Abstract
Sandoz-adalimumab (SDZ-ADL; Hyrimoz®, GP2017) is an adalimumab (ADL) biosimilar approved for the treatment of immune-mediated inflammatory diseases. Here, we review the available literature on SDZ-ADL from controlled and real-world evidence studies. A literature search was carried out to identify articles published up to July 2023 reporting data on efficacy, safety, immunogenicity, and treatment retention rates for SDZ-ADL. In randomized clinical trials, the efficacy, safety, and immunogenicity of SDZ-ADL were comparable to those observed for reference-adalimumab (ref-ADL) and not altered after single or multiple drug switches. Real-world studies confirmed the effectiveness and safety of treatment initiation with SDZ-ADL and of switching to SDZ-ADL from ref-ADL or from other ADL biosimilars. This literature review provides evidence that SDZ-ADL is as effective and safe as ref-ADL in both biologic-naïve and biologic-experienced patients.
Keywords: Adalimumab, Arthritis, Biosimilar, GP2017, Hyrimoz, Inflammatory bowel disease, Psoriasis, Sandoz-adalimumab, Spondyloarthritis
Key Summary Points
| Sandoz-adalimumab (SDZ-ADL; Hyrimoz®, GP2017) is an adalimumab (ADL) biosimilar approved for the treatment of immune-mediated inflammatory diseases, including psoriasis, inflammatory bowel disease, and several inflammatory rheumatic diseases |
| This literature review covers published clinical and real-world evidence on the efficacy and safety of SDZ-ADL across approved indications in detail |
| In clinical trials, the efficacy, safety, and immunogenicity of SDZ-ADL have been shown to match those observed for reference adalimumab (ref-ADL) and are not impacted by single or multiple drug switches |
| These clinical observations are substantiated by evidence from real-world studies, confirming the effectiveness and safety of SDZ-ADL in both biologic-naïve patients and those switching from ref-ADL or other ADL biosimilars |
| The findings of this literature review support that SDZ-ADL is as effective and safe as ref-ADL in both biologic-naïve and biologic-experienced patients and should enhance confidence in SDZ-ADL among clinicians and patients |
Introduction
Cost-effective therapeutics are desirable for any healthcare system. Despite having revolutionized the management of immune-mediated inflammatory diseases, a major drawback of biologic medicines is their significant cost burden on healthcare systems. Biosimilars are more affordable, because they benefit from abbreviated clinical development programs and extrapolation of indications, and they can improve patient care by increasing access to biologic therapy [1, 2]. Biosimilars undergo a rigorous evaluation procedure following the European Medicines Agency (EMA)-, US Food and Drug Administration (FDA)-, and World Health Organization-guided approach of stepwise totality of evidence, and approved biosimilars have been shown to match their reference medicines in terms of efficacy, safety, and immunogenicity [2–4].
Adalimumab (ADL) is an anti-tumor necrosis factor (TNF)-α monoclonal antibody that binds to TNF-α and blocks its interaction with the TNF receptor [5]. ADL biosimilars first entered the European market in 2018, after patent loss of the reference product Humira® (AbbVie) [6]. In the USA, ADL biosimilars were approved by the FDA several years ago but have only recently become commercially available because of longstanding patent disputes [6]. Biosimilars were reported to have captured around 35% of the European ADL market within a year of their introduction, with a 40% decrease in cost compared to reference-ADL (ref-ADL) reported for some biosimilars [6]. In some European countries, discounts on the reference medicine of up to 90% have been reported following the introduction of biosimilars [7]. In many countries, ADL biosimilars are commonly prescribed in biologic-naïve patients, in patients switched from ref-ADL, and, more recently, in patients experiencing multiple switches between ADL biosimilars. Healthcare professionals required to switch patients to biosimilars due to prescription and reimbursement policies, and physicians with limited clinical experience in prescribing ADL biosimilars, still may have concerns about effectiveness and/or increased safety or immunogenicity signals following switching.
The ADL biosimilar Sandoz-adalimumab (SDZ-ADL; Hyrimoz®, GP2017) was approved in the European Union (EU) in July 2018 for use in all licensed indications of ref-ADL [8, 9]. The FDA has also granted approval of SDZ-ADL for a range of indications, including rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), psoriatic arthritis (PsA), plaque psoriasis (PsO), ankylosing spondylitis (AS), adult and pediatric Crohn’s disease (CD), ulcerative colitis (UC), and, most recently, hidradenitis suppurativa (HS) [10]. The recent development of the high-concentration, citrate-buffer-free formulation (HCF) of SDZ-ADL was undertaken to provide options for low-volume dosing and improved patient convenience [11]. Since the confirmatory phase III trials and approval of SDZ-ADL, numerous real-world studies have provided valuable insights into the effectiveness, safety, and retention rates of SDZ-ADL [12–14]. Here, published clinical and real-world evidence on the use of SDZ-ADL in biologic-naïve patients, and in patients who switched from ref-ADL or from other ADL biosimilars, is reviewed in detail.
Methods
Data Sources and Search Strategy
A literature search was conducted via OVID in MEDLINE, EMBASE, Cochrane database of systematic reviews, and BIOSIS previews to identify articles covering SDZ-ADL data up to July 31, 2023, with no other date restrictions. The search terms “Hyrimoz,” “GP2017,” “SDZ-ADA,” and “SDZ-ADL” were used, with searches restricted to English language and human studies. Hand-searching was performed to identify additional articles. Publications recommended by the authors, based on their awareness of the literature, were also considered for inclusion.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Literature Selection
Abstracts of all articles retrieved by the searches were reviewed. Peer-reviewed articles and congress abstracts containing efficacy/effectiveness, safety, and immunogenicity data in patients initiating on, or switching to, SDZ-ADL in clinical trials (phase I and III) and real-world settings were included. Publications of relevance to the health economics of SDZ-ADL were also included. Case studies, case series (without novel insight), reviews, and editorials were excluded. Full-text screening followed by qualitative assessment of the shortlisted articles was performed. Full-text publications were preferred over previously published congress abstracts. In the case of identical data reporting, multiple publications from the same study were considered as a single record. Relevant data from the included publications are summarized here in a descriptive manner.
Results
A total of 182 articles were identified. After removing duplicates and screening, 15 original peer-reviewed articles and 6 congress abstracts containing relevant SDZ-ADL data were selected for inclusion (Table 1). Four congress abstracts of relevance to the health economics of SDZ-ADL were also identified.
Table 1.
