Abstract
Thyroid hormone (TH) is required for limb development in Xenopus laevis. Specific cell types in the growing limb were targeted for expression of a dominant negative form of the TH receptor by sperm-mediated transgenesis. Limb muscle development, the innervation of muscle from the spinal cord, and cartilage growth can be inhibited without affecting patterning of the limb or differentiation of other cell types. Remodeling of the skin occurs late in metamorphosis after the limb has formed. The coordination of these independent programs is affected in part by the control that TH exerts over DNA replication in all cell types of the limb.
Keywords: transgenesis, cell autonomy, thyroid hormone receptor, DNA replication
Specification of the vertebrate limb has been one of the most extensively studied problems in developmental biology (1–4). Cell division, growth, patterning of the limb axes, and cell-type-specific differentiation are seamlessly orchestrated to produce a complex organ. Many of the crucial genes that determine limb pattern encode secreted proteins that interact with receptors on neighboring cells and influence their future fate (5). In anurans, such as Xenopus laevis, external hind limb buds appear in tadpoles ≈2 weeks after the completion of embryogenesis. By this early stage, when the limb buds are spherical [Nieuwkoop and Faber stage (NF) 50 (6); see Fig. 1], the future axes have been established (7). The hind limb bud elongates in the next few weeks (NF 52) and forms a typical vertebrate limb. By the onset of metamorphic climax (NF 59) the limb is morphologically complete but still nonfunctional. The tadpole switches abruptly at NF 60 from tail to leg swimming (8) and then completes metamorphosis by resorbing its tail. Limb development in frogs is distinguished from that in higher vertebrates because it is controlled by thyroid hormone (TH).
Fig. 1.
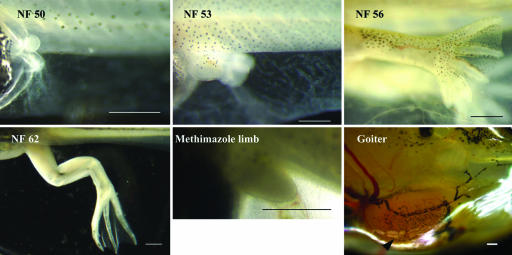
Hind limb development in X. laevis tadpoles. The NF numbers shown are those assigned by Nieuwkoop and Faber (6) to normal hind limb development. An arrested hind limb bud of a tadpole grown continuously in the presence of 1 mM methimazole (a goitrogen) for 16 months is shown. The inhibitor was added at the end of embryogenesis (1 week after fertilization, NF 46), when the tadpole begins to feed. This tadpole developed a large goiter (arrow). (Scale bar: 0.5 mm.)
How TH is involved in limb development is not known. In keeping with the cell–cell interactions that are essential for establishing the limb's pattern, TH might control one cell type, which in turn influences the other cells in the limb. Alternatively, multiple cell-autonomous programs are coordinated to form a limb. To investigate this possibility we have analyzed the role of TH in the differentiation of individual cell types of the developing limb by sperm-mediated transgenesis (9) using cell-type specific promoters to drive a dominant negative form of the TH receptor (TRDN). Because TH receptors are not secreted proteins and reside constitutively in the nucleus, the effect of the TRDN is cell-autonomous. Even though limb development is a synchronized process, we find that differentiation of each of the major cell types is under the cell-autonomous control of TH.
