pattern of endogenous SnoN in normal tissues, we carried out immunofluorescent staining of normal human mammary tissue sections by using antibodies raised against a C-terminal (Fig. 1a) or N-terminal (Fig. 1b) peptide of SnoN, as well as a third antibody recognizing the C-terminal half of SnoN (data not shown) (10). SnoN was expressed in the luminal epithelial cells lining the mammary duct and was, surprisingly, predominantly cytoplasmic. Of the cells expressing SnoN, 51% exhibited exclusively cytoplasmic localization, 34% expressed both cytoplasmic and nuclear SnoN, and only 15% of cells showed exclusively nuclear SnoN. When tissue sections were stained with the C-terminal SnoN antibody in the presence of a peptide competitor, the fluorescent signal was reduced dramatically (Fig. 1c), suggesting that the antibody recognizes endogenous SnoN with high specificity. Similar to what was observed in mammary tissue, SnoN was exclusively cytoplasmic in normal human epidermis (Fig. 1d). Interestingly, in tissue sections derived from two invasive mammary ductal carcinomas (Fig. 1e and data not shown), SnoN was exclusively nuclear in 93% of cells. Thus, SnoN is predominantly cytoplasmic in normal tissues and becomes exclusively nuclear in cancer cells.
Fig. 1.
SnoN localization varies in nontumorigenic versus malignant tissues and cell lines. Tissue sections derived from normal human mammary tissue (a–c), normal skin tissue (d), and stage II invasive ductal carcinoma (e) were stained for SnoN by using an antibody against the C (a, c–i) or N(b) terminus of SnoN. (c) The SnoN antibody was preincubated with a blocking peptide, as described in Methods. Endogenous SnoN was visualized in the nontumorigenic HMT-3522-S1 cell line (f), the invasive mammary carcinoma MDA-MB-231 cell line (g), primary HEKa (h), and the malignant melanoma A375 cell line (i). In all images, the nuclei were visualized by staining with Hoechst fluorescent dye.
We next surveyed established human and mouse cell lines to discern whether a similar pattern of SnoN localization exists in tissue culture cells. In many untransformed cell lines that have been passaged for many generations in tissue culture and have lost their functional capacities in comparison with their in vivo counterpart (such as NIH 3T3 cells), SnoN is localized in the nucleus (data not shown). However, in primary HEKa, as well as in at least two nontumorigenic mammary epithelial cell lines [HMT3522 S1 (24) and 184 (26)] that retain the ability to differentiate morphologically into acinus-like structures, localization of SnoN was observed in both the cytoplasm and nucleus (Fig. 1 f and h, and data not shown). Similar to what was observed in cancer tissues, exclusively nuclear localization of SnoN was observed in breast cancer cell lines and melanoma cells (Fig. 1 g and i, and data not shown).
Mapping of Nuclear Translocation Sequence in SnoN. To examine the function of cytoplasmic SnoN with regard to its ability to repress TGF-β signaling, we needed to generate a form of SnoN that localized exclusively in the cytoplasm. Because the mechanism that retains SnoN in the cytoplasm in normal cells is not known, we identified the nuclear localization sequence in SnoN. Deletion mutants of SnoN were generated (Fig. 2a) and introduced into 293T cells by transfection, and their intracellular localization was determined by immunofluorescence with an anti-Flag antibody. As described in refs. 12 and 20, ectopically expressed WT SnoN was nuclear (Fig. 2b). Deletion of the first 96 aa of SnoN resulted in exclusively cytoplasmic localization, whereas SnoN lacking the first 11 aa remained nuclear (Fig. 2b). Thus, the nuclear localization signal appeared to reside between residues 12 and 97. A closer examination of the sequence of this region revealed two lysine pairs (K16,17 and K30,31) that could potentially serve as part of a monopartite or a bipartite nuclear localization signal (Fig. 2a). Mutation of lysines 16 and 17 to asparagines had no effect on nuclear localization of SnoN, whereas mutation of lysines 30 and 31 to asparagines resulted in exclusively cytoplasmic localization (Fig. 2b). Therefore, lysines 30 and 31 are required for the nuclear localization of SnoN, and mutation of these residues (SnoN K30,31N) allows us to examine the activity of cytoplasmic SnoN.