Summary of clinical studies and real-world evidence of SDZ-ADL
| No. | Author, year | Study design | Treatment of interest | Patient numbers by indication | Efficacy outcomes | Safety | Immunogenicity |
|---|---|---|---|---|---|---|---|
| Clinical studies | |||||||
| 1 | Blauvelt et al. 2018 [15] | Phase III, randomized, double-blinded | SDZ-ADL ref-ADL |
SDZ-ADL: 231 PsO pts ref-ADL: 234 PsO pts |
PASI 75 response at Wk 16, adjusted response rate, % ± SE SDZ-ADL: 66.8 ± 3.3 ref-ADL: 65.0 ± 3.4 Δ, % ± SE (95% CI): 1.8 ± 4.8 (− 7.5; 11.2) (equivalence margin of ± 18%) Percentage change from baseline to Wk 16 in PASI, LSM, % ± SE SDZ-ADL: − 60.7 ± 1.5 ref-ADL: − 61.5 ± 1.6 Δ, % ± SE (95% CI): 0.8 ± 2.0 (− 3.2; 4.8) (equivalence margin of ± 15%) |
Pts with ≥ 1 AE up to Wk 17, % SDZ-ADL: 50.2 ref-ADL: 52.6 Pts with ≥ 1 AE between Wk 17–51, % Cont. SDZ-ADL: 52.4 Cont. ref-ADL: 55.9 Switched to SDZ-ADL: 46.0 Switched to ref-ADL: 57.0 |
Pts ADA-positive up to Wk 17, % SDZ-ADL: 36.8 ref-ADL: 34.1 Pts ADA-positive between Wk 17–51, % Cont. SDZ-ADL: 35.8 Cont. ref-ADL: 45.1 Switched to SDZ-ADL: 39.0 Switched to ref-ADL: 47.0 |
| 2 | Blauvelt et al. 2021 [16] | Two phase III, randomized, double-blinded studies |
SDZ-ADL ref-ADL |
ADACCESS study SDZ-ADL: 231 PsO pts ref-ADL: 234 PsO pts ADMYRA study SDZ-ADL: 177 RA pts ref-ADL: 176 RA pts |
ADACCESS study PRO assessments at Wk 17, mean (SD) DLQI: SDZ-ADL: 4.2 (5.7) ref-ADL: 3.9 (5.3) EQ-5D-5L: SDZ-ADL: 80.6 (17.6) ref-ADL: 80.9 (19.4) HAQ-DI: SDZ-ADL: 0.5 (0.6) ref-ADL: 0.5 (0.6) PRO assessments at Wk 51, mean (SD) DLQI: Cont. SDZ-ADL: 2.4 (4.1) Cont. ref-ADL: 2.3 (3.2) Switched to SDZ-ADL: 4.1 (5.3) Switched to ref-ADL: 3.1 (5.0) EQ-5D-5L: Cont. SDZ-ADL: 87.4 (10.2) Cont. ref-ADL: 88.3 (10.9) Switched to SDZ-ADL: 85.0 (16.5) Switched to ref-ADL: 85.5 (12.4) HAQ-DI: Cont. SDZ-ADL: 0.4 (0.5) Cont. ref-ADL: 0.4 (0.6) Switched to SDZ-ADL: 0.5 (0.8) Switched to ref-ADL: 0.7 (0.6) ADMYRA study PRO assessments at Wk 24, mean (SD) HAQ-DI: SDZ-ADL: 0.8 (0.6) ref-ADL: 0.9 (0.6) FACIT-Fatigue score: SDZ-ADL: 37.1 (9.2) ref-ADL: 38.1 (9.3) PRO assessments at Wk 48, mean (SD) HAQ-DI: SDZ-ADL: 0.9 (0.7) ref-ADL to SDZ-ADL switch: 0.8 (0.7) FACIT-Fatigue score: SDZ-ADL: 36.2 (9.8) ref-ADL to SDZ-ADL switch: 37.0 (9.7) |
– | – |
| 3 | Wiland et al. 2020 [17] | Phase III, randomized, double-blinded |
SDZ-ADL ref-ADL |
SDZ-ADL: 177 RA pts ref-ADL: 176 RA pts |
Change from baseline to Wk 12 in DAS28-CRP, LSM ± SE SDZ-ADL: − 2.16 ± 0.11 ref-ADL: − 2.18 ± 0.11 Δ (95% CI): 0.02 (− 0.24; 0.27) Time-averaged change from baseline to Wk 24 in DAS28‑CRP, LSM ± SE SDZ-ADL: − 1.85 ± 0.10 ref-ADL: − 1.93 ± 0.09 Δ (95% CI): 0.08 (− 0.11; 0.27) Change from baseline to Wk 48 in DAS28-CRP, mean ± SD SDZ-ADL: − 3.09 ± 1.09 ref-ADL/switched to SDZ-ADL: − 3.05 ± 1.27 ACR20/50/70 response rates at Wk 48, % SDZ-ADL: 86.1/66.7/45.4 ref-ADL/switched to SDZ-ADL: 88.1/64.3/43.7 |
Pts with ≥ 1 TEAE up to Wk 48, % SDZ-ADL: 70.6 ref-ADL/switched to SDZ-ADL: 68.8 Pts with ≥ 1 SAE up to Wk 48, % SDZ-ADL: 4.0 ref-ADL/switched to SDZ-ADL: 5.7 |
Pts ADA-positive up to Wk 48, % SDZ-ADL: 24.2% (72.5% of these patients were nADA-positive) Ref-ADL/switched to SDZ-ADL: 25.6% (79.1% of these patients were nADA-positive) Pts ADA-positive after switch at Wk 24 up to Wk 48, % SDZ-ADL: 24.0 (72.2% of these pts were nADA-positive) Ref-ADL/switched to SDZ-ADL: 26.3 (81.0% of these pts were nADA-positive) |
| 4 | Baraliakos et al. 2022 [18] | Phase III, randomized, open-label | SDZ-ADL Secukinumab |
SDZ-ADL: 286 axSpA pts SEC (150 mg): 287 axSpA pts SEC (300 mg): 286 axSpA pts |
Pts with no radiographic progression (mSASSS ≤ 0.5) at Wk 104, est. mean % (95% CI) SDZ-ADL: 65.1 (58.8; 71.5) SEC (150 mg): 66.6 (60.7; 72.5) SEC (300 mg): 66.8 (60.5; 73.1) Change from baseline to Wk 16 in MRI SIJ score, mean ± SE SDZ-ADL: –1.51 ± 0.14 SEC (150 mg): –1.22 ± 0.14 SEC (300 mg): –1.10 ± 0.14 Change from baseline to Wk 16 in MRI spine score, mean ± SE SDZ-ADL: –2.31 ± 0.15 SEC (150 mg): –1.43 ± 0.14 SEC (300 mg): –1.59 ± 0.15 Change from baseline to Wk 104 in mSASSS, LSM ± SE SDZ-ADL: 0.72 ± 0.18 SEC (150 mg): 0.54 ± 0.18 SEC (300 mg): 0.55 ± 0.18 Pts with no new syndesmophytes at Wk 104 (in pts with ≥ 1 syndesmophyte at baseline), % SDZ-ADL: 53.3 SEC (150 mg): 56.9 SEC (300 mg): 53.8 |
Pts with ≥ 1 AE, % SDZ-ADL: 84.2 SEC (150 mg): 79.7 SEC (300 mg): 81.8 Pts with ≥ 1 SAE, % SDZ-ADL: 11.2 SEC (150 mg): 14.0 SEC (300 mg): 10.2 |
– |
| 5 | von Richter et al. 2019 [20] | Phase I, randomized, double-blinded |
SDZ-ADL ref-ADL |
SDZ-ADL: 107 subjects ref-ADL: 211 subjects |
GMR (90% CI) of SDZ-ADL/EU ref-ADL Cmax: 1.05 (0.99 to 1.11) ng/ml AUC0–inf: 1.04 (0.96 to 1.13) ng × h/ml GMR (90% CI) of SDZ-ADL/US ref-ADL Cmax: 1.00 (0.94 to 1.06) ng/ml AUC0–inf: 1.08 (1.00 to 1.18) ng × h/ml (prespecified PK similarity margin 0.80–1.25) |
Subjects with ≥ 1 TEAE, % SDZ-ADL: 71.0 US ref-ADL: 68.6 EU ref-ADL: 70.8 |
SDZ-ADL ADA: 57.9% nADA: 54.2% US ref-ADL ADA: 69.5% nADA: 62.9% EU ref-ADL ADA: 69.8% nADA: 64.2% |
| 6 | von Richter et al. 2019 [19] | Two phase I, single-center, randomized, double-blinded studies | SDZ-ADL | 197 subjects |
GMR (90% CI) between two studies SDZ-ADL exposure parameters Cmax: 0.95 (0.89 to 1.01) ng/ml AUC0–last: 0.80 (0.72 to 0.90) ng × h/ml AUC0–360 h: 0.92 (0.86 to 0.99) ng × h/ml AUC0–inf: 0.80 (0.72 to 0.89) ng × h/ml |
Subjects with ≥ 1 AE, % GP17-103 study: 45.6 GP17-104 study: 71.0 |
ADA-positive pts, % GP17-103 study: 71.1 GP17-104 study: 57.9 |
| 7 | von Richter et al. 2023 [11] | Phase I, randomized, multicenter, double-blinded |
SDZ-ADL (HCF vs. LCF) |
SDZ-ADL-HCF: 162 subjects SDZ-ADL-LCF: 168 subjects |
GMR (90% CI) of HCF vs. LCF Cmax: 1.02 (0.95 to 1.10) ng/ml AUC0–last: 1.04 (0.94 to 1.16) ng × h/ml AUC0–360 h: 1.03 (0.96 to 1.11) ng × h/ml AUC0–inf: 1.06 (0.98 to 1.15) ng × h/ml (prespecified PK similarity margin 0.80–1.25) |