Materials and Methods
Transgenic X. laevis Tadpoles. Transgenic X. laevis were prepared by the sperm-mediated method that employs the restriction enzyme NotI (9). Three different promoters were used to control the expression of a TRDN transgene. The TRDN is a deletion of 12 aa from the C terminus of X. laevis thyroid receptor α. The constitutive versions of the X. laevis neural β-tubulin (NβT) (9) and the mouse minimal collagen promoter (10) in the plasmid pCS2+ constructs have been described (8, 11). In these plasmids GFP is fused to the N terminus of the TRDN. Expression of the third promoter used in these experiments, the X. laevis “cardiac actin” promoter, has been placed under control of the tetracycline-inducible system (12). A description of the two plasmids has been reported (13). GFP was fused to the C terminus of TRDN with a small flexible linker forming the plasmid called pCS2+(tetO)TRDN/GFP. This plasmid was cotransfected with pCar/rTA2S-M2. A doubly transgenic male frog was raised to sexual maturity and bred with a wild-type female. Half of the progeny were transgenic for both plasmids. After 1 week of embryogenesis, 20 feeding F1 tadpoles were raised in 4 liters of 0.1 MMR (10 mM NaCl/0.2 mM KCl/0.1 mM MgCl2/0.2 mM CaCl2/0.5 mM Hepes, pH 7.5). The transgene was induced with 50 μg/ml doxycycline (Sigma) added to the rearing water. The medium was changed twice a week. The cell expression pattern of each transgenic experiment was verified by fluorescence and also by immunostaining of limb sections with an antibody to GFP (Torrey Pines Biolabs, San Diego) (14).
In Situ Hybridization. Fixation in paraformaldehyde, sectioning, and in situ hybridization with digoxygenin-labeled antisense RNAs has been described (15). The probes that were used are identified in each experiment by the accession number of the cDNA.
Results
TH-Controlled DNA Replication and Growth of the Limb. Stages of normal limb development during tadpole growth are shown in Fig. 1. The thyroid gland of X. laevis started to function 3 days after the tadpole began to feed (10 days after fertilization, NF 46), weeks before there was an external hind limb bud. A tadpole reared in the continuous presence of the goitrogen methimazole continued to eat and grow even though DNA replication and muscle and cartilage development in the limb ceased. The hind limb of such a tadpole arrested at the morphological equivalent of an NF 52 tadpole (Fig. 1). The future axes of the limb have been determined by this stage (7). After 16 months of continuous growth in methimazole, this tadpole formed a large goiter (Fig. 1), attesting to the effective shutoff of its endogenous TH production.
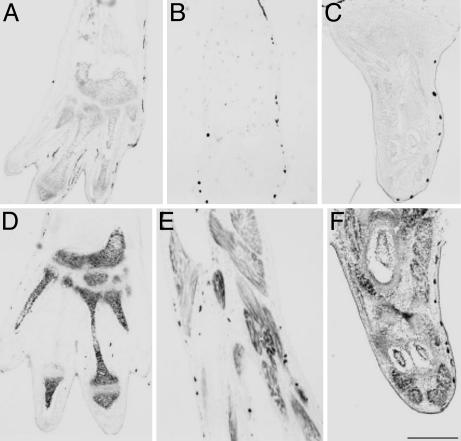
During spontaneous metamorphosis, differentiated muscle fibers appear at around NF 53, when the limb has developed into a dumbbell-shaped structure. Peripheral nerves begin to penetrate the developing limb at this stage. Metamorphic changes including limb development can be arrested by methimazole at any stage up to around NF 58 (16). After that stage, the endogenous TH concentration is high enough to complete metamorphosis even if the goitrogen is added. TH induces DNA replication in limbs (11). We have found in microarray experiments (B.D., L.C., and D.D.B., unpublished data) that many cell-cycle-related genes are induced in the limb by TH treatment. This up-regulation occurs in all limb cell types as demonstrated here by in situ hybridization with a probe for the cell cycle gene cyclin D2 (Fig. 2). We used a cartilage-specific probe (sox-9) and a muscle-specific probe (actin) to demonstrate that TH-induced gene activation occurs in both cell types (Fig. 2).
Fig. 2.
TH controls bone and cartilage formation (A and D), muscle development (B and E), and DNA replication (C and F) in the limb. Premetamorphic NF 55 tadpoles were grown for 1 week in 1 mM methimazole. Then 10 nM T3 was added to the rearing water of one group of tadpoles (D–F) but not others (A–C) for 3 days. The hind limbs were fixed, and frontal sections were prepared for in situ hybridization (15). sox-9 (CF285736; A and D), alpha 1 skeletal muscle actin (BC046739; B and E), and cyclin D2 (CA792959; C and F) were used. (Scale bar: 200 μm.)
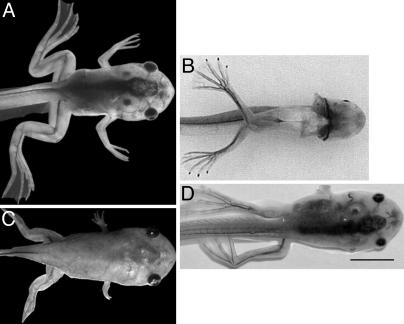
Muscle Differentiation in the Limb Is Cell-Autonomous. The X. laevis promoter of the cardiac actin gene (pCar) drives a reporter in skeletal, cardiac, and smooth tadpole muscle (17). We have demonstrated previously (18) that the TRDN transgene directed to muscle inhibits the TH-controlled death of the tail muscle in a cell-autonomous manner. The limbs of high-expressing individuals form cartilage that becomes ossified at the same time as control tadpoles. However, these limbs lack most of their muscle and are completely paralyzed (18) (Fig. 3D). We introduced the transgene under the control of the tetracycline-inducible system (13). When the TRDN transgene was induced even as late as NF 56, a stage at which limb muscle had already begun to form, the preexisting muscle degenerated even while the limbs continued to grow (L.C., B.D., and D.D.B., unpublished data). The paralyzed animals died at metamorphic climax, but the shape and size of their limbs, including cartilage formation, were normal.
Fig. 3.
Transgenic NF 63 tadpoles at the climax of metamorphosis showing the typical limb phenotypes that occur when these three promoters drive a TRDN transgene. (A) Wild type. (B) Constitutive NβT/TRDN paralyzed phenotype (ventral view). (C) Constitutive collagen/TRDN “pig” phenotype. (D) Inducible pCar/TRDN paralyzed phenotype. This F1 progeny was induced with 50 μg/ml doxycycline from NF 52 to NF 63. (Scale bar: 2 mm.)
Innervation of the Limb Is Cell-Autonomous. The X. laevis NβT promoter (9) expresses specifically in neural tissue of the tadpole (8, 19). Tadpoles transgenic for the TRDN gene controlled by this neural-specific promoter developed normally to the climax of metamorphosis. The most striking phenotype of these tadpoles was their quadraplegia (Fig. 3B). This phenotype was caused by the interference of TH-dependent changes in the spinal cord (8). These paralyzed animals had normal muscle and cartilage/bone formation.
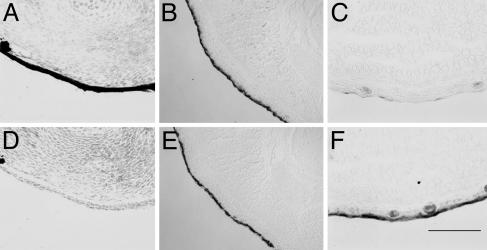
Replacement of the Tadpole Limb Epidermis by Adult Skin. The self-replicating tadpole skin changes to a germinative frog epidermis at the climax of metamorphosis (20). The TH-induced death of the larval (tadpole) epidermis of the body is apoptotic and cell-autonomous (14). Even though the limb is a developing adult structure, it is covered by tadpole skin (larval keratin) throughout its development. The change of limb skin to an adult epidermis (adult keratin) occurs at metamorphic climax, exactly when that covering the body changes (Fig. 4). We conclude that the TH-induced transition of tadpole skin to adult skin is independent of the other differentiating limb cell types; it occurs after the other limb cell types have formed.
Fig. 4.
Tadpole skin covering the limb changes to adult skin at the climax of metamorphosis. Shown are in situ hybridizations of limb cross sections (14) with larval keratin (X04807; A–C) and adult keratin (X02895; D–F). Stages were as follows: NF 53 (A and D), NF 59 (B and E), and NF 62 (C and F). (Scale bar: 200 μm.)
Inhibition of Limb Cartilage Growth. Formation of the cartilage precedes ossification just as it does in the growth of other vertebrate limbs. By the climax of metamorphosis, ossification is advanced except at the epiphyses, which remain cartilaginous until a frog has reached maturity. A mouse collagen enhancer fused to a minimal collagen α2 (1) promoter (10) drives transgene expression in many tissues of developing tadpoles (11). Expression in the growing limb is active in the cartilage, especially in the epiphyseal regions, by NF 56. The transgene is also expressed in limb muscle. These tadpoles developed to climax with stunted limbs and died at around NF 63, before completing metamorphosis (Fig. 3). Despite their short limbs, they converted from tail to leg swimming before death, allowing us to conclude that there was no impairment of muscle development or its innervation from the spinal cord. The expression in muscle may have caused some damage, but the level of the transgene was not high enough in the muscle to cause the phenotype seen in the pCar/TRDN animals. We conclude that the growth of limb cartilage is another cell-autonomous target of TH.
Discussion
Limb development is one of the earliest TH-controlled events in metamorphosis. In the absence of the hormone, development of a limb bud is arrested before the differentiation of muscle and cartilage (NF 52) (Fig. 1). By the climax of metamorphosis (NF 59), a tadpole has developed a completely formed limb but still swims with its tail. One day later the tadpole shifts abruptly from tail to leg swimming (8). Another feature of TH-controlled limb development is the continuous requirement for the hormone. Interruption of TH synthesis by the goitrogen methimazole at any time from NF 52 to 57 will arrest limb development at that stage (16).
Most frog organs are formed from a preexisting tadpole organ that has served a similar function, but the developing limb has no function in a tadpole. In that respect, the limb's developmental profile resembles that of an insect imaginal disk that resides in the larva in an undifferentiated yet fully determined state until the metamorphosing hormone ecdysone stimulates its development to an adult structure.
This study concentrated on the hind limb. The X. laevis forelimb is smaller and less accessible. However, in each case that we examined the forelimb, it showed the same features as the hind limb: namely, paralysis in the case of the neural and muscle promoters and shortening when the collagen promoter drove the TRDN transgene (Fig. 3).
A functional thyroid gland first appears 3 days after the tadpole begins to feed (10 days after fertilization, NF 46). As early as NF 51, before TH begins to control subsequent limb development, polarizing zones are present in the X. laevis hind limb (7). The localized expression of sonic hedgehog denoting the zone of polarizing activity is detected as early as NF 50 (21, 22) and ends at around NF 53. Markers of the apical ectodermal ridge such as Fgf8 are actively expressed in the limb bud by NF 50 and silent by NF 55 (22). Grafted tissue from NF 52 or earlier produces organized limb structures.
Up to this point, anuran limb formation is much like that of other vertebrates. Then TH controls the subsequent cell proliferation, growth, and muscle and cartilage formation. Exogenous TH induces muscle- and cartilage-specific gene expression in 72 h (23) (Fig. 2). TH controls innervation of the limb by acting within the spinal cord (8). When the expression of the NβT/TRDN transgene was controlled by the tetracycline-inducible system, two TH-sensitive steps of the innervation process were revealed (13). The increase of limb motor neurons that occurs very early in tadpole life is inhibited by the NβT/TRDN transgene. When the transgene is expressed throughout limb development (NF 52–56), the fully developed limbs are paralyzed. However, the inhibition of functional limb innervation has no effect on the gross morphology of limb muscle or cartilage development (Fig. 3).
The most remarkable of these cell-autonomous phenotypes occurs when the TRDN transgene is directed to muscle. The cardiac actin promoter controls a terminally differentiated muscle gene; therefore, the transgene is not expressed detectably in the limb before NF 54. Limb myogenesis begins before the transgene is activated. Yet the constitutive expression of TRDN driven by the muscle promoter results in an otherwise normal-looking limb with almost no muscle (18). This paradox has been resolved by placing the muscle-specific transgene under the control of an inducible promoter (13). When the transgene is activated after limb muscle has formed, the preexisting muscle decays, yielding the paralyzed phenotype shown in Fig. 3D. The resulting limbs have normal long bones (18). The inhibitory transgene selectively induces muscle degeneration; therefore, TH acts in the muscle lineage at least at two points. TH controls DNA replication of muscle progenitors along with the other limb cell types (Fig. 2), and then TH promotes survival of differentiated muscle fibers. Frogs that are transgenic for the pCar/TRDN under control of the tetracycline system have been reared for >2 months in high concentrations of doxycycline beginning several weeks after metamorphosis. They eat and grow normally but develop abnormal hind limbs. The limbs have not been analyzed histologically, but the frogs have restricted movement from the hip to the knee even though they can still swim with their feet. This much reduced phenotype in frogs agrees with the general resistance of frogs to TH compared with the sensitivity of tadpoles to the hormone. The cardiac actin promoter drives expression in all types of tadpole muscle, including the heart and the smooth muscle of the intestine. The effect of the transgene expressed in smooth muscle on intestinal remodeling is very minimal (A.M.S. and D.D.B., unpublished data).
The mouse collagen promoter drives transgene expression widely in young tadpoles (11). Late in tadpole development, limb expression is restricted to cartilage and muscle (data not shown). These animals die at the climax of metamorphosis, before resorbing their tails. They differ from the pCar/TRDN phenotype in that their limbs are shortened dramatically (Fig. 3) yet function just before they die. The shortened limb phenotype caused by the collagen promoter controlling the transgene is presumed to be due to inhibition of long bone growth by expression of the transgene in the growing cartilage. The concentration of the transgene in muscle is too low to cause a muscle phenotype.
The conclusion that skin remodeling is independent of the rest of limb development is based on the fact that the down-regulation of larval-specific genes and the up-regulation of adult-specific genes occur in the limb at climax when epidermal remodeling happens throughout the body (Fig. 4). By this stage, the limb has developed extensively (Fig. 1).
The participation of multiple independent TH-controlled programs in the developmental fate of an organ at metamorphosis has been demonstrated also for tail resorption (18). The tail muscle and fibroblasts are separate targets of the hormone.
Research on the vertebrate limb has emphasized the importance of cell–cell interaction in the early establishment of the several axes that will underlie the limb's pattern (1). The ability to intervene in the differentiation of selected limb cell types provides a powerful tool to investigate later stages of TH-induced limb differentiation. Here, we present evidence that anuran limb muscle, innervation of the limb, cartilage growth, and skin development are TH-dependent yet cell-autonomous programs. Although these programs can be inhibited independently, they participate in the formation of a correctly proportioned integrated organ. The control by TH of DNA replication and limb growth encompasses all cell types of the limb throughout the entire development of the limb and therefore must play an essential role in the integration of the cell-autonomous programs involved in limb development. It is not likely that the specific inhibition of DNA replication can itself account for the TH-controlled programs. The muscle promoter is late and not activated until cell replication has stopped and fusion and differentiation of muscle fibers has begun. The NβT/TRDN does interfere with replication of limb motor neurons very early in tadpole life (8), but it also has an essential role in the late innervation of limb muscle (13). In a methimazole-arrested limb, the timing of the response to added TH demonstrates that the activation of muscle-specific and bone-specific genes occurs at least as rapidly as the activation of DNA synthesis.
Acknowledgments
We thank Drs. Chen-Ming Fan, Marnie Halpern, and Cliff Tabin for their critical reading of the manuscript. This research was supported by grants from the National Institutes of Health and the G. Harold and Leila Y. Mathers Charitable Trust (to D.D.B.).
Author contributions: D.D.B., L.C., B.D., N.M.-A., and A.M.S. designed research; D.D.B., L.C., B.D., N.M.-A., A.M.S., and R.J. performed research; D.D.B., L.C., B.D., N.M.-A., and A.M.S. analyzed data; and D.D.B. wrote the paper.
Abbreviations: TH, thyroid hormone; TRDN, dominant negative form of the TH receptor; NF, Nieuwkoop and Faber stage; NβT, neural β-tubulin.
References
- 1.Dudley, A. T., Ros, M. A. & Tabin, C. J. (2002) Nature 418, 539–544. [DOI] [PubMed] [Google Scholar]
- 2.Tickle, C. (2003) Dev. Cell 4, 449–458. [DOI] [PubMed] [Google Scholar]
- 3.Hinchliffe, J. R. (2002) Int. J. Dev. Biol. 46, 835–845. [PubMed] [Google Scholar]
- 4.Koussoulakos, S. (2004) Anat. Embryol. 209, 93–105. [DOI] [PubMed] [Google Scholar]
- 5.Capdevila, J. & Izpisua Belmonte, J. C. (2004) Annu. Rev. Cell Dev. Biol. 17, 87–132. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwkoop, P. D. & Faber, J. (1956) Normal Table of Xenopus laevis (Daudin) (Elsevier/North-Holland, New York).
- 7.Cameron, J. & Fallon, J. F. (1977) Dev. Biol. 55, 320–330. [DOI] [PubMed] [Google Scholar]
- 8.Marsh-Armstrong, N., Cai, L. & Brown, D. D. (2004) Proc. Natl. Acad. Sci. USA 101, 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroll, K. & Amaya, E. (1996) Development (Cambridge, U.K.) 122, 3173–3183. [DOI] [PubMed] [Google Scholar]
- 10.Bou-Gharios, G., Garrett, L. A., Rossert, J., Niederreither, K., Eberspaecher, H., Smith, C., Black, C. & Crombrugghe, B. (1996) J. Cell Biol. 134, 1333–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schreiber, A., Das, B., Huang, H., Marsh-Armstrong, N. & Brown, D. D. (2001) Proc. Natl. Acad. Sci. USA 98, 10739–10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urlinger, S., Baron, U., Thellmann, M., Hasan, M. T., Bujard, H. & Hillen, W. (2000) Proc. Natl. Acad. Sci. USA 97, 7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das, B. & Brown, D. D. (2004) Proc. Natl. Acad. Sci. USA 101, 4839–4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber, A. & Brown, D. D. (2003) Proc. Natl. Acad. Sci. USA 100, 1769–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry, D. L., Schwartzman, R. A. & Brown, D. D. (1998) Dev. Biol. 203, 12–23. [DOI] [PubMed] [Google Scholar]
- 16.Elinson, R. P., Remo, B. & Brown, D. D. (1999) Dev. Biol. 215, 243–252. [DOI] [PubMed] [Google Scholar]
- 17.Mohun, T. J., Garrett, N. & Gurdon, J. B. (1986) EMBO J. 5, 3185–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das, B., Schreiber, A. M., Huang, H. & Brown, D. D. (2002) Proc. Natl. Acad. Sci. USA 99, 12230–12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh-Armstrong, N., Huang, H., Berry, D. L. & Brown, D. D. (1999) Proc. Natl. Acad. Sci. USA 96, 14389–14393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshizato, K. (1992) Dev. Growth Differ. 34, 607–612. [DOI] [PubMed] [Google Scholar]
- 21.Endo, T., Tamura, K. & Ide, H. (2000) Dev. Biol. 220, 296–306. [DOI] [PubMed] [Google Scholar]
- 22.Endo, T., Yokoyama, H. & Tamura, K. (1997) Dev. Dyn. 209, 227–232. [DOI] [PubMed] [Google Scholar]
- 23.Buckbinder, L. & Brown, D. D. (1992) J. Biol. Chem. 267, 25786–25791. [PubMed] [Google Scholar]