Fig. 2.
Lysines 30 and 31 are required for nuclear localization of SnoN. (a) Schematic representations of SnoN deletion and point mutations. nuc, nuclear; cyto, cytoplasmic. (b) Localization of WT SnoN and SnoN mutant proteins. 293T cells were transfected with WT SnoN or the indicated SnoN mutants and stained with anti-Flag to determine the localization of ectopically expressed SnoN proteins. Nuclei were visualized by staining with Hoechst dye.
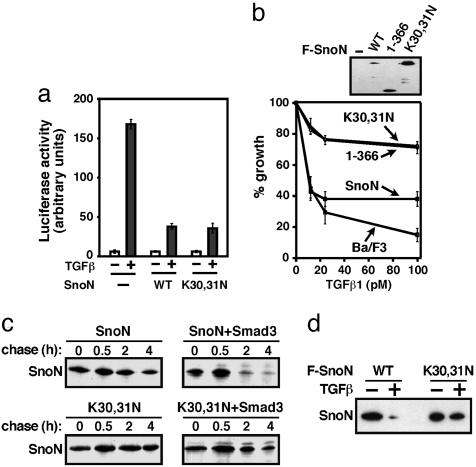
Cytoplasmic SnoN Is Able to Repress TGF-β Signaling. We first examined the ability of cytoplasmic SnoN to affect the transcription of TGF-β-target genes by using a luciferase reporter assay. SnoN K30,31N readily repressed TGF-β-induced transactivation when cotransfected with the p3TP-lux reporter construct into TGF-β-responsive Hep3B cells (Fig. 3a). In multiple experiments, this mutant exhibited enhanced repression of TGF-β-induced transcription relative to WT SnoN. To examine whether cytoplasmic SnoN was capable of repressing TGF-β-induced growth inhibition, stable cell lines expressing either WT or K30,31N SnoN were generated by retroviral infection and tested in a growth-inhibition assay (Fig. 3b). As described in ref. 10, WT SnoN is degraded in response to TGF-β treatment, and therefore repressed TGF-β-mediated growth inhibition only moderately. Cytoplasmic SnoN (K30,31N) greatly enhanced the ability of SnoN to repress TGF-β-induced growth arrest (Fig. 3b), similar to that observed with a truncated form of SnoN (SnoN 1–366) that is resistant to TGF-β-induced degradation and therefore blocked TGF-β-induced growth inhibition more potently than WT SnoN. These data suggest that targeting SnoN to the cytoplasm may potentiate its ability to repress TGF-β-signaling responses.
Fig. 3.
Activity and properties of cytoplasmic SnoN. (a) Cytoplasmic localization of SnoN potently represses TGF-β-elicited transcriptional activation. Hep3B cells were cotransfected with p3TP-lux together with empty vector, Flag-tagged WT SnoN, or SnoN K30,31N, as described in Methods. At 24 h after transfection, cells were serum-starved for 8 h and then treated with 50 pM TGF-β for 16 h before luciferase activity was measured. (b) Cytoplasmic localization of SnoN results in increased repression of TGF-β-induced growth arrest. Parental Ba/F3 cells, or Ba/F3 cells stably expressing Flag-tagged WT SnoN, SnoN 1–366, or SnoN K30,31N were treated with increasing concentrations of TGF-β and cultured for 4 days. Cell growth was calculated by cell counting and expressing the cell number as a percentage of the number of cells in unstimulated samples. SnoN expression was assessed by immunoblotting with anti-Flag antiserum. (c) Cytoplasmic SnoN is resistant to Smad3-mediated degradation in a pulse–chase assay. 293T cells were transfected with HA-SnoN and Flag-Smad3 and subjected to pulse–chase assays, as described in Methods. Smad3-bound SnoN was isolated by immunoprecipitation with anti-Flag antisera. SnoN was directly immunoprecipitated from 293T cells singly transfected with Flag-tagged SnoN (F-SnoN) as a control. (d) Cytoplasmic SnoN is resistant to TGF-β-induced degradation. Ba/F3 cells stably expressing Flag-tagged WT SnoN or SnoN K30,31N were treated with TGF-β for 1 h. SnoN levels were determined by anti-Flag immunoprecipitation, followed by immunoblotting with anti-Flag antiserum.
Cytoplasmic SnoN Is Resistant to TGF-β-Mediated Degradation. The increased repressive activity of cytoplasmic SnoN may result from a higher level of expression, because the steady-state level of SnoN K30,31N in infected cells was consistently higher than that of WT SnoN, especially in the presence of TGF-β (Fig. 3 b and d). Therefore, we compared the half-life of cytoplasmic SnoN with that of WT SnoN in the absence or presence of Smad3 by using pulse–chase assays. In the absence of Smad3, cytoplasmic SnoN appeared to be slightly more stable than nuclear WT SnoN (Fig. 3c). However, in the presence of Smad3, the half-life of nuclear WT SnoN was reduced dramatically as shown in refs. 10, 13, 14, and 16, whereas that of SnoN K30,31N was unaffected (Fig. 3c). This finding indicates that cytoplasmic SnoN is resistant to Smad3-mediated degradation. Similarly, TGF-β treatment resulted in degradation of nuclear WT SnoN but had no effect on the level of SnoN K30,31N (Fig. 3d). These results suggest that degradation of SnoN probably occurs in the nucleus and that cytoplasmic SnoN is resistant to TGF-β-elicited degradation, resulting in a higher level of SnoN in the presence of TGF-β and, consequently, stronger repression of TGF-β signaling.
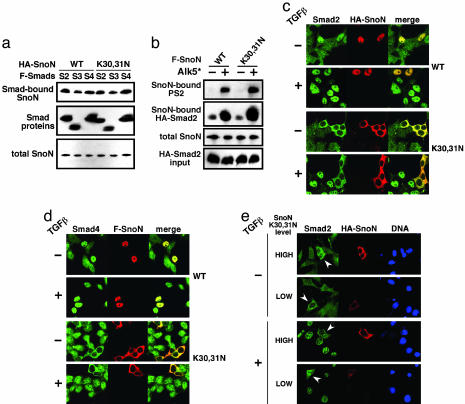
Cytoplasmic SnoN Sequesters Smad Proteins in the Cytoplasm to Repress TGF-β Signaling. The identified (8–11) mechanisms of repression of TGF-β signaling by SnoN occur largely in the nucleus. To determine how cytoplasmic SnoN represses TGF-β signaling, we examined the ability of cytoplasmic SnoN to bind to Smad proteins and affect their phosphorylation. In 293T cells transfected with Flag-Smad proteins together with HA-tagged WT or cytoplasmic SnoN, cytoplasmic SnoN bound to Smad2, Smad3, and Smad4 as efficiently as WT SnoN (Fig. 4 a and b). Similarly, in stable cell lines expressing WT or K30,31N SnoN, no significant difference in the binding affinity of WT versus cytoplasmic SnoN for Smad4 was detected (data not shown). Cytoplasmic SnoN also did not affect receptor-mediated phosphorylation of the receptor-regulated Smad (R-Smad) proteins. In 293T cells transfected with a SnoN construct, phosphorylation of Smad2 by the active type I TGF-β receptor, TβRI (Alk5*), was unaffected by expression of either WT SnoN or SnoN K30,31N, suggesting that cytoplasmic SnoN does not antagonize TGF-β signaling by preventing R-Smad phosphorylation (Fig. 4b).
Fig. 4.
Mechanism of repression of TGF-β signaling by cytoplasmic SnoN. (a) Cytoplasmic localization of SnoN does not disrupt interaction with Smad proteins. Flag-tagged Smad2, Smad3, or Smad4 (F-Smads) were cotransfected with HA-tagged WT or mutant SnoN K30,31N in 293T cells. Levels of SnoN bound to Smad proteins were assessed by blotting the anti-Flag immunoprecipitates with anti-HA antiserum. Anti-HA immunoprecipitates were directly blotted with anti-HA antisera to detect expression of the SnoN proteins. (b) Cytoplasmic SnoN does not inhibit receptor-mediated phosphorylation of receptor-regulated Smad (R-Smad) proteins. We transfected 293T cells with Flag-tagged WT SnoN (F-SnoN) or SnoN K30,31N, as well as HA-Smad2 and the constitutively active TGF-β type I (Alk5*) receptor, where indicated. Levels of phospho-Smad2 bound to SnoN were detected by immunoblotting with anti-phospho-Smad2 antibody. The level of SnoN-bound total Smad2 was monitored by reblotting with anti-HA antibody. (c and d) Cytoplasmic SnoN sequesters Smad proteins in the cytoplasm and prevents TGF-β-induced nuclear translocation. Hep3B cells and NIH 3T3 cells (data not shown) were transfected with HA- or Flag-tagged SnoN proteins and treated with 200 pM TGF-β for 1 h, as indicated. Localization of endogenous Smad2 (c) and Smad4 (d) was determined by immunostaining with anti-Smad2 or anti-Smad4 antibodies, respectively. SnoN-transfected cells were identified by costaining with anti-HA or anti-Flag, as indicated. (e) Sequestration of Smad2 by cytoplasmic SnoN depends on SnoN expression level. Low (0.25 μg) or high (1.0 μg) levels of SnoN (K30,31N) were transfected into NIH 3T3 cells. At 48 h after transfection, cells were treated with 200 pM TGF-β before immunostaining. Transfected SnoN K30,31N was detected with anti-HA antibody, and endogenous Smad2 was stained with anti-Smad2. White arrowheads indicate transfected cells. Nuclei were visualized with Hoechst dye.
Next, we examined whether cytoplasmic SnoN affects the nuclear translocation of the Smad proteins. In untransfected cells, endogenous Smad proteins are localized throughout the cell in the absence of TGF-β and concentrate in the nucleus upon TGF-β treatment (Fig. 4 c and d, and data not shown). Overexpression of WT SnoN (nuclear) caused the Smad proteins to concentrate in the nucleus. When cytoplasmic SnoN (K30,31N) was overexpressed, all three Smad proteins were retained exclusively in the cytoplasm, even in the presence of TGF-β (Fig. 4 c and d, and data not shown). Thus, cytoplasmic SnoN appears to sequester Smad proteins in the cytoplasm and prevent their nuclear translocation in response to TGF-β. This sequestration could be the underlying mechanism by which cytoplasmic SnoN represses TGF-β signaling.
This sequestration model predicts that, in cells with cytoplasmic SnoN, the level of nuclear Smad complexes may depend on the relative amounts of SnoN and Smad proteins. In cells with a high ratio of cytoplasmic SnoN to Smad proteins, SnoN may efficiently block the TGF-β-induced nuclear translocation of Smad proteins, leading to inhibition of TGF-β signaling. However, if the level of cytoplasmic SnoN is sufficient to sequester only a fraction of the Smad proteins, the rest of the Smad proteins should undergo nuclear translocation. To test this hypothesis, we examined the localization of endogenous Smad2 in cells expressing either low or high levels of SnoN K30,31N in the absence or presence of TGF-β. In cells that expressed a high level of SnoN K30,31N, Smad2 was completely sequestered in the cytoplasm and excluded from the nucleus even in the presence of TGF-β (Fig. 4e). In contrast, SnoN K30,31N expressed at low levels retained only a portion of Smad2 in the cytoplasm, and a significant amount of Smad2 translocated to the nucleus upon stimulation with TGF-β (Fig. 4e). Thus, in cells expressing cytoplasmic SnoN, the magnitude of downstream TGF-β responses may be regulated in part by the stoichiometric ratio of SnoN to Smad proteins.
Intracellular Localization of SnoN Is Altered upon Cell Differentiation. Because SnoN can localize in both the cytoplasm and nucleus, we sought to determine how this localization is regulated. We had observed that localization of SnoN in normal mammary tissue exhibited a certain degree of heterogeneity (Fig. 1a) and wondered whether this heterogeneity is related to the differentiation states of cells. To test this hypothesis, we used a tissue culture differentiation system by using S1 mammary epithelial cells. When harvested from 2D culture and embedded in Matrigel (3D), S1 cells undergo morphological differentiation to form a multicellular acinus-like structure composed of 5–10 cells positioned in a polarized, spherical arrangement (25). After 10 days in 3D culture, the acinus-like structures formed by S1 cells were cryosectioned, and localization of SnoN was examined by immunofluorescence and compared with that of S1 cells cultured in 2D. Whereas SnoN in cells grown in 2D culture is distributed throughout the cytoplasm and nucleus, it is located exclusively in the nucleus after differentiation in 3D cultures (Fig. 5a). Thus, intracellular localization of SnoN is regulated during cell differentiation.
Fig. 5.
SnoN localization is nuclear in 3D cultures and cells that have withdrawn from the cell cycle. (a) SnoN is exclusively nuclear in differentiated 3D HMT-3522 S1 (S1) cultures. S1 cells were allowed to undergo morphological differentiation in 3D cultures for 10 days. Cryosections of 3D cultures were subjected to immunofluorescent staining as described in Methods. (b) SnoN localization throughout 3D morphological differentiation. S1 cells were allowed to differentiate in 3D cultures for various lengths of time. After the indicated number of days, 3D cellular structures were subjected to immunostaining for SnoN and Ki67, as described in Methods. (c) Cell-cycle withdrawal induces nuclear localization of SnoN. S1 cells were plated in complete medium for 3 days and then placed in medium lacking EGF (starvation) to induce withdrawal from the cell cycle. After 3 days, SnoN localization was visualized by immunofluorescent staining, and cells were costained with anti-Ki67 to confirm cell-cycle withdrawal. (d) ECM signaling has no effect on localization of SnoN. S1 cells were grown in complete medium for 3 days and then stimulated for an additional 3 days by the addition of reconstituted basement membrane. SnoN localization was then determined by immunofluorescent staining. (e) Cell-cycle arrest in G1 induces nuclear localization of SnoN. S1 cells were grown in complete medium for 3 days, and then placed in medium lacking EGF (starvation) or medium containing 10 μM either LY294002 (phosphatidylinositol 3-kinase inhibitor) or SP600125 (c-Jun N-terminal kinase inhibitor). After 3 days, SnoN localization was determined by immunostaining.
To determine when the shift to nuclear localization occurs during differentiation, we carried out a time-course study. S1 cells were embedded in 3D Matrigel. At different time points, cells were harvested and stained for SnoN and Ki67, which is a marker for proliferating cells. SnoN was distributed in both the cytoplasm and nucleus during the first 3 days of 3D culture, similar to what was observed in undifferentiated S1 cells in 2D culture (Fig. 5b and data not shown). By day 5, localization of SnoN shifted to the nucleus, and SnoN remained nuclear thereafter (Fig. 5b). Interestingly, the transition of SnoN localization from cytoplasmic and nuclear to exclusively nuclear correlated with the loss of Ki67 expression (Fig. 5b), suggesting that localization of SnoN may be related to cell-cycle withdrawal or arrest.
Morphological differentiation of S1 cells in 3D culture is induced by signals from the extracellular matrix (ECM) and accompanied later by withdrawal of cells from the cell cycle (25). To examine which of the two processes is responsible for the nuclear localization of SnoN, S1 cells were grown in medium lacking EGF to induce growth arrest or stimulated with reconstituted basement membrane to mimic ECM signaling. S1 cells cultured for 3 days in medium without EGF resulted in virtually complete withdrawal of cells from the cell cycle, as evidenced by the absence of staining for Ki67 (Fig. 5c). Interestingly, costaining with anti-SnoN revealed a marked shift in SnoN localization from predominantly cytoplasmic to predominantly nuclear localization of SnoN, with 85% of the cells exhibiting exclusively nuclear SnoN (Fig. 5c). In contrast, stimulation by ECM for 3 days did not affect the localization of SnoN (Fig. 5d). Therefore, withdrawal from the cell cycle appears to induce a shift from cytoplasmic to nuclear SnoN localization.
To determine whether, in addition to withdrawal from the cell cycle, cell-cycle arrest in G1 may alter SnoN localization, S1 cells in 2D culture were treated with pharmacological inhibitors of phosphatidylinositol 3-kinase (LY294002) or c-Jun N-terminal kinase (SP600125) for 3 days. Under these treatments, cells were arrested in G1, as confirmed by flow cytometry (data not shown), and SnoN was found to concentrate in the nucleus, similar to what was observed during cell-cycle withdrawal (Fig. 5e). Therefore, cell-cycle withdrawal and arrest both result in the accumulation of SnoN in the nucleus.
Discussion
In this article, we demonstrate differential regulation of SnoN activity in normal versus tumor tissues and cells at the level of intracellular localization. In normal tissues and cells, SnoN is localized predominantly in the cytoplasm and becomes nuclear during morphological differentiation upon withdrawal from the cell cycle or during cell-cycle arrest. When localized to the cytoplasm, SnoN still represses TGF-β signaling by sequestering the Smad proteins in the cytoplasm to prevent their nuclear translocation. Interestingly, cytoplasmic SnoN is resistant to degradation in response to TGF-β signaling and, therefore, appears to be more potent than nuclear SnoN in antagonizing TGF-β signaling.
Whereas SnoN localization in nontumorigenic cells is subject to regulation, the localization of SnoN in tumor cells appears constitutively nuclear. The regulatory processes linking SnoN localization and cell proliferation have likely been lost in tumor cells, leading to exclusively nuclear localization in tumor cells. This phenomenon is not unique to SnoN. Differential regulation of subcellular localization in normal and tumor cells has also been reported for the c-Abl tyrosine kinase (27). c-Abl is located in both the nucleus and cytoplasm, but nuclear c-Abl is active only in cycling cells, whereas cytoplasmic c-Abl functions in quiescent cells (28, 29). In contrast, in proliferating tumor cells, the transforming forms of Abl (v-Abl and Bcr-Abl) are exclusively cytoplasmic (30, 31). Therefore, cellular processes regulating the localization of a protooncoprotein in nonmalignant cells may be quite distinct from those that regulate localization of the cognate oncoprotein in tumor cells.
To our knowledge, the mechanisms that regulate the intracellular localization of SnoN have not been identified. We speculate that SnoN may localize to the cytoplasm through association with a cytoplasmic retention protein that masks the residues required for nuclear translocation (K30 and K31) in SnoN. This protein may be present only in normal tissues and cells and is likely to be absent in most immortalized cell lines. During cell differentiation and in tumor cells, this protein may be degraded or inactivated, allowing SnoN to migrate into the nucleus. Alternatively, SnoN may need the help of a nuclear translocation factor that recognizes sequences surrounding K30 and K31 in SnoN to facilitate its translocation into the nucleus. This translocation factor may be absent in normal cells but up-regulated in tumor cells or in normal cells undergoing differentiation.
Although both can repress TGF-β signaling, the cytoplasmic population of SnoN differs from the nuclear SnoN in the mechanism of repression and in its ability to undergo degradation in response to TGF-β. Cytoplasmic SnoN is resistant to TGF-β-induced degradation and inhibits Smad proteins through physical sequestration. Because a fraction of the Smad proteins may be sequestered in the cytoplasm, cells expressing cytoplasmic SnoN may pose a higher threshold for activation of TGF-β target genes as well as reduce the magnitude of Smad activities in the cells. Given that different expression levels and activities of intracellular signaling molecules can influence the duration of downstream signaling, which in turn affects the eventual signaling specificity (32, 33), this difference in the magnitude of TGF-β signaling due to differential localization of SnoN could result in activation of a separate set of target genes and influence the decision of cells to proliferate or differentiate. In tissues that undergo repeated periodic or chronic cycles of proliferation and differentiation, such as mammary gland or skin, this regulatory mechanism could have an important role in maintaining the appropriate functional state of these epithelial cells.
Acknowledgments
We thank Dr. Joe Gray (Lawrence Berkeley National Laboratory) and the University of California, San Francisco, Tissue Core for providing human mammary tissue sections; and Eva Lee and other members of the M.J.B. laboratory for advice and technical support. This work was supported by National Institutes of Health Grant CA87940 (to K.L.), Department of Energy Office of Biological and Environmental Research Grant DE-AC03-76SF00099 (to M.J.B. and K.L.), the France–Berkeley Fund (K.L. and A.M.), Institut National de la Santé et de la Recherche Médicale (A.M.), and a predoctoral fellowship from the Department of Defense Breast Cancer Research Program (to A.R.K.).
Author contributions: K.L. designed research and analyzed data; A.R.K. and J.L. performed research; A.M. and M.J.B. contributed new reagents/analytic tools and provided discussion of the project and critical reading and revision of the manuscript; and A.R.K. and K.L. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: HA, hemagglutinin; ECM, extracellular matrix; HEKa, adult human epidermal keratinocytes.
References
- 1.Roberts, A. B. & Sporn, M. B. (1990) in Peptide Growth Factors and Their Receptors, eds. Sporn, M. B. & Roberts, A. B. (Springer, Heidelberg), pp. 419–472.
- 2.Derynck, R., Akhurst, R. J. & Balmain, A. (2001) Nat. Genet. 29, 117–129. [DOI] [PubMed] [Google Scholar]
- 3.Moustakas, A., Pardali, K., Gaal, A. & Heldin, C. H. (2002) Immunol. Lett. 82, 85–91. [DOI] [PubMed] [Google Scholar]
- 4.Derynck, R. & Zhang, Y. E. (2003) Nature 425, 577–584. [DOI] [PubMed] [Google Scholar]
- 5.Shi, Y. & Massague, J. (2003) Cell 113, 685–700. [DOI] [PubMed] [Google Scholar]
- 6.Liu, X., Sun, Y., Weinberg, R. A. & Lodish, H. F. (2001) Cytokine Growth Factor Rev. 12, 1–8. [DOI] [PubMed] [Google Scholar]
- 7.Luo, K. (2004) Curr. Opin. Genet. Dev. 14, 65–70. [DOI] [PubMed] [Google Scholar]
- 8.Akiyoshi, S., Inoue, H., Hanai, J., Kusanagi, K., Nemoto, N., Miyazono, K. & Kawabata, M. (1999) J. Biol. Chem. 274, 35269–35277. [DOI] [PubMed] [Google Scholar]
- 9.Luo, K., Stroschein, S. L., Wang, W., Chen, D., Martens, E., Zhou, S. & Zhou, Q. (1999) Genes Dev. 13, 2196–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroschein, S. L., Wang, W., Zhou, S., Zhou, Q. & Luo, K. (1999) Science 286, 771–774. [DOI] [PubMed] [Google Scholar]
- 11.Wu, J. W., Krawitz, A. R., Chai, J., Li, W., Zhang, F., Luo, K. & Shi, Y. (2002) Cell 111, 357–367. [DOI] [PubMed] [Google Scholar]
- 12.He, J., Tegen, S. B., Krawitz, A. R., Martin, G. S. & Luo, K. (2003) J. Biol. Chem. 278, 30540–30547. [DOI] [PubMed] [Google Scholar]
- 13.Bonni, S., Wang, H. R., Causing, C. G., Kavsak, P., Stroschein, S. L., Luo, K. & Wrana, J. L. (2001) Nat. Cell Biol. 3, 587–595. [DOI] [PubMed] [Google Scholar]
- 14.Stroschein, S. L., Bonni, S., Wrana, J. L. & Luo, K. (2001) Genes Dev. 15, 2822–2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun, Y., Liu, X., Ng-Eaton, E., Lodish, H. F. & Weinberg, R. A. (1999) Proc. Natl. Acad. Sci. USA 96, 12442–12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wan, Y., Liu, X. & Kirschner, M. W. (2001) Mol. Cell 8, 1027–1039. [DOI] [PubMed] [Google Scholar]
- 17.Nomura, N., Sasamoto, S., Ishii, S., Date, T., Matsui, M. & Ishizaki, R. (1989) Nucleic Acids Res. 17, 5489–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imoto, I., Pimkhaokham, A., Fukuda, Y., Yang, Z. Q., Shimada, Y., Nomura, N., Hirai, H., Imamura, M. & Inazawa, J. (2001) Biochem. Biophys. Res. Commun. 286, 559–565. [DOI] [PubMed] [Google Scholar]
- 19.Edmiston, J. S., Yeudall, W. A., Chung, T. D. & Lebman, D. A. (2005) Cancer Res. 65, 4782–4788. [DOI] [PubMed] [Google Scholar]
- 20.Boyer, P. L., Colmenares, C., Stavnezer, E. & Hughes, S. H. (1993) Oncogene 8, 457–466. [PubMed] [Google Scholar]
- 21.Zhang, F., Monkkonen, M., Roth, S. & Laiho, M. (2002) FEBS Lett. 527, 58–62. [DOI] [PubMed] [Google Scholar]
- 22.Reed, J. A., Bales, E., Xu, W., Okan, N. A., Bandyopadhyay, D. & Medrano, E. E. (2001) Cancer Res. 61, 8074–8078. [PubMed] [Google Scholar]
- 23.Zhang, F., Lundin, M., Ristimaki, A., Heikkila, P., Lundin, J., Isola, J., Joensuu, H. & Laiho, M. (2003) Cancer Res. 63, 5005–5010. [PubMed] [Google Scholar]
- 24.Briand, P., Petersen, O. W. & Van Deurs, B. (1987) In Vitro Cell Dev. Biol. 23, 181–188. [DOI] [PubMed] [Google Scholar]
- 25.Petersen, O. W., Ronnov-Jessen, L., Howlett, A. R. & Bissell, M. J. (1992) Proc. Natl. Acad. Sci. USA 89, 9064–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond, S. L., Ham, R. G. & Stampfer, M. R. (1984) Proc. Natl. Acad. Sci. USA 81, 5435–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pendergast, A. M. (2002) Adv. Cancer Res. 85, 51–100. [DOI] [PubMed] [Google Scholar]
- 28.Taagepera, S., McDonald, D., Loeb, J. E., Whitaker, L. L., McElroy, A. K., Wang, J. Y. & Hope, T. J. (1998) Proc. Natl. Acad. Sci. USA 95, 7457–7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welch, P. J. & Wang, J. Y. (1995) Mol. Cell. Biol. 15, 5542–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Etten, R. A., Jackson, P. & Baltimore, D. (1989) Cell 58, 669–678. [DOI] [PubMed] [Google Scholar]
- 31.McWhirter, J. R. & Wang, J. Y. (1991) Mol. Cell. Biol. 11, 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicolas, F. J. & Hill, C. S. (2003) Oncogene 22, 3698–3711. [DOI] [PubMed] [Google Scholar]
- 33.Marshall, C. J. (1995) Cell 80, 179–185. [DOI] [PubMed] [Google Scholar]