Subjects with ≥ 1 TEAE, % SDZ-ADL-HCF: 49.4 SDZ-ADL-LCF: 56.5 |
Overall results (up to Day 72) HCF SDZ-ADL ADA: 69.1% nADA: 63.0% LCF SDZ-ADL ADA: 64.9% nADA: 61.3% |
| Real-world studies | |||||||
| 8 | Puig et al. 2022 [14] | Prospective registry study | SDZ-ADL |
46 PsO pts biologic-naïve 90 PsO pts biologic-switch |
Pts with PASI ≤ 2, % biologic-naïve vs. biologic-switch pts Month 6: 77.3 vs. 76.9 Month 12: 84.6 vs. 76.9 Pts with DLQI 0 or 1, % biologic-naïve vs. biologic-switch pts Month 6: 42.9 vs. 80.0 Month 12: 70.0 vs. 75.0 |
No. of events; IR/1000 (95% CI) PY Total serious events: 4; 31.5 (8.6; 80.7) Other serious infection: 2; 15.7 (1.9; 56.9) Malignant events: 0; 0.0 (0.0; 13.4) |
– |
| 9 | Loft et al. 2021 [26] | Cohort study from a prospective registry (DERMBIO) |
SDZ-ADL ref-ADL SB5 |
Patients with PsO Ref-ADL: 378 pts Switched from ref-ADL to SDZ-ADL: 186 pts Switched from ref-ADL to SB5: 162 pts |
Change from baseline in PASI following switch, median (IQR) Switched to ADL biosimilar (SDZ-ADL and SB5 combined): 0.0 (–0.3 to 0.2) Continued ref-ADL: 0.0 (–0.3 to 0.2) Change from baseline in DLQI following switch, median (IQR) Switched to ADL biosimilar (SDZ-ADL and SB5 combined): 0.0 (–1.0 to 0.0) Continued ref-ADL: 0.0 (0.0 to 0.0) |
Any AE, n (%) Switched to ADL biosimilar (SDZ-ADL and SB5 combined): 29 (9.1) Continued ref-ADL: 18 (5.0) Continued ref-ADL sensitivity cohort: 38 (10.5) |
– |
| 10 | Nabi et al. 2021 [12] | Observational cohort study |
SDZ-ADL SB5 |
SDZ-ADL: 214 RA pts 148 PsA pts 261 axSpA pts SB5: 253 RA pts 173 PsA pts 269 axSpA pts |
1-year treatment withdrawal rate (age/gender adjusted) SDZ-ADL vs. SB5, HR (95% CI) RA: 0.50 (0.28; 0.90)* PsA: 0.97 (0.46; 2.02) AxSpA: 0.75 (0.42; 1.33) 6 months’ remission rate (fully adjusted) SDZ-ADL vs. SB5, OR (95% CI) RA: 1.81 (1.07; 3.06)* PsA: 1.79 (0.88; 3.66) AxSpA: 1.87 (1.04; 3.34)* Disease activity scores, median (IQR) DAS 28 RA baseline: 2.3 (1.7 to 2.8) 6 months post-switch: 2.0 (1.6 to 2.6) PsA baseline: 2.1 (1.6 to 2.6) 6 months post-switch: 1.9 (1.5 to 2.6) HAQ (0–3) RA baseline: 0.6 (0.2 to 1.1) 6 months post-switch: 0.6 (0.2 to 1.1) PsA baseline: 0.5 (0.1 to 0.9) 6 months post-switch: 0.5 (0.0 to 1.0) BASDAI, cm AxSpA: baseline: 2.5 (1.0 to 4.6) 6 months post-switch: 2.5 (1.1 to 4.5) |
– | – |
| 11 | Colaci et al. 2021 [27] | Observational, single center study | SDZ-ADL |
25 RA pts 32 PsA pts 31 axSpA pts |
Disease activity scores, median (IQR) DAS28-CRP Baseline (ref ADL): 1.03 (0.96 to 3.43) 6 months post-switch: 1.21 (0.0 to 3.7) DAPSA Baseline (ref ADL): 0 (0.0 to 12.2) 6 months post-switch: 0 (0.0 to 15.0) BASDAI Baseline (ref ADL): 0 (0.0 to 4.3) 6 months post-switch: 0 (0.0 to 6.4) Retention rate: 93.2% |
Disease reactivation n = 1 (1.1%), subjective/nocebo effect n = 5 (5.7%); (general malaise and transient increase of blood pressure n = 1; dizziness, paresthesia, arthralgia, headache n = 1; itch n = 1; subjective worsening n = 2) | Not assessed |
| 12 | Di Giuseppe et al. 2022 [13] | Observational cohort study |
SDZ-ADL ref-ADL SB5 ABP 501 MSB11022 |
SDZ-ADL (ADL-naïve pts): 1044 428 RA pts 240 PsA pts 261 axSpA pts 115 other pts SDZ-ADL (pts switched from ref-ADL): 42 RA pts 53 PsA pts 60 axSpA pts 2 other pts ref-ADL (ADL-naïve pts): 2619 pts |
1-year retention rate (all pts), % (95% CI) SDZ-ADL: 76 (71; 80) ref-ADL: 78 (77; 80) 1-year retention rate (switched pts), % (95% CI) ref-ADL to SDZ-ADL: 90 (79; 95) ref-ADL continued: 95 (88; 98) |
– | – |
| 13 | Jyssum et al. 2023 [28] | Observational study (NOR-DMARD) |
SDZ-ADL ref-ADL |
SDZ-ADL: 64 SpA, 39 RA, 29 PsA, 6 other pts ref-ADL: 113 SpA, 59 RA, 41 PsA, 27 other pts |
Responders at 3 months (SpA/RA/PsA/other), % SDZ-ADL: 49/72/79/80 ref-ADL: 48/61/61/63 |
– |
Pts ADA-positive, n (%) SDZ-ADL: 6 (4) ref-ADL: 33 (14) p = 0.004 |
| 14 | Mocci et al. 2022 [30] | Retrospective, multicenter study |
SDZ-ADL ref-ADL |
SDZ-ADL: 20 UC pts 42 CD pts ref-ADL: 21 UC pts 51 CD pts |
Clinical remission rate, % SDZ-ADL: 82.3; ref-ADL: 75.0 (p = 0.31) Clinical response rate, % SDZ-ADL: 87.1; ref-ADL: 84.7 (p = 0.69) Mucosal healing rate, % SDZ-ADL: 89.2; ref-ADL: 60.0 (p = 0.003) (median follow-up time of 12 months) |
AEs, n (%) UC Total AEs SDZ-ADL: 0 (0); ref-ADL: 2 (9.5) Severe AEs SDZ-ADL: 0 (0); ref-ADL: 1 (4.8) CD Total AEs SDZ-ADL: 1 (2.4); ref-ADL: 2 (3.9) Severe AEs SDZ-ADL: 1 (2.4); ref-ADL: 1 (2.0) |
– |
| 15 | Wasserbauer et al. 2022 [31] | Prospective observational, multicenter study |
SDZ-ADL FKB327 |
SDZ-ADL: 28 IBD pts FKB327: 22 IBD pts |
Remission or partial response after 12 weeks, % SDZ-ADL: 75.0; FKB327: 81.8 (p = 0.73) CDAI in pts with CD treated with SDZ-ADL, median Baseline: 251.0; Week 12: 110.5 (p = 0.003) MSS value in pts with UC treated with SDZ-ADL, median Baseline: 8.0; Week 12: 4.0 (p = 0.01) |
Total AEs: seven (for all treatments) Four mild complications (one patient each with skin eczema, COVID-19, mycotic infection, pneumonia) Two allergic reactions One neurologic complication |
– |
| 16 | Gros et al. 2022 [32] | Prospective, observational study | SDZ-ADL |
6 UC pts 34 CD pts |
Pts in clinical remission at Month 9: 94.9% Laboratory values prior (pts on ref-ADL) and after switching to SDZ-ADL (median follow-up 10.6 months): ADL levels (7.7 vs. 5.9 mg/ml; p = 0.001) CRP levels (0.4 vs. 0.7 g/l; p = 0.02) Albumin levels (4.4 vs. 4.6 g/l; p = 0.02) |
No severe AEs reported | – |
| 17 | Lontai et al. 2022 [29] | Prospective, observational, multicenter study |
SDZ-ADL ref-ADL ABP 501 MSB11022 |
ref-ADL to SDZ-ADL switch: 174 IBD pts (41 UC, 133 CD) ADL biosimilars to SDZ-ADL switch: 102 IBD pts (30 UC, 72 CD) |
Clinical remission rates, % ref-ADL to ADL biosimilar switch: pre-switch, 87.3; at switch, 88.5; Weeks 8–12, 86.5; Weeks 20–24, 85.7 ADL biosimilar to ADL biosimilar switch: pre-switch, 74.5; at switch, 78.4; Weeks 8–12, 85.3; Weeks 20–24, 79.8 Drug survival probability, % ± SE ref-ADL to ADL biosimilar switch: 20 weeks: 95.4 ± 1.6; 40 weeks: 91.6 ± 2.2 ADL biosimilar to ADL biosimilar switch: 20 weeks: 94.1 ± 2.3; 40 weeks: 87.0 ± 3.4 |
Treatment-related AEs Five events in five pts Two skin erythema (1 SDZ-ADL, 1 MSB11022) One liver enzyme elevation (SDZ-ADL) One ADL-induced psoriasis (SDZ-ADL) One late hypersensitivity (SDZ-ADL) |
- |
| 18 | Tursi et al. 2022 [34] | Retrospective, observational, multicenter study |
SDZ-ADL ABP 501 SB5 MSB11022 |
Pts switched from ref-ADL to: SDZ-ADL: 7 IBD pts ABP 501: 78 IBD pts SB5: 65 IBD pts MSB11022: 3 IBD pts |
Maintenance of remission in switched patients after median follow-up of 12 months, % SDZ-ADL: 66.7 (n = 4/7) ABP 501: 84.6 (n = 66/78) SB5: 78.5 (n = 51/65) MSB11022: 100 (n = 3/3) |
Total AEs SDZ-ADL: one mild-moderate allergy |
– |
| 19 | Tursi et al. 2023 [33] |
Retrospective, observational, multicenter study (same study as Tursi 2022) |
SDZ-ADL ABP 501 SB5 MSB11022 |
Switched and naïve pts treated with: SDZ-ADL: 49 IBD pts (13 UC, 36 CD) ABP 501: 259 IBD pts SB5: 214 IBD pts MSB11022: 11 IBD pts |
Clinical remission obtained or maintained (all patients), % SDZ-ADL, 77.5; ABP 501, 78.3; SB5, 75.2; MSB11022, 81.8 Clinical remission obtained (biologic-naïve pts), % SDZ-ADL, 82.1; ABP 501, 77.7; SB5, 80.2; MSB11022, 100 Clinical remission maintained (switched pts), % SDZ-ADL, 66.7; ABP 501, 83.5; SB5, 78.5; MSB11022, 100 Clinical response obtained or maintained (all patients), % SDZ-ADL, 91.8; ABP 501, 89.3; SB5, 90.2; MSB11022, 90.9 |
Total AEs SDZ-ADL: one (2.0%; allergy) |
– |
ACR American College of Rheumatology, ADA anti-drug antibody, ADL adalimumab, AE adverse event, AUC area under the curve, axSpA axial spondyloarthritis, BASDAI Bath Ankylosing Spondylitis Disease Activity Index, CD Crohn’s disease, CDAI Crohn’s Disease Activity Index, CI confidence interval, Cmax maximum concentration, COVID-19 coronavirus disease 2019, CRP C-reactive protein, DAPSA Disease Activity Index for Psoriatic Arthritis, DAS28 Disease Activity Score (four variables), DLQI Dermatology Life Quality Index, EQ-5D-5L Euro-QoL five-dimensional five-level, EU European Union, FACIT Functional Assessment of Chronic Illness Therapy, GMR geometric mean ratio, HAQ Health Assessment Questionnaire, HAQ-DI Health Assessment Questionnaire-Disability Index, HCF high-concentration formulation, HR hazard ratio, IBD inflammatory bowel disease, IQR interquartile range, IR incidence rate, LCF low-concentration formulation, LSM least squares mean, MRI magnetic resonance imaging, mSASSS modified Stoke Ankylosing Spondylitis Spinal Score, MSS Mayo scoring system, nADA neutralizing anti-drug antibody, OR odds ratio, PASI Psoriasis Area and Severity Index, PK pharmacokinetics, PRO patient-reported outcome, PsA psoriatic arthritis, PsO psoriasis, pt patient, PY patient-year, RA rheumatoid arthritis, ref-ADL reference-adalimumab, SAE serious adverse event, SD standard deviation, SDZ-ADL Sandoz-adalimumab, SE standard error, SEC secukinumab, SIJ sacroiliac joint, TEAE treatment-emergent adverse event, UC ulcerative colitis, US United States, Wk week
*p < 0.05. Δ = treatment difference
Clinical Trials
Three phase III trials published in three articles and one congress abstract and four phase I studies published in three articles discussed the efficacy, safety, and immunogenicity of SDZ-ADL [11, 15–20]. All patients included in the phase III trials were ADL-naïve patients from across the EU and USA. From a total of 1677 patients participating in the three phase III trials, 923 were exposed to SDZ-ADL, among whom 292 experienced switching between SDZ-ADL and ref-ADL [15, 17, 18].
Plaque PsO and PsA
The phase III ADACCESS trial was a 51-week, randomized, double-blinded, active-controlled trial designed to explore the equivalence in efficacy, safety, and immunogenicity between SDZ-ADL and ref-ADL. Four switches between ref-ADL and SDZ-ADL were included in the trial design to evaluate the impact of multiple switches. A total of 465 patients with moderate-to-severe plaque PsO (21.1% of patients had concomitant PsA) were randomized at baseline to SDZ-ADL (n = 231) or ref-ADL (n = 234). Both treatments were equivalent in terms of the Psoriasis Area and Severity Index (PASI) 75 response at Week 16 (primary endpoint; SDZ-ADL, 66.8%; ref-ADL, 65.0%) and mean percentage change from baseline to Week 16 in PASI (key secondary endpoint; SDZ-ADL, − 60.7%; ref-ADL, − 61.5%) in the per-protocol set. Near-identical mean absolute PASI and mean percentage change from baseline in PASI were observed between SDZ-ADL and ref-ADL up to Week 16. At Week 17, patients were re-randomized to either undergo four subsequent switches between SDZ-ADL and ref-ADL or to continue SDZ-ADL or ref-ADL treatment up to Week 51. No significant differences were observed in PASI outcomes (mean absolute PASI, mean percentage change from baseline in PASI, and PASI 50/75/90/100 response rates) between treatment groups. Starting from Week 3 (first dose assessment) and over the 51-week treatment period, mean trough serum drug concentrations remained steady and within reference medicine label ranges for all treatment arms [15]. The impact of SDZ-ADL on quality of life (QoL) and patient-reported outcomes (PROs) was assessed as part of the ADACCESS trial. Assessed PROs included Dermatological Life Quality Index (DLQI) and Euro-QoL five-dimensional five-level (EQ-5D-5L), which improved from baseline (with improvements sustained up to Week 51) and were comparable regardless of the treatment or switch between treatments. In patients with PsA at baseline, the mean Health Assessment Questionnaire-Disability Index (HAQ-DI) decreased in both groups, with comparable scores between treatment arms at Week 17 and post-switch at Week 51 [16].
RA
ADMYRA was a randomized, double-blinded, multicenter, phase III trial designed to demonstrate similarity in efficacy and compare the safety and immunogenicity of SDZ-ADL to ref-ADL in patients with moderate-to-severe RA with an inadequate response to disease-modifying antirheumatic drugs, including methotrexate. The trial also evaluated the impact of a single switch from ref-ADL to SDZ-ADL on efficacy, safety, and immunogenicity. The primary endpoint was the change from baseline in Disease Activity Score using 28 joints with C-reactive protein (DAS28-CRP) at Week 12. No significant difference was observed in the least squares mean (LSM) change from baseline in DAS28-CRP scores between the 177 patients randomized to SDZ-ADL and the 176 patients randomized to ref-ADL (LSM difference = 0.02; 95% confidence interval [CI] − 0.24; 0.27, within the prespecified equivalence margin of ± 0.60). This similarity in response in terms of DAS28-CRP continued for up to 24 weeks and was maintained up to Week 48 after the switch to SDZ-ADL in the ref-ADL arm (switch occurred at Week 24). The American College of Rheumatology 20/50/70 responses and mean proportions of patients achieving remission, according to the European Alliance of Associations for Rheumatology (EULAR) and Boolean definitions, were comparable between the treatment groups throughout the trial. After the single switch at Week 24, the proportions of patients who achieved EULAR good or moderate responses at Week 48 based on DAS28-CRP were similar between the SDZ-ADL and ref-ADL/switched SDZ-ADL groups (EULAR good/moderate response rates: 69.2%/29.0% vs. 68.0%/29.6%, respectively) [17]. QoL and PRO outcomes with SDZ-ADL versus ref-ADL were assessed in a post hoc analysis. Mean HAQ-DI decreased from baseline to Week 24, indicating an improvement in QoL. Changes from baseline in HAQ-DI were comparable between the treatment groups at Week 24 (SDZ-ADL: HAQ-DI score, 0.84 [change from baseline, 0.65]; ref-ADL: HAQ-DI, 0.85 [change from baseline, 0.61]) and Week 48 (continued SDZ-ADL: HAQ-DI, 0.85 [change from baseline, 0.63]; ref-ADL/switched to SDZ-ADL: HAQ-DI, 0.82 [change from baseline, 0.62]). Mean Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue scores at baseline (24.7 for SDZ-ADL and 25.3 for ref-ADL) increased to 37.1 and 38.1, respectively, at Week 24 and were sustained at Week 48 (continued SDZ-ADL, 36.2; ref-ADL/switched to SDZ-ADL, 37.0) [16].
Spondyloarthritis
The SURPASS trial was a randomized, multicenter, partially blinded (secukinumab dose-blinded; SDZ-ADL open-label), head-to-head, phase III trial designed to evaluate the effect of secukinumab (150 mg and 300 mg) and SDZ-ADL on the 2-year evolution of spinal radiographic progression in patients with radiographic axial spondyloarthritis with high risk of radiographic progression [18, 21]. At Week 104, the mean ± standard error (SE) changes from baseline in modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) were 0.72 ± 0.18, 0.54 ± 0.18, and 0.55 ± 0.18 in the SDZ-ADL, secukinumab 150 mg, and secukinumab 300 mg arms, respectively. The proportions of patients with no radiographic progression (change from baseline in mSASSS of ≤ 0.5) were 66.1% (secukinumab 150 mg), 66.9% (secukinumab 300 mg), and 65.6% (SDZ-ADL), with no significant differences between the interventions. Similarly, secondary endpoints were comparable for SDZ-ADL and secukinumab, including rates of change from baseline in mSASSS, proportions of patients with ≥ 1 syndesmophyte(s) at baseline with no new syndesmophyte(s) at Week 104, and changes from baseline in magnetic resonance imaging (MRI) Berlin sacroiliac joint inflammation score and the AS spine MRI-activity Berlin modification score [18, 22].
Subgroup analyses were carried out to evaluate the efficacy of SDZ-ADL and secukinumab in patients with factors at baseline predictive of spinal radiographic progression (presence of syndesmophytes ≥ 1 and elevated C-reactive protein [CRP; ≥ 5 mg/l]). Of the 859 patients, 653 (76.0%) had elevated CRP levels, 627 (73.0%) had syndesmophytes, and 466 (54.2%) had elevated CRP and syndesmophytes at baseline. At Week 104, there were no notable differences in radiographic outcomes between SDZ-ADL and secukinumab, regardless of the presence or absence of specific predictive factors. Irrespective of treatment arms, rates of radiographic progression were higher in patients with predictive factors versus those without (proportions of patients with no radiographic progression with secukinumab 150 mg/secukinumab 300 mg/SDZ-ADL for each subgroup: elevated CRP: 64.4%/66.3%/66.8%; no elevated CRP: 75.0%/74.5%/71.2%; syndesmophytes: 58.4%/58.6%/58.2%; no syndesmophytes: 91.8%/92.5%/96.6%; elevated CRP and syndesmophytes: 54.9%/55.2%/55.8%) [22].
Safety and Immunogenicity
In clinical trials, the safety and tolerability profile of SDZ-ADL was similar to that of ref-ADL. In patients receiving SDZ-ADL, the proportions of patients reporting ≥ 1 adverse event (AE) ranged from 52.4% to 84.2%, and the proportions of patients reporting ≥ 1 serious AE (SAE) ranged from 2.4% to 11.2% [15, 17, 18]. In the ADACCESS trial, no meaningful differences in the proportions of patients with ≥ 1 AE were observed between SDZ-ADL (50.2%) and ref-ADL (52.6%) during the pre-switch treatment period up to Week 17. Similarly, there were no meaningful differences in the frequency of AEs between the different arms after the switch (Week 17–51: continued SDZ-ADL 52.4%; continued ref-ADL 55.9%; SDZ-ADL to ref-ADL switch 57.0%; ref-ADL to SDZ-ADL switch 46.0%). Infections and infestations were the most commonly reported AEs by Medical Dictionary for Regulatory Activities (MedDRA) system organ class up to Week 17 (SDZ-ADL 23.8%; ref-ADL 23.9%), with nasopharyngitis the most commonly reported AE by preferred term (SDZ-ADL 5.6%; ref-ADL 6.4%) [15].
In the ADMYRA trial, the incidence of AEs and SAEs was low and similar between SDZ-ADL and ref-ADL to SDZ-ADL switch groups (AEs: 70.6% vs. 68.8%, SAEs: 4.0% vs. 5.7%). The most frequently reported treatment-emergent AEs (TEAEs) were infections and infestations (SDZ-ADL: 35.6%, ref-ADL: 36.9%, continued SDZ-ADL: 15.7%, ref-ADL to SDZ-ADL: 16.3%). The highest incidence was observed for viral upper respiratory tract infections (16.4% vs. 10.8%) and upper respiratory tract infection (7.9% vs. 5.1%) in the SDZ-ADL versus ref-ADL to SDZ-ADL treatment arms, respectively. Most patients experienced AEs of mild or moderate severity [17]. In SURPASS, 79.7%, 81.8%, and 84.2% of patients had ≥ 1 AE, and 14.0%, 10.2%, and 11.2% of patients had ≥ 1 SAE in the secukinumab 150 mg, secukinumab 300 mg, and SDZ-ADL arms, respectively, over 2 years [18].
A clinical pharmacokinetic (PK) bridging study was a regulatory requirement for the approval of the HCF of SDZ-ADL by the FDA (March 2023) and EMA (May 2023) [23, 24]. The study demonstrated PK similarity between HCF SDZ-ADL (100 mg/ml) and low-concentration formulation (LCF SDZ-ADL: 50 mg/ml) at the 40 mg dose in healthy participants, with comparable safety and immunogenicity profiles between the two formulations. Safety profiles of a single dose of HCF SDZ-ADL or LCF SDZ-ADL were similar, with 49.4% and 56.5% of participants reporting ≥ 1 AE, respectively. General disorders and administration-site conditions, together with injection-site reactions (all mild in severity), were the most common MedDRA system organ class and preferred term AEs observed in both arms. Differences in the severity and rates of injection-site reactions were not observed between HCF and LCF SDZ-ADL [11].
Anti-drug antibody (ADA) formation can impact treatment exposure and influence treatment outcomes. However, cross-study comparisons of ADAs may only be appropriate if the same assay is used across studies [25]. In two different phase I studies, ADAs to SDZ-ADL developed at an incidence rate comparable to ref-ADL. In two different single-dose PK studies in healthy participants, the incidence rate of ADAs for SDZ-ADL ranged from 57.9% to 71.1%. The rate of ADAs was expected to be high, as the results were observed in healthy participants presenting with primary immune response kinetics. The difference in immunogenicity between the two studies was not product-related, because the same batch of drug was used in both studies, but possibly emerged because of the heterogeneity of the two study populations [19]. In two PK studies, one also reported the immunogenicity of SDZ-ADL compared to two batches of ref-ADL (US and EU). The rates of ADAs were similar across all arms, with ref-ADL rates ranging between 69.5% and 69.8%, and SDZ-ADL at 57.9% [20]. The immunogenicity results for HCF SDZ-ADL were similar to those obtained in previous studies, and the difference in incidence rate of ADAs in the LCF versus HCF was insignificant at 64.9% versus 69.1% (of all patients treated with LCF vs. HCF, 61.3% vs. 63.0% had neutralizing ADAs) [11].
In the 48-week results from the ADMYRA trial, the incidence rate of ADAs with SDZ-ADL (24.2%) was similar to that in the ref-ADL/switch SDZ-ADL arm (25.6%), with no meaningful differences between the two groups in rates of neutralizing ADAs (72.5% vs. 79.1%, respectively). The switch to SDZ-ADL at Week 24 did not affect immunogenicity, with an ADA incidence rate after Week 24 of 24.0% in the continued SDZ-ADL arm compared with 26.3% in the ref-ADL/switched to SDZ-ADL arm [17].
Real-World Evidence
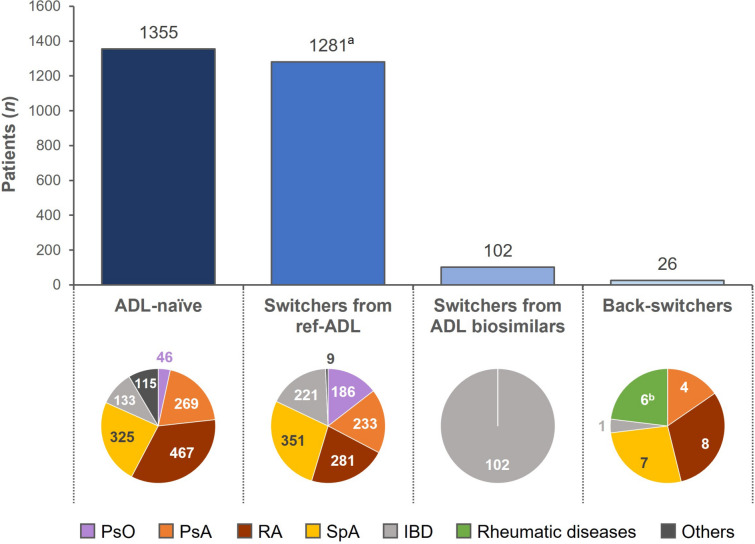
Eleven real-world observational studies in nine full publications and three congress abstracts assessed clinical outcomes and safety after initiating treatment with SDZ-ADL or switching to SDZ-ADL from ref-ADL or another ADL biosimilar. These studies included data from a total of 1355 ADL-naïve patients treated with SDZ-ADL and 1473 patients switching to SDZ-ADL (1281 from ref-ADL, 102 from other ADL biosimilars, and 90 from other biologics) (Fig. 1; Table 1).
Fig. 1.
Pooled number of patients receiving SDZ-ADL from real-world evidence by type of treatment and by indications. ADL adalimumab, IBD inflammatory bowel disease, n number of patients, PsA psoriatic arthritis, PsO psoriasis, RA rheumatoid arthritis, ref-ADL reference-adalimumab, SDZ-ADL Sandoz-adalimumab, SpA spondyloarthritis. Data stratified by patients who were ADL-naïve, those who switched from ref-ADL, those who switched from other ADL biosimilars, and those who switched back to ref-ADL. Figure excludes 90 patients with PsO reported by Puig et al. [14], who switched from a previous biologic treatment to SDZ-ADL. aIncludes 186 patients from pooled DERMBIO data who switched to SDZ-ADL, bColaci et al. [27] report back-switchers from all chronic arthritis indications combined
PsO
The British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) is a large, prospective pharmacovigilance registry of patients with PsO in the UK and Republic of Ireland. A real-world study based on the BADBIR registry showed that treatment initiation in patients with PsO who were either receiving SDZ-ADL as their first biologic, or were treated with another biologic prior to switching to SDZ-ADL, resulted in 77.3% (n = 17) of biologic-naïve patients and 76.9% (n = 20) of biologic-switched patients achieving PASI ≤ 2 after 6 months, which increased to 84.6% (n = 11) and 76.9% (n = 10), respectively, after 12 months. Additionally, complete normalization of DLQI (0–1) was achieved by 42.9% (n = 9) of biologic-naïve and 80.0% (n = 8) of biologic-switched patients after 6 months, and 70.0% (n = 7) and 75.0% (n = 3) of patients, respectively, after 12 months [14].
An analysis of the Danish DERMBIO (Biological Treatment in Danish Dermatology) registry was carried out to assess outcomes following a mandatory switch from ref-ADL to ADL biosimilars in patients with moderate-to-severe PsO. In total, 348 patients were included in the ADL biosimilar cohort (186 switched to SDZ-ADL and 162 to SB5 [Imraldi®; Samsung Bioepis]), and 378 patients were included in a historical cohort of patients treated with ref-ADL. The 1-year drug retention rates were 92.1% (95% CI 89.4; 94.8) for the ref-ADL cohort and 92.0% (95% CI 89.0; 94.9) for the ADL biosimilar cohort. In total, 28 patients (8%) in the ADL biosimilar cohort stopped treatment within 12 months, and 9 of these patients switched back to ref-ADL. Similarly, in the ref-ADL cohort group, 30 patients (7.9%) discontinued treatment within 12 months. The crude hazard ratios (HRs) for discontinuation of ADL biosimilar versus ref-ADL were 1.02 (95% CI 0.61; 1.70) for all causes and 0.82 (95% CI 0.39; 1.73; p = 0.60) for ineffectiveness. PASI and DLQI were unchanged following a switch from ref-ADL to ADL biosimilar [26].
Inflammatory Rheumatic Diseases
An observational and geographically clustered study based on the Danish DANBIO registry population evaluated 1318 patients with rheumatic diseases (RA, PsA, and axSpA) who underwent a mandatory switch from ref-ADL to either SDZ-ADL (n = 623; 47.3%) or ADL biosimilar SB5 (n = 695; 52.7%). In the total population, the 1-year crude treatment retention rate for the two biosimilars was 89.5%, with the estimated risk of withdrawal lower with SDZ-ADL versus SB5 (HR = 0.60; 95% CI 0.42; 0.86). Similar results were seen for patients with RA (HR = 0.50; 95% CI 0.28; 0.90). The 6-month remission rate was higher with SDZ-ADL versus SB5 in the total population (odds ratio [OR] = 1.72; 95% CI 1.25; 2.37; p = 0.001); similar trends were seen across the indications, but statistical significance was only reached for patients with RA (OR = 1.81; 95% CI 1.07; 3.06; p = 0.027).
Disease activity was assessed following the switch from ref-ADL to SDZ-ADL in the different indications. In the 148 patients with PsA, clinical outcomes were comparable from baseline to 6 months post-switch. The median difference between baseline and 6 months post-switch ranged between 0.0 and 0.3 on different scales for disease activity scores (DAS28, HAQ, Clinical Disease Activity Index [CDAI]). Median CRP levels and a visual analog scale (VAS) of patient-assessed pain and fatigue were also comparable from baseline to 6 months after the switch. Among the 214 patients with RA who switched from ref-ADL to SDZ-ADL, the median difference between baseline and 6 months post-switch was 0.0 for DAS28, HAQ-DI, CDAI, and CRP levels; VAS for patient global assessment of pain and fatigue followed a similar trend, with median change from baseline to 6 months ranging between 0 and 2 across all VAS scales.
In the 261 patients with axSpA who switched to SDZ-ADL, the median change in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Ankylosing Spondylitis Disease Activity Score, and CRP from baseline to 6 months post-switch was 0.0 for all parameters. At 6 months post-switch, there was no change from baseline in median VAS for patient global assessment of pain and fatigue. In the observed period, 2.7% (4/148) of patients with PsA, 3.7% (8/214) of patients with RA, and 2.7% (7/261) of patients with axSpA switched back from SDZ-ADL to ref-ADL. Across all indications, the reasons for switching back from SDZ-ADL to ref-ADL were loss of efficacy in 47% of patients and AEs in 37% of patients [12].
The effectiveness and safety of switching from ref-ADL to SDZ-ADL was investigated in 32 patients with PsA, 25 patients with RA, and 31 patients with axSpA treated at a single center in Italy. The retention rate for SDZ-ADL was 93.2% across all indications. In patients with PsA, no statistically significant differences were observed in median Disease Activity Index for Psoriatic Arthritis between baseline (0 [0–12.2]) and 6 months after switching to SDZ-ADL (0 [0–15]). In patients with RA, no significant differences were observed in median [interquartile range] DAS28-CRP/CDAI/Simple Disease Activity Index (SDAI) values at baseline (1.0 [1.0–3.4]/0.0 [0.0–15.0]/0.0 [0.0–15.0]) and after 6 months (1.2 [0.0–3.7]/0.0 [0.0–17.0]/0.5 [0.0–17.5]). In patients with axSpA, there was no significant difference in the median BASDAI values between baseline (0.0 [0.0–4.3]) and 6 months after switching (0.0 [0.0–6.4]). From the overall 88 patients who switched to SDZ-ADL, 6.8% switched back to ref-ADL (because of disease reactivation in one patient and for subjective reasons in five patients) [27].
The Swedish Rheumatology Quality (SRQ) register is a collection of national data from patients with immune-mediated rheumatic diseases that was initiated in 1995. An analysis was conducted on treatment retention of patients starting SDZ-ADL (n = 1044) and ref-ADL (n = 2619) for the first time. No difference was observed in treatment retention between SDZ-ADL and ref-ADL (1-year retention rate for first-time users: 76% vs. 78%, respectively [HR = 0.97; 95% CI 0.79; 1.20]). Results were similar when stratified by disease indication (RA, HR = 0.86 [95% CI 0.62; 1.19]; PsA, HR = 1.44 [95% CI 0.94; 2.20]; axSpA, HR = 0.84 [95% CI 0.51; 1.37]). Treatment retention in switchers from ref-ADL to SDZ-ADL (n = 157) was also investigated, revealing no significant difference in the 1-year retention rate between switchers to SDZ-ADL (90%) and matched controls who continued ref-ADL (95%; HR = 1.52 [95% CI 0.28; 8.41]) [13].
The NOR-DMARD study is an observational study from Norway that includes patients with inflammatory joint diseases starting treatment with disease-modifying anti-rheumatic drugs. An analysis from the NOR-DMARD study included 378 patients (PsA, n = 70; RA, n = 98; SpA, n = 177; other, n = 33) treated with ref-ADL (n = 240; 63%) or SDZ-ADL (n = 138; 37%). There was no significant difference between ref-ADL and SDZ-ADL in the proportions of patients with a response at 3 months in patients with PsA (61% vs. 79%; p = 0.12), RA (61% vs. 72%; p = 0.29), and SpA (48% vs. 49%; p = 0.86). Drug survival during the first 2 years of treatment was not significantly different between ref-ADL and SDZ-ADL (p = 0.14) [28].
Inflammatory Bowel Disease
A prospective, observational, multicenter study conducted in Hungary was designed to evaluate switching from ref-ADL to SDZ-ADL, and between ADL biosimilars, in patients with inflammatory bowel disease (IBD). In total, 174 patients switched from ref-ADL to SDZ-ADL (including 12 patients who initially switched to other ADL biosimilars [ABP 501 and MSB11022] and underwent a second switch to SDZ-ADL due to renewed regulations during the inclusion period), and 102 patients transitioned from ABP 501 or MSB11022 to SDZ-ADL. Drug survival calculated from Kaplan–Meier curves revealed no significant difference between the ref-ADL-to-biosimilar and biosimilar-to-biosimilar switch cohorts (log-rank: 0.96; p = 0.33). Drug survival probabilities of patients in the ref-ADL-to-biosimilar and biosimilar-to-biosimilar switch cohorts were comparable (estimated probability to stay on therapy: 95%–94% after 20 weeks and 92%–87% after 40 weeks, respectively). Proportions of patients in clinical remission remained unchanged at Week 8–12 pre-switch/at switch/Week 8–12/Week 20–24 in the ref-ADL-to-biosimilar switch cohort (87%/89%/87%/86%; p = 0.89) and in the biosimilar-to-biosimilar switch cohort (75%/78%/85%/80%; p = 0.29), respectively. Clinical disease activity scores based on median Crohn’s Disease Activity Index (Crohn’s CDAI) in patients with CD and partial Mayo Score (pMayo) in patients with UC remained stable in the ref-ADL-to-biosimilar and biosimilar-to-biosimilar switch cohorts. Corticosteroid use increased numerically from time of switch to Weeks 20–24 post-switch in the ref-ADL-to-biosimilar switch cohort (4.0% to 6.7%), whereas it decreased in the biosimilar-to-biosimilar switch cohort (8.8% to 2.2%); these changes were not significant. One of the 276 patients who switched to SDZ-ADL switched back to ref-ADL [29].
A retrospective, multicenter, observational study conducted across nine IBD centers in Italy was designed to investigate the effectiveness and safety of SDZ-ADL versus ref-ADL in real-world settings. From a total of 134 patients (93 with CD, 41 with UC), 62 (46.3%), and 72 (53.7%) received SDZ-ADL and ref-ADL, respectively. The median follow-up time was 12 months. The primary endpoint was the rate of clinical remission (defined as a Mayo Clinic score ≤ 2 in patients with UC and Harvey–Bradshaw Index [HBI] ≤ 5 in patients with CD), which was comparable between patients treated with SDZ-ADL and ref-ADL (82.3% vs. 75.0%; p = 0.31). Most patients were biologic-naïve (n = 118), of whom 39 of 46 patients (84.8%) treated with SDZ-ADL and 54 of 72 patients (75.0%) treated with ref-ADL achieved clinical remission. Among 16 patients naïve to ADL, but exposed previously to biologic therapies (anti-TNFs and anti-integrins), 12 (75.0%) achieved clinical remission, all in the SDZ-ADL group. Reductions of steroid use and clinical response, defined as a decrease of ≥ 2 points in the Mayo score in patients with UC and ≥ 3 points in the HBI in patients with CD, were achieved equally in both treatment cohorts, with no significant differences (p = 0.91 and p = 0.69, respectively). However, there was a significant difference (p = 0.003) in mucosal healing (defined as a Mayo subscore for endoscopy of ≤ 1 for UC and Simple Endoscopic Score for Crohn’s Disease [SES-CD] of ≤ 2 for CD; evaluated only in patients naïve to SDZ-ADL who underwent colonoscopy), which was achieved in a higher proportion of patients treated with SDZ-ADL (n = 33/37 patients; 89.2%) compared with ref-ADL (n = 30/50 patients; 60.0%). No information on the methodology used to balance treatment groups was given [30].
A prospective, observational study conducted in seven gastroenterology departments across the Czech Republic collected effectiveness data on 28 biologic-naïve patients with CD or UC initiating treatment with SDZ-ADL. After 12 weeks of therapy, remission (Crohn’s CDAI < 150 [CD]; pMayo score ≤ 2 points [UC]) or partial response (≥ 70-point decrease from baseline in Crohn’s CDAI [CD]; ≥ 2-point decrease from baseline in pMayo score [UC]) was achieved in 75% of patients. The median Crohn’s CDAI for patients with CD decreased significantly from 251.0 at baseline to 110.5 at Week 12 (p = 0.003). Similarly, the median Mayo scoring system value in patients with UC decreased from 8.0 at baseline to 4.0 (p = 0.001) at Week 12 [31].
A prospective, observational study conducted in a tertiary IBD referral center in Spain evaluated the switch from ref-ADL to SDZ-ADL in 40 patients (34 with CD, six with UC) who were in clinical remission (HBI ≤ 4 or pMayo score ≤ 2) with or without biologic or endoscopic remission. After a follow-up of ≥ 9 months, 37 patients (94.9%) maintained clinical remission status. Significant differences were observed in levels of ADL (7.7 vs. 5.9 mg/ml; p = 0.001), CRP (0.4 vs. 0.7 g/l; p = 0.02), and albumin (4.4 vs. 4.6 g/l; p = 0.02) between prior to and after switching. The authors stated that any impact of these differences, however, was not observable [32].
In an Italian retrospective, multicenter, observational analysis, patients with CD or UC in clinical remission who were treated with various ADL biosimilars, including SDZ-ADL, were assessed for induction or maintenance of remission, and safety. Patients who were biologic- or ADL-naïve were assessed for induction of remission, and those who switched to SDZ-ADL were assessed for maintenance of remission. Remission was defined as a Mayo score ≤ 2 in patients with UC and a HBI ≤ 5 in patients with CD. Of the 49 patients receiving SDZ-ADL, 38 (77.5%) achieved clinical remission. Among these patients, 32 of the 39 biologic-naïve patients (82.1%) and two of the four ADL-naïve patients (50.0%) achieved clinical remission with SDZ-ADL. Clinical response (defined as a decrease from baseline of ≥ 2 points in Mayo score [UC] or ≥ 3 points in the HBI [CD]) with SDZ-ADL was obtained in 45 of 49 patients, and mucosal healing (defined as a Mayo subscore for endoscopy of ≤ 1 [UC] and SES-CD ≤ 2 [CD]) was obtained in 24 of 28 patients (85.7%). For the seven patients who switched from ref-ADL to SDZ-ADL, at a median follow-up time of 12 months, four patients (66.7%) maintained clinical remission [33, 34].
Safety and Immunogenicity
In real-world settings in patients with immune-mediated inflammatory diseases, the frequency of AEs with SDZ-ADL ranged between 0% and 14.3%, and the frequency of SAEs ranged between 0% and 2.4% [30, 31, 33, 34]. The incidence rate of SAEs reported with SDZ-ADL in the BADBIR registry was 31.5 per 1000 patient-years [14]. In the DERMBIO registry in patients with PsO, there was no significant difference in the rate of discontinuation due to AEs between patients switched from ref-ADL to ADL biosimilar (SDZ-ADL or SB5) versus a historical cohort of patients treated with ref-ADL (HR = 1.41; 95% CI 0.52; 3.77; p = 0.50]) [26]. The incidence of AEs over 12 months was higher in patients switched to ADL biosimilar versus those treated with ref-ADL (9.1% vs. 5.0%; p = 0.04), but this pattern was not observed in a sensitivity analysis using a different historical cohort of patients treated with ref-ADL (9.1% vs. 10.5%; p = 0.53). Over the same period, rates of dermatologic AEs were similar in patients switched to ADL biosimilar versus those treated with ref-ADL (1.3% vs. < 3%; p = 0.19) [26].
The DANBIO analysis reported a withdrawal rate of 8.5% for SDZ-ADL over 1 year of follow-up. Among the 53 patients who withdrew from treatment, AEs were the reason for withdrawal in 15 of these individuals (28.3%) [12]. The SRQ register reported 148 discontinuations of SDZ-ADL among first-time users, 35% of which were due to AEs. The same study reported similar rates of discontinuation due to AEs in patients who switched to SDZ-ADL (2 out of 10 discontinuations; 20.0%) and matched controls who continued ref-ADL (2 out of 12 discontinuations; 16.7%) [13]. In the observational study from Hungary in 276 patients with IBD switched to SDZ-ADL, 29 patients discontinued SDZ-ADL, with AEs the reason for discontinuation in four patients (13.8%) [29]. In an observational study conducted in patients with IBD in Italy, rates of AEs were low and not significantly different with ref-ADL versus SDZ-ADL (UC: 9.5% [n = 2/21] vs. 0% [n = 0/20]; CD: 3.9% [n = 2/51] vs. 2.4% [n = 1/42]; p = not significant for both). Rates of treatment discontinuation due to AEs were also low and comparable between treatment groups (two patients treated with ref-ADL and one patient treated with SDZ-ADL) [30]. In a second observational study in Italy in patients with IBD, rates of AEs were higher with SDZ-ADL (14.3%) than with other ADL biosimilars (ABP 501: 7.6%; SB5: 7.7%) [34].
Immunogenicity and ADAs are not routinely measured and reported in clinical practice, which likely accounts for the paucity of data from real-world settings. In patients with RA, PsA, SpA, adult JIA, or unspecified polyarthritis enrolled in the observational NOR-DMARD study, those who initiated treatment with ref-ADL had significantly higher rates of ADA formation over 2 years (14% vs. 4%; p = 0.004) and significantly lower serum drug levels (6.5 mg/l vs. 8.3 mg/l; p = 0.0003) compared with patients who received SDZ-ADL [28]. These differences were evident across indications and after adjustment for confounding factors. The authors stated, however, that there were no significant differences in clinical outcomes between the two groups [28].
Health Economics
A budget impact model of SDZ-ADL for the treatment of patients with RA, PsA, AS, CD, UC, uveitis, and HS over a 5-year period in the Middle East region estimated that the adoption of SDZ-ADL could achieve substantial cost savings of up to US$ 181 million (39%) compared with the cost of ref-ADL treatment, assuming that all patients were switched to SDZ-ADL. Moreover, 45% of expert respondents were likely to choose the biosimilar over the reference product [35]. A similar analysis from the Spanish health system predicted cost savings of 11.5% (relative to the cost of ref-ADL) if SDZ-ADL was introduced for new prescriptions only, and savings of up to 30% if all patients were prescribed SDZ-ADL [36]. A US budget impact analysis of transitioning from ref-ADL to ADL biosimilar for the treatment of RA, JIA, AS, PsA, PsO, UC, and CD, based on approximately 5232 treatments over a 3-year time horizon, estimated total savings of between US$ 4.2 and 21.0 million, depending on the speed of switching [37]. An analysis of 1318 patients with RA, PsA, or axSpA in the DANBIO registry who underwent a mandatory switch from ref-ADL to SDZ-ADL or SB5 found that monthly hospital costs at 9 months post-switch were similar or decreased compared with the 9-month period prior to the switch [38].
Discussion
Over the 5-year period since its initial approval in Europe in 2018, SDZ-ADL has been evaluated in numerous clinical and real-world studies. This review summarizes data from 923 patients in controlled trials and 2828 patients in observational studies and patient registries across Europe treated with SDZ-ADL, including both ADL-naïve patients and those switching to SDZ-ADL from ref-ADL or another ADL biosimilar. The clinical and real-world evidence highlights the similarity of SDZ-ADL to ref-ADL in terms of clinical outcomes. In the following paragraphs, we will collate and compare the available clinical data and real-world evidence of SDZ-ADL in terms of efficacy/effectiveness, safety, immunogenicity, and treatment retention.
Efficacy/Effectiveness
Overall, the efficacy of SDZ-ADL was comparable to ref-ADL in biologic-naïve patients and unaltered after single and multiple switches between ref-ADL and SDZ-ADL as assessed in randