Abstract
Reduction of prefrontal cortex glutamic acid decarboxylase (GAD67) and reelin (mRNAs and proteins) expression is the most consistent finding reported by several studies of postmortem schizophrenia (SZ) brains. Converging evidence suggests that the reduced GAD67 and reelin expression in cortical GABAergic interneurons of SZ brains is the consequence of an epigenetic hypermethylation of RELN and GAD67 promoters very likely mediated by the overexpression of DNA methyltransferase 1 in cortical GABAergic interneurons. Studies of the molecular mechanisms (DNA methylation plus related chromatin remodeling factors) that cause the down-regulation of reelin and GAD67 in SZ brains have important implications not only to understand the disease pathogenesis but also to improve present pharmacological interventions to treat SZ. The mouse treated with l-methionine models some of the molecular neuropathologies detected in SZ, including the hypermethylation of RELN promoter CpG islands and the down-regulation of reelin and GAD67 expression. We now report that in these mice, RELN and GAD67 promoters express an increased recruitment of methyl-CpG binding domain proteins. In these mice the histone deacetylase inhibitor valproate, which increases acetylated histone content in cortical GABAergic interneurons, also prevents MET-induced RELN promoter hypermethylation and reduces the methyl-CpG binding domain protein binding to RELN and GAD67 promoters. These findings suggest that DNA hypermethylation and the associated chromatin remodeling may be critically important in mediating the epigenetic down-regulation of reelin and GAD67 expression detected in cortical GABAergic interneurons of SZ patients.
Keywords: DNA methyltransferase1, l-methionine, methyl binding domain proteins, valproate, chromatin
Schizophrenia (SZ) pathophysiology is characterized by a down-regulation of several GABAergic neuronal markers including GAD67 and reelin mRNAs and proteins (1-8).
Reelin is an extracellular matrix protein, synthesized and secreted from cortical GABAergic interneurons (9-12), that surrounds apical and basal dendritic spines of pyramidal cortical neurons (13-14). This protein not only plays a defined role in prenatal central nervous system development (13-15) but also plays an important role in the adult brain by modulating cortical pyramidal neuron dendritic spine expression density, the branching of dendrites, and the expression of long-term potentiation (14, 16, 17). Very likely, reelin has a role in regulating the event-related increase of protein synthesis mediated by the dendritic translation of cytosolic mRNAs (18). In this scenario, the down-regulation of reelin expression in neocortices and hippocampi of SZ patients (SZP) (1, 5, 19) may be important in mediating the down-regulation of pyramidal neuron dendritic branching and spine expression and in the neuropil hypoplasticity typical of SZ (20-22).
GAD67 is one of two molecular forms of the GABA synthesizing enzymes expressed in GABAergic interneurons (23). In SZP, the down-regulation of this enzyme is very likely responsible for the deficit in the inhibitory tone supporting the disruption of the intermittent high-frequency synchronized firing of cortical pyramidal neurons, which contributes to the working memory impairment (24).
Epigenetic DNA modifications, characterized by 5-methylation of cytosine (5mC) expressed in RELN and GAD67 CpG island promoters, have been implicated in the transcription down-regulation of these two genes detected in the prefrontal cortices (PFCs) of SZP (6, 25-29). It is likely that RELN and GAD67 promoter hypermethylation in PFC of SZP is mediated by the overexpression of DNA methyltransferase 1 occurring in SZ cortical GABAergic interneurons (6-8, 27, 28).
DNA promoter cytosine hypermethylation favors gene transcriptional repression either by a direct interference of hypermethylated CpGs with the binding of transcription modulatory factors or by attracting a family of methyl-CpG binding domain (MBD) proteins that by recruiting histone deacetylases (HDACs) and corepressors (i.e., Sin3A) induces chromatin remodeling (30).
Among the most abundant members of the MBD proteins expressed in the brain are MeCP2 and MBD2 (30, 31). Both protein families bind to selective gene promoters with a high affinity to symmetrically hypermethylated CpG dinucleotides (30). This background helps to explain why MeCP2 gene mutations are linked to Rett syndrome, a severe form of neurodevelopmental mental retardation (32). Moreover, transgenic mice, which express MeCP2 at ≈2-fold of wild-type levels, develop seizures associated with hypoactivity, and ≈30% of them are dead at 1 year of age (33).
Chen et al. (34) and Martinowich et al. (35), experimenting with neuronal cultures, revealed that an up-regulation of MeCP2 binding is associated with hypermethylation of BDNF CpG island promoters. The possibility that MBD proteins contribute to the hypermethylation of specific promoter genes (i.e., RELN and GAD67) in cortical GABAergic interneurons is supported by the immunohistochemical findings that MeCP2 is highly expressed in GABAergic interneurons of primate and mouse frontal cortices (FCs) (36).
Protracted treatment (3 to 4 weeks) of SZP with high doses of l-methionine (MET), a precursor of the methyl donor S-adenosyl-methionine that is a requirement for DNA methyltransferase 1 catalytic activity, causes a recrudescence of the psychopathology in 79% of these patients (for a review, see ref. 37). We have previously reported (38, 39) that the protracted administration to mice of large doses of MET generates behavioral endophenotypes reminiscent of the psychosis recrudescence induced by MET in SZP. These psychotic-like behavioral endophenotypes are associated with RELN promoter hypermethylation and with the down-regulation of reelin and GAD67 expression in GABAergic neurons.
This study tests the hypothesis that in mice, the down-regulation of reelin and GAD67 expression elicited by MET treatment induces hypermethylation of RELN and GAD67 promoters, which is associated with an increased binding of MeCP2 or MBD2 proteins. All these changes can be abrogated by pretreatment with valproate (VPA), a HDAC inhibitor (38, 39). Hence, these studies provide convincing evidence that the extent of MeCP2 and MBD2 binding to RELN and GAD67 promoters relates to their methylation intensity.
Materials and Methods
Drug Administration Schedule and Brain Dissection. Swiss Albino ND4 mice of ≈20 g (Harlan, Indianapolis) received s.c. either MET (5.2 mmol/kg), or VPA (2 mmol/kg), or a combination of MET and VPA dissolved in saline (0.1 ml/10 g of body weight, twice daily) for a period of 3, 5, 6, 10, and 15 days. Two hours after the last injection, mice were decapitated and the FCs were dissected for chromatin immunoprecipitation (ChIP) assay. FC is defined as the area of the neocortex that extends 2 mm anterior to the bregma.
RNA Extraction and Quantitative RT-PCR Analysis. Total RNA was extracted from FC, and reelin mRNA content was measured by quantitative competitive RT-PCR with internal standards as described by Tremolizzo et al. (38).
Western Blot Analysis. Reelin, GAD67, or GAD65 were extracted from the FC samples homogenized in Laemmli buffer (100 μl/10 mg of tissue). MeCP2 was extracted in Laemmli buffer from crude FC nuclear fractions (900 × g pellet of a 0.32 M sucrose homogenate). Proteins were separated by SDS/PAGE (7.5% acrylamide gel for reelin and 10-20% gradient for MeCP2, GAD65, and GAD67) and blotted overnight onto Hybond ECL nitrocellulose membranes (Amersham Pharmacia). The membrane blots were reacted for 6 h at 25°C with: (i) G-10 anti-reelin monoclonal antibody, diluted 1:5,000 (12); (ii) GAD67/GAD65 anti-rabbit polyclonal antibody (Chemicom), diluted 1:2,000; and (iii) MeCP2 polyclonal antibody (Upstate Cell Signaling Solutions, Charlottesville, VA), diluted 1:1,000.
The intensity of β-actin immunofluorescence was determined on the same blot with β-actin monoclonal antibody (1:3,000) (Clone AC-15, Sigma-Aldrich) and used for a comparative estimation of the protein amount applied to the gels.
ChIP. About 10 mg of FC tissue was used for this procedure. Tissue slices (0.3 × 0.3 mm) were incubated with 400 μl of PBS containing 1% formaldehyde at 37°C for 15 min, supplemented with protease inhibitors (1 mM PMSF/1 μg/ml aprotinin/1 μg/ml pepstatin) to crosslink MeCP2, MBD2, and acetylated histone 3 (Upstate Cell Signaling Solutions) with the target genomic DNAs. After being washed six times with cold PBS containing protease inhibitors, slices were homogenized in 200-400 μl of SDS lysis buffer (supplied by ChIP kit, Upstate Cell Signaling Solutions). To obtain consistent chromatin fragmentation, the lysates were sonicated by a Sonic Dismembrator, Model 500 (Fisher Scientific) at 70% of output power for 10 s on ice and repeated 4 times. The sizes of the majority of sonicated genomic DNA fragments included 250 to 500 bp (Fig. 6A, which is published as supporting information on the PNAS web site). The ChIP procedure was carried out by using the ChIP assay kit and protocol (Upstate Cell Signaling Solutions no. 17-295). The antibody concentration used was that suggested by the manufacturer. In preliminary experiments, it was empirically established that in a given amount of tissue extract, the amount of reelin or GAD67 promoters precipitated by the antibodies failed to increase when the antibody concentration was increased by 10-fold.
An aliquot (1-2%) of the sonicated lysate without antibody (Input) was used to quantitate the total amount of DNA present in different sample extracts before immunoprecipitation. At the end of the ChIP procedure, the protein/DNA cross-linked nucleosomal chromatin complex immunoprecipitated by specific antibodies was reverse cross-linked with NaCl at a final concentration of 100 mM at 65°C for 8-12 h.
Samples were then treated with proteinase-K. Protein-free DNA was extracted in phenol/chloroform and precipitated and washed in ethanol. This extract was used for detection and quantification of RELN, GAD67, GAD65, and β-globin regulatory regions.
Measurements of RELN, GAD67, GAD65, and β-Globin Promoter Fragments by Quantitative Competitive PCR. Primer pair design. For routine purposes, we performed PCR amplification reactions of CpG-rich RELN promoter region from -520 to -225 bp (forward primer: 5′-cgcgcgcggggcaccgtc-3′; reverse primer: 5′-agagaccgacgggctgcc-3′). In some experiments, we also PCR-amplified RELN promoter regions from -234 to +59 bp or from -772 to -493 bp. We PCR amplified the GAD67 promoter region from -760 to -329 bp (forward primer: 5′-agcggcactcgtgcgtgttattaa-3′ and reverse primer: 5′-tgttgggtgagggcaagggaaaat-3′). In some experiments, GAD67 promoter regions from -490 to +60 bp or from -184 to +183 bp were also amplified.
The GAD65 promoter region was amplified by using forward primer, 5′-tctcttcagccgtcagtcaaaacc-3′; and reverse primer, 5′-cacgtgtgcatcgattggctcatt-3′. The β-globin regulatory region (GenBank accession no. LCR/AF071080, bp 61551-61876) was amplified by using forward primer, 5-actgcatctgcaagcctttt-3′; and reverse primer, 5′-gatgtgcctaaagttgccca-3′.
Design of the internal standards (ISs). To quantify by PCR analyses RELN, GAD67, GAD65, and β-globin target promoter sequences, we used appropriate ISs. The ISs were designed by deleting 100-150 bp fragments from the middle of the target gene sequence (as shown in Fig. 7 A and C, which is published as supporting information on the PNAS web site) and were generated by overlap extension PCR reaction with internal deletion primers (40).
Quantification of target promoters by competitive PCR. The ISs contain 3′ and 5′ terminal nucleotide sequences identical to the 3′ and 5′ terminal sequences of the target promoters. They show identical amplification kinetics to the ChIP promoters (Fig. 7B). By virtue of these properties, the internal standard template is amplified by and competes with the target promoter template for the same primers. The amplification products of the IS and of the target gene can be separated by agarose gel electrophoresis taking advantage of the different sizes (Fig. 7B). A representative quantitative PCR analysis of RELN promoter by using its IS is shown in Fig. 7C.
Statistical Analysis. Experimental results are expressed as mean ± SEM. Student's t test and one-way or two-way ANOVA, followed by Dunnett's multiple comparison test, were used to assess the significance of the differences between groups. The criteria of significance (P < 0.05 or <0.01) are indicated in the figure legends.
Results
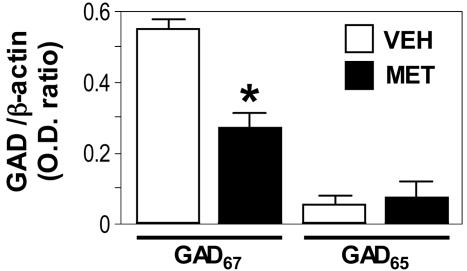
MET-Induced Down-Regulation of Reelin and GAD67 Expression. MET (5.2 mmol/kg, s.c. twice daily), administered to mice for 3, 5, 6, 10, and 15 days down-regulates reelin mRNA and protein expression in a manner related to the treatment duration. Table 1 shows that in FC of MET-treated mice, there is a nonsignficant decrease of reelin mRNA expression after 5 days, but this decrease becomes significantly greater (≈30%) after 10 days and even greater (≈45%) after 15 days of treatment. Table 1 shows a significant decrease in the immunoreactivity levels of the 400-kDa reelin fragment expressed in the FC after 6 and 15 days of MET-treatment compared to the vehicle-treated group. In mice treated with MET for 15 days, GAD67, but not GAD65, was also decreased (Fig. 1; see also Fig. 8, which is published as supporting information on the PNAS web site).
Table 1. MET induces a time-dependent down-regulation of reelin mRNA and protein expression in the frontal cortex of mice.
| VEH
|
Methionine 5.2 mmol/kg twice a day
|
|||
|---|---|---|---|---|
| MET-drug targets | 15-day | 3-day | 6-day | 15-day |
| Reelin mRNA, attomole/μg RNA | 170 ± 16 | 135 ± 33 | 117 ± 7* | 97 ± 7** |
| Reelin/β-actin, OD ratio | 0.54 ± 0.02 | 0.42 ± 0.012 | 0.29 ± 0.04* | 0.22 ± 0.03** |
Each value is the mean ± SE of five mice. *, P < 0.05 vehicle vs. MET; **, P < 0.01 vehicle vs. MET.
Fig. 1.
GAD67 but not GAD65 protein expression is down-regulated in FC of MET-treated mice. GAD67/β-actin and GAD65/β-actin OD ratios were obtained after Western blot of GAD67 and GAD65 on a 10-20% SDS/PAGE gradient. Each value is the mean ± SE of VEH and MET (5.2 mmol/kg/s.c. twice a day for 15 days)-treated mice (n = 3). *, P < 0.01 VEH vs. MET (Student's t test).
As a positive control for MET, we administered glycine (13 mmol/kg twice a day for 15 days). This amino acid in a dose twice the equimolar dose of MET failed to change reelin and GAD67 expression in FC (38).
MET Treatment Recruits MBD Proteins to RELN and GAD67 Hypermethylated Promoters. To test in mice receiving MET for 3, 6, or 15 days whether the increased number of 5mC expressed in CpG islands of RELN or GAD67 promoters recruit MBD proteins, we studied the extent of MeCP2 and MBD2 protein association with RELN and GAD67 promoters by using ChIP assay.
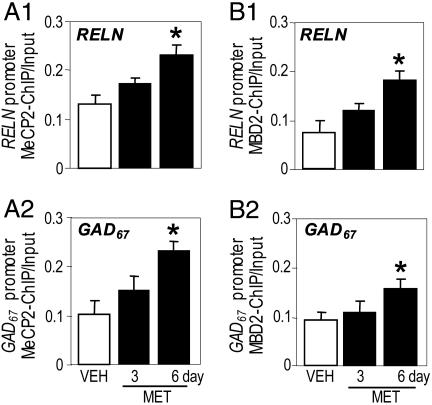
As shown in Fig. 2 A1 and A2, quantitative competitive PCR measurements of RELN (sequence from -520 to -225 bp) and GAD67 (sequences from -760 to -329) promoter fragments immunoprecipitated with a MeCP2-specific antibody indicate that the amount of RELN and GAD67 promoters immunoprecipitated by the MeCP2 antibody is slightly but insignificantly increased after 3 days of MET treatment. In contrast, however, it is significantly increased (≈ 2-fold) in FC extracts from mice treated for 6 days with MET. A 15-day MET treatment increases MeCP2 binding to RELN and GAD67 promoters to an extent similar to that measured after a 6-day treatment with MET (Fig. 3).
Fig. 2.
MET treatment induces a time-related increase in the amount of FC RELN and GAD67 promoters immunoprecipitated with MeCP2 or MBD2 antibodies. RELN (-520 to -225 bp) and GAD67 (-760 to -329 bp) promoter fragments were quantified by using competitive PCR with internal standards (see Materials and Methods). (A1 and A2) The ratios are depicted between the amount of RELN (A1) and GAD67 (A2) promoters immunoprecipitated with MeCP2 antibody (MeCP2-ChIP) and the amount of RELN and GAD67 promoter fragments in the initial nonimmunoprecipitated extract (Input). (B1 and B2) The ratios are depicted between the amount of RELN (B1) and GAD67 (B2) promoters immunoprecipitated with MBD2 antibody (MBD2-ChIP) and the amount of RELN and GAD67 promoter fragments in the initial nonimmunoprecipitated extract (Input). VEH, vehicle; MET, methionine (5.2 mmol/kg s.c. twice a day; the last injection of MET was administered 2 h before killing). The data represent mean ± SE of three mice. *, P < 0.05 vs. vehicle treated group. ANOVA is followed by Dunnett's test.
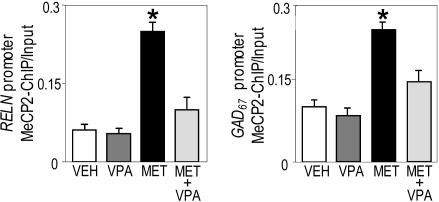
Fig. 3.
VPA prevents the MET-induced increase in the amount of FC RELN (Left) and GAD67 (Right) promoters immunoprecipitated with MeCP2 antibody. In ordinates depicted are the ratios between the amount of RELN and GAD67 promoters immunoprecipitated with MeCP2 antibody (MeCP2-ChIP) and the amount of RELN and GAD67 promoter fragments in the initial nonimmunoprecipitated extract (Input). Treatments: VEH, vehicle; MET, methionine 5.2 mmol/kg s.c. twice a day for 15 days; VPA, valproate 2 mmol/kg s.c. twice a day for 15 days. The data represent mean ± SE of three mice. *, P < 0.05 vs. VPA plus MET-treated group; ANOVA is followed by Dunnett's test.
Comparable results were obtained for the RELN promoter region amplification including sequences from -234 to + 59, or from -772 to -493 bp, or for GAD67 promoter region amplification, including sequences from -490 to + 60 or -184 to + 183 bp.
Importantly, although the amount of RELN and GAD67 promoter DNA immunoprecipitated by MeCP2 antibody dramatically increased in the MET-treated mice, the overall FC MeCP2 levels failed to change after 15 days of MET treatment (Fig. 9, which is published as supporting information on the PNAS web site).
After 6 days but not after 3 days of MET treatment, there was also an ≈2-fold increase of RELN promoter that was immunoprecipitated with the MBD2 antibody (Fig. 2 B1 and B2). However, the extent of the increase of GAD67 promoter immunoprecipitated by the MBD2 antibody was smaller (only 50%) than that immunoprecipitated with the MeCP2 antibody. Overall, the amount of RELN and GAD67 promoter immunoprecipitated with MBD2 antibody was smaller than that immunoprecipitated with MeCP2 antibody.
As a positive control for MET, a group of mice was injected twice a day for 15 days with 13 mmol/kg glycine. This treatment failed to elicit any change in the amount of RELN promoter immunoprecipitated with the MeCP2-specific antibodies [Ratio RELN promoter immunoprecipitated by MeCP2 antibody/RELN promoter input: vehicle (VEH) = 0.070 ± 0.0050, glycine = 0.060 ± 0.0025, n = 3].
MET Fails to Change MeCP2 Interactions with GAD65 Promoter Fragments and β-Globin Regulatory Sites. In MET-treated mice, in addition to the MeCP2 associated with RELN and GAD67 promoters, we also studied two additional genes, GAD65 and the β-globin.
The expression of GAD65 mRNA and protein is not changed by MET treatment (Fig. 1 and ref. 38), whereas the expression of the β-globin gene could not be studied because the cognate protein of this gene is not expressed in the brain of adult mice (41, 42). As shown in Table 2, MET treatment failed to change the amount of GAD65 promoter or β-globin regulatory intragenic region immunoprecipitated by the MeCP2 antibody.
Table 2. MET fails to change the interaction of MeCP2 with GAD65 promoter or β-globin regulatory region.
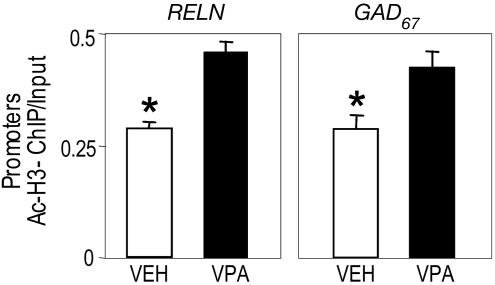
Valproate Regulates the Targeting of MeCP2 to the RELN and GAD67 Promoters. VPA administered to mice in doses of 2 mmol/kg dramatically increases brain nucleosomal acetylated-H3 content (38, 39). The ChIP assay also shows that VPA induces an increased level of acetylated H3 flanking RELN and GAD67 promoter sites (Fig. 4). Moreover, Fig. 3 shows that VPA administered with MET abates the amount of RELN and GAD67 promoter immunoprecipitated with MeCP2 to levels similar to those measured in VEH-treated groups. VPA also induces a small decrease of RELN and GAD67 promoter associated with MeCP2 in VEH-treated groups.
Fig. 4.
VPA enhances the amount of FC RELN and GAD67 promoters immunoprecipitated with acetyl histone (H3) antibody. Depicted are the ratios between the amount of RELN (Left) and GAD67 (Right) promoters immunoprecipitated with acetyl-histone3 (H3) antibody (AcH3-ChIP) and the amount of RELN and GAD67 promoter fragments in the initial nonimmunoprecipitated extract (Input). Mice were injected with VEH or VPA (2 mmol/kg s.c.) 2 hr before killing. The data represent mean ± SE of three experiments *, P < 0.05, Student's t test.
VPA, in doses that increase brain acetylated H3, also exhibits anticonvulsant activity (43). To test whether the HDAC activity or the anticonvulsant activity of VPA is responsible for the decrease in MeCP2 binding to RELN or GAD67 promoters that are elevated in MET-treated mice, we compared the action of VPA with that of imidazenil. This imidazobenzodiazepine is a potent anticonvulsant that positively and allosterically potentiates the action of GABA at GABAA receptors containing α5 subunits but is devoid of intrinsic activity at GABAA receptors expressing α1 subunits (4). Doses of imidazenil that possess a potent anticonvulsant activity (up to 1 mg/kg s.c. 60 min before) failed to increase brain (cortex, hippocampus, and striatum) acetylated H3 content. Moreover, when imidazenil (1 mg/kg s.c. twice a day for 6 days) was administered with MET, it failed to abate the FC increase in RELN promoter that is immunoprecipitated with MeCP2 antibodies (Ratio RELN promoter immunoprecipitated by MeCP2 antibody/RELN promoter input: VEH = 0.075 ± 0.0035, MET = 0.22 ± 0.02, imidazenil 0.13 ± 0.01, imidazenil plus MET = 0.30 ± 0.037; each value is the mean ± SE of three mice). The differences between VEH and imidazenil and between MET and MET plus imidazenil were not significant.
Valpromate, an analogue of VPA that lacks HDAC inhibitory activity in vitro (27), cannot be used as a positive control for VPA because when injected in mice in doses equimolar to that of VPA, it increases brain acetylated H3 content. Most likely, valpromate is metabolized in vivo into VPA.
Discussion
The MET-treated mouse is an incomplete model of SZ morbidity. Nevertheless, it allows the study of epigenetic factors that alter the transcriptional regulation of specific genes operative in cortical GABAergic neurons. These genes include reelin and GAD67, which in our mouse model were found to be epigenetically down-regulated by an extent similar to that reported to occur in cortical GABAergic neurons of SZP.
MeCP2 and MBD2 Binding to Hypermethylated RELN and GAD67 Promoters. Protracted MET treatment, by increasing mouse brain S-adenosyl-methionine (38, 39), induces a hypermethylation of cytosines embedded in the CpG islands of the RELN promoter (Fig. 5 and refs. 38 and 39) that very likely mediate the down-regulation of cortical reelin mRNA and protein expression (38, 39). MET treatment for 15 days also decreases GAD67 mRNA (38) and protein expression (Fig. 1), suggesting that GAD67 promoter hypermethylation may be operative in the transcriptional repression of this gene as well.
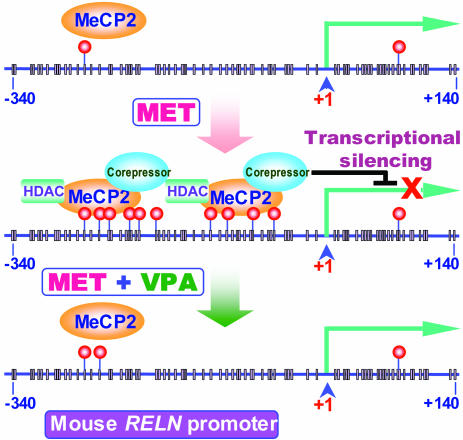
Fig. 5.
Proposed mechanism by which mouse RELN promoter hypermethylation and recruitment of chromatin remodeling complexes (MeCP2, HDACs, and corepressors) regulate reelin gene expression. The mouse reelin (RELN) promoter region depicted here is that reported by Tremolizzo et al. (39). Vertical bars represent CpG dinucleotides present in this region. Pink dots denote 5mC present in the sequence. Note the increase of 5mC in MET (methionine)-treated mice and the prevention of this increase in MET plus VPA (valproate)-treated mice. MeCP2 recruits corepressor complexes including HDACs and induces a state of gene repression. VPA induces loss of MeCP2 binding to the promoter and enhances transcription.
Here, we report that by immunoprecipitating chromatin nucleosomal fragments (250-500 bp) with MeCP2 or MBD2 antibodies and measuring with appropriate internal standards the amount of RELN or GAD67 promoter DNA immunoprecipitated with these antibodies, one finds that in frontal cortex of MET-treated mice, there is an increase MeCP2 or MBD2 binding to RELN or GAD67 promoters. The extent of this increase is related to the duration of MET treatment. It begins to appear at 3 days, reaches a maximum (2-fold increase) between 6 and 15 days of MET treatment, and precedes the reelin mRNA and protein down-regulation that becomes evident between 6 and 10 days and reaches a maximum after 15 days of MET treatment. Importantly, the MET-induced increase of MeCP2 binding to RELN and GAD67 promoter sites is expressed in the absence of an increase of the total MeCP2 protein content.
Based on these data, we propose (Fig. 5) that the increased binding of MeCP2 or MBD2 to the RELN promoter in MET-treated mice reflects an increased amount of 5mC in the CpG island-rich RELN promoter. Likewise, we suggest that the increased binding of MeCP2 or MBD2 to the GAD67 promoter reflects an increase in the number of 5mC in the CpG island of this promoter even though GAD67 promoter methylation has not been studied directly. To obtain further indirect support for the concept that the extent of MBD protein binding to RELN and GAD67 promoters depends on the number of the 5-methylated cytosines, we attempted to reverse the effect of MET with VPA. We show that VPA, at a dose that inhibits HDACs, can increase DNA demethylase activity (44), which prevents the hypermethylation of the RELN promoter induced by MET (Fig. 5 and refs. 38 and 39) and also prevents the MET-induced increase in MeCP2 binding to RELN and GAD67 promoters (Fig. 3).
Taken together, these results are consistent with the view that hypermethylation of RELN and GAD67 promoters represses gene transcription by recruiting chromatin remodeling protein complexes likely including specialized MBD repressor proteins (MeCP2-MBD2) and HDACs (Fig. 5).
Despite the high VPA dose used, its effect on MET-induced MeCP2 binding to RELN and GAD67 promoters appears specifically related to the ability of VPA to inhibit HDACs (38, 39) and/or to induce promoter demethylation (44) but not to its anticonvulsant action (43). Presumably, the action of VPA on MeCP2 binding to RELN promoters is not mimicked by an anticonvulsant drug such as imidazenil, which is a potent positive allosteric modulator of GABA action at specific GABAA receptor subtypes (4) devoid of HDACs inhibitory action.
VPA is an antiepileptic and mood stabilizer that, after protracted treatment at pharmacologically relevant doses, increases reelin and GAD67 expression (38, 39) and enhances GABAergic transmission (43). Interestingly, VPA also accelerates the onset of the beneficial actions of antipsychotics in the treatment of SZ and bipolar disorder patients with psychosis (45).
Whether antipsychotic drugs facilitate the chromatin remodeling action of VPA on RELN or GAD67 promoters should be investigated, although preliminary experiments suggest that treatment (7 days) with haloperidol (1 mg/kg s.c.) or clozapine (10 mg/kg s.c.) fail to change per se the MET-induced increase of MeCP2 binding to RELN promoter.
Differences in MeCP2 and MBD2 Binding to RELN and GAD67 Promoters. Under basal conditions, the binding of MBD2 to GAD67 and RELN promoters is generally lower (≈7%) than that of MeCP2 (≈15%), but the binding of both MBD proteins is increased after MET treatment. After 6 days of MET treatment, the binding of MeCP2 and MBD2 to the RELN promoter increased by ≈200%. Also, the binding of MeCP2 to the GAD67 promoter increased by ≈200%, whereas that of MBD2 to the GAD67 promoter increased by only 50%.
There are a number of features that distinguish MeCP2 and MBD2 in their ability to form HDACs/corepressor complexes and in their affinity to bind to target promoters. For example, MeCP2 binds to monomethylated cytosine with high affinity and recruits Sin 3A, HDACs, and DNA methyltransferase 1, whereas MBD2 is associated with HDAC1 in the MeCP1 corepressor complex, but poorly methylated genes do not provide a good substrate for MBD2 binding. In fact, MBD2 binds only to highly methylated promoters (30, 46). Whether MBD2 and MeCP2 contribute differently, synergistically, or competitively to the down-regulation of reelin and GAD67 expression elicited by MET remains to be clarified. It is likely that both factors cooperate in the chromatin remodeling and in the down-regulation of gene expression. In recent studies (44), it has been suggested that MBD2 may also possess DNA demethylase activity. Thus, the type of MBD protein that interacts with methylated RELN and GAD67 promoters may have a role in the dynamic equilibrium and stability of gene methylation.
Specificity of MET-Induced Increase of MBD Proteins Binding to RELN and GAD67 Promoters. MeCP2 has emerged as an important signal for gene silencing that usually ensures long-term inhibition of methylation-dependent gene expression (30, 31, 47). The question arises as to whether there are other promoters that are the targets of MBD proteins in MET-treated mice GABAergic neurons, in addition to the RELN and GAD67 promoters.
Although we expect that MET treatment may increase methylation of other gene promoters, it is likely that the increase in MeCP2 binding to GAD67 and RELN promoters after MET treatment does not indiscriminately extend to the entire genome.
For example, in the FC and hippocampus of MET-treated mice, reelin and GAD67 expression are down-regulated, but GAD65, which is also expressed in GABAergic neurons, and NSE, which is also a neuronal marker, fail to show changes after MET treatment (38). Accordingly, the GAD65 promoter expresses a low basal level of MeCP2 binding, and although embedded in a CpG island, fails to increase MeCP2 binding in MET-treated mice. Likewise, a gene that is not expressed in the brain (β-globin) fails to show changes in MeCP2 binding in MET-treated mice.
Thus, these data and complementary literature incontrovertibly establish that MeCP2 targets specific genes (30, 34, 35), presumably those that can be hypermethylated, working in concert with other factors (i.e., HDACs) as part of a multiprotein repressor complex.
Conclusions
Recent studies indicate that the RELN promoter is hypermethylated in SZ brains (6, 28). This hypermethylation is likely due to the increased expression of DNA methyltransferase 1 in cortical GABAergic interneurons (7, 8). Therefore, to validate the MET-treated mouse as a partial model of SZ, in the future it would be important to establish in the cortical GABAergic neurons of SZP whether hypermethylated RELN or GAD67 promoters are targeted by MBD proteins and whether this targeting is also required for reelin and GAD67 down-regulation. Additionally, it would be important to study whether changes in promoter remodeling complexes can be responsible for the down-regulation of other genes expressed in GABAergic neurons of SZP, for instance, N-methyl-d-Aspartate receptor subunit NR2A (2), or α7 nicotinic receptor subunits (48).
GAD67, reelin (1, 2), and NR2A (2) are also down-regulated in cortical GABAergic neurons of bipolar depressed patients with psychosis. In the hippocampus of bipolar disorder patients GAD65 expression is also down-regulated (49). Thus, further studies should address the question of whether all these gene promoters are hypermethylated or whether other regulatory mechanisms are responsible for their down-regulation.
GABAergic dysfunction is presumed to play a role in the disruption of the intermittent high frequency synchronized firing of cortical pyramidal neurons that results in disorders of perception and cognition (24). Hence, it is puzzling that SZ and bipolar disorders are still treated with dopaminergic receptor blockers and not with GABAergic receptor modulators. Such a change also appears in order because after many years, we can conclude that dopamine receptor blockers do not treat psychosis satisfactorily. One strategy that could be adopted to reduce the consequences of GABAergic tone down-regulation in SZ could be to enhance the defective GABAergic transmission with positive allosteric modulators of GABAA receptors such as imidazenil. Imidazenil acts as a positive allosteric modulator of GABA action selective at GABAA receptor subtypes including α5 subunits, and it is inactive at GABAA receptors expressing α1 subunits (4). Because of its subunit selective mode of action, imidazenil is therefore devoid of sedative, amnestic, and tolerance liabilities.
An alternative strategy stems from the findings presented in this study that VPA up-regulates reelin and GAD67 expression by decreasing DNA methylation-dependent chromatin remodeling. These findings suggest that to correct the decrease of reelin and GAD67 expression found in cortical GABAergic neurons of SZP, one should consider an “epigenetic pharmacological treatment,” including inhibition of HDACs that very likely induces a postulated DNA demethylase activation (44). This activity should be studied and defined.
Supplementary Material
Acknowledgments
We thank Dr. Francine M. Benes (Laboratories for Structural Neuroscience and the Department of Psychiatry, Harvard Medical School, Boston) and Dr. Brian C. Roth (Department of Biochemistry, Case Western Reserve University Medical School, Cleveland) for their constructive criticisms and suggestions. This work was supported in part by National Institutes of Mental Health Grants MH62188 and MH70855 (to A.G.), MH62090 and MH71667 (to E.C.), and MH462682 (to D.R.G.).
Author contributions: E.D., D.R.G., E.C., and A.G. designed research; E.D., R.C.A.-B., M.V.S., and A.G. performed research; E.D., R.C.A.-B., M.V.S., and D.R.G. contributed new reagents/analytic tools; E.D., R.C.A.-B., M.V.S., D.R.G., E.C., and A.G. analyzed data; and E.D., R.C.A.-B., M.V.S., D.R.G., E.C., and A.G. wrote the paper.
Abbreviations: ChIP, chromatin immunoprecipitation; FC, frontal cortex; 5mC, 5-methylation of cytosine; GAD67, glutamic acid decarboxylase; HDAC, histone deacetylase; IS, internal standard; MBD, methyl binding domain; MET, l-methionine; SZ, schizophrenia; SZP, SZ patients; VEH, vehicle; VPA, valproate.
References
- 1.Guidotti, A., Auta, J., Davis, J. M., DiGiorgi-Gerevini, V., Dwivedi, Y., Grayson, D. R., Impagnatiello, F., Pandey, G., Pesold, C., Sharma, R., et al. (2000) Arch. Gen. Psychiatry 57, 1061-1069. [DOI] [PubMed] [Google Scholar]
- 2.Woo, T. U., Walsh, J. P. & Benes, F. M. (2004) Arch. Gen. Psychiatry 61, 649-657. [DOI] [PubMed] [Google Scholar]
- 3.Lewis, D. A., Hashimoto, T. & Volk, D. W. (2005) Nat. Rev. Neurosci. 6, 312-324. [DOI] [PubMed] [Google Scholar]
- 4.Guidotti, A., Auta, J., Davis, J., Dong, E., Grayson, D. R., Veldic, M., Zhang, X. & Costa E. (2005) Psychopharmacology 180, 191-205. [DOI] [PubMed] [Google Scholar]
- 5.Impagnatiello, F., Guidotti, A. R., Pesold, C., Dwivedi, Y., Caruncho, H., Pisu, M. G., Uzunov, D. P., Smalheiser, N. R., Davis, J. M., Pandey, G. N., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 15718-15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdolmaleky, H. M., Cheng, K. H., Russo, A., Smith, C. L., Faraone, S. V., Wilcox, M., Shafa, R., Glatt, S. J., Nguyen, G., Ponte, J. F., et al. (2005) Am. J. Med. Genet. B. Neuropsychiatr. Genet. 16, 60-66. [DOI] [PubMed] [Google Scholar]
- 7.Veldic, M. Caruncho, H. J., Liu, W. S., Davis, J., Satta, R., Grayson, D. R., Guidotti, A. & Costa, E. (2004) Proc. Natl. Acad. Sci. USA 101, 348-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veldic, M., Guidotti, A., Maloku, E., Davis, J. M. & Costa, E. (2005) Proc. Natl. Acad. Sci. USA 102, 2152-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pesold, C., Impagnatiello, F., Pisu, M. G., Uzunov, D. P., Costa, E., Guidotti, A. & Caruncho, H. J. (1998) Proc. Natl. Acad. Sci. USA 95, 3221-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pesold, C., Liu, W. S., Guidotti, A., Costa, E. & Caruncho, H. J. (1999) Proc. Natl. Acad. Sci. USA 96, 3217-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez, M. A., Pesold, C., Liu, W. S., Kriho, V., Guidotti, A., Pappas, G. D. & Costa, E. (2000) Proc. Natl. Acad. Sci. USA 97, 3550-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacor, P. N., Grayson, D. R., Auta, J., Sugaya, I., Costa, E. & Guidotti, A. (2000) Proc. Natl. Acad. Sci. USA 97, 3556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Arcangelo, G., Miao, G. G., Chen, S. C., Soares, H. D., Morgan, J. I. & Curran, T. (1995) Nature 374, 719-723. [DOI] [PubMed] [Google Scholar]
- 14.Costa, E., Davis, J., Grayson, D. R., Guidotti, A., Pappas, G. D. & Pesold, C. (2001) Neurobiol. Dis. 8, 723-742. [DOI] [PubMed] [Google Scholar]
- 15.Niu, S., Renfro, A., Quattrocchi, C. C., Sheldon, M. & D'Arcangelo, G. (2004) Neuron 41, 71-84. [DOI] [PubMed] [Google Scholar]
- 16.Liu, W. S., Pesold, C., Rodriguez, M. A., Carboni, G., Auta, J., Lacor, P., Larson, J., Condie, B. G., Guidotti, A. & Costa, E. (2001) Proc. Natl. Acad. Sci. USA 98, 3477-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weeber, E. J., Beffert, U., Jones, C., Christian, J. M., Forster, E., Sweatt, J. D. & Herz, J. (2002) J. Biol. Chem. 277, 39944-39952. [DOI] [PubMed] [Google Scholar]
- 18.Dong, E., Caruncho, H., Liu, W. S., Smalheiser, N. R., Grayson, D. R., Costa, E. & Guidotti, A. (2003) Proc. Natl. Acad. Sci. USA 100, 5479-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fatemi, S. H., Earle, J. A. & McMenomy, T. (2000) Mol. Psychiatry 5, 654-663. [DOI] [PubMed] [Google Scholar]
- 20.Goldman-Rakic, P. S. & Selemon, L. D. (1997) Schizophr. Bull. 23, 437-458. [DOI] [PubMed] [Google Scholar]
- 21.Glantz, L. A. & Lewis, D. A. (2001) Arch. Gen. Psychiatry 58, 203. [DOI] [PubMed] [Google Scholar]
- 22.Black, J. E., Kodish, I. M., Grossman, A. W., Klintsova, A. Y., Orlovskaya, D., Vostrikov, V., Uranova, N. & Greenough W. T. (2004) Am. J. Psychiatry 161, 742-744. [DOI] [PubMed] [Google Scholar]
- 23.Soghomonian, J. J. & Martin, D. L. (1998) Trends Pharmacol. Sci. 19, 500-505. [DOI] [PubMed] [Google Scholar]
- 24.Spencer, K. M., Nestor, P. G., Perlmutter, R., Niznikiewicz, M. A., Klump, M. C., Frumin, M., Shenton, M. E. & McCarley, R. W. (2004) Proc. Natl. Acad. Sci. USA 101, 17288-17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costa, E., Davis, J. M., Dong, E., Grayson, D. R., Guidotti, A., Tremolizzo, L. & Veldic, M. (2004) Crit. Rev. Neurobiol. 16, 1-23. [DOI] [PubMed] [Google Scholar]
- 26.Costa, E., Chen, Y., Davis, J., Dong, E., Noh, J. S., Tremolizzo, L., Veldic, M., Grayson, D. R. & Guidotti, A. (2002) Mol. Interv. 2, 47-57. [DOI] [PubMed] [Google Scholar]
- 27.Chen, Y., Sharma, R., Costa, R. H., Costa, E. & Grayson, D. R. (2002) Nucleic Acids Res. 30, 2930-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grayson, D. R., Jia, X., Chen, Y., Sharma, R. P., Mitchell, C. P., Guidotti, A. & Costa, E. (2005) Proc. Natl. Acad. Sci. USA 102, 9341-9346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell, C. P., Chen, Y., Kundakovic, M., Costa, E. & Grayson, D. R. (2005) J. Neurochem. 93, 483-492. [DOI] [PubMed] [Google Scholar]
- 30.Fan, G. & Hntnick, L. (2005) Cell Res. 15, 255-261. [DOI] [PubMed] [Google Scholar]
- 31.Ng, H. H. & Bird, A. (1999) Curr. Opin. Gene Dev. 9, 158-163. [DOI] [PubMed] [Google Scholar]
- 32.Tucker, K. L. (2001) Neuron 30, 649-652. [DOI] [PubMed] [Google Scholar]
- 33.Collins, A. L., Levenson, J. M., Vilaythong, A. P., Richman, R., Armstrong, D. L., Noebels, J. L., David Sweatt, J. & Zoghbi, H. Y. (2004) Hum. Mol. Genet. 13, 2679-2689. [DOI] [PubMed] [Google Scholar]
- 34.Chen, W. G., Chang, Q., Lin, Y., Meissner, A., West, A. E., Griffith, E. C., Jaenisch, R. & Greenberg, M. E. (2003) Science 302, 885-889. [DOI] [PubMed] [Google Scholar]
- 35.Martinowich, K., Hattori, D., Wu, H., Fouse, S., He, F., Hu, Y., Fan, G. & Sun, Y. E. (2003) Science 302, 890-893. [DOI] [PubMed] [Google Scholar]
- 36.Akbarian, S., Chen, R. Z., Gribnau, J., Rasmussen, T. P., Fong, H., Jaenisch, R. & Jones, E. G. (2001) Neurobiol. Dis. 8, 784-791. [DOI] [PubMed] [Google Scholar]
- 37.Wyatt, A., Benedict, R. G. & Davis, J. (1971) Schiz. Bull. 4, 10-44. [Google Scholar]
- 38.Tremolizzo, L., Carboni, G., Ruzicka, W. B., Mitchell, C. P., Sugaya, I., Tueting, P., Sharma, R., Grayson, D. R., Costa, E. & Guidotti, A. (2002) Proc. Natl. Acad. Sci. USA 99, 17095-17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tremolizzo, L., Doueiri, M. S., Dong, E., Grayson, D. R., Davis, J. M., Pinna, G., Tueting, P., Rodriguez-Menendez, V., Costa, E. & Guidotti, A. (2005) Biol. Psychiatry 57, 500-509. [DOI] [PubMed] [Google Scholar]
- 40.Auta, J., Chen, Y., Ruzicka, W. B. & Grayson, D. R. (2005) in Practical Neurochemistry: Methods, Handbook of Neurochemistry and Molecular Neurobiology, eds. Baker, G. B., Dunn, S. M. J. & Holt, A. (Kluver, New York), in press.
- 41.Tolhuis, B., Palstra, R. J., Splinter, E., Grosveld, F. & de Laat, W. (2002) Mol. Cell 10, 1453-1465. [DOI] [PubMed] [Google Scholar]
- 42.Forsberg, E. C., Downs, K. M. & Bresnick, E. H. (2000) Blood 96, 334-339. [PubMed] [Google Scholar]
- 43.Loscher, W. (2002) CNS Drugs 16, 669-694. [DOI] [PubMed] [Google Scholar]
- 44.Detich, N., Bovenzi, V. & Szyf, M. (2003) J. Biol. Chem. 278, 27586-27592. [DOI] [PubMed] [Google Scholar]
- 45.Wassef, A., Baker, J. & Kochan, L. D. (2003) J. Clin. Psychopharmacol. 23, 601-640. [DOI] [PubMed] [Google Scholar]
- 46.Sarraf, S. A. & Stancheva, I. (2004) Mol. Cell 15, 595-605. [DOI] [PubMed] [Google Scholar]
- 47.Klose, R. & Bird, A. (2003) Science 302, 793-795. [DOI] [PubMed] [Google Scholar]
- 48.Martin, L. F., Kem, W. R. & Freedman, R. (2004) Psychopharmacology 174, 54-56. [DOI] [PubMed] [Google Scholar]
- 49.Heckers, S., Stone, D., Walsh, J., Shick, J., Koul, P. & Benes, F. M. (2002) Arch. Gen. Psychiatry 59, 521-529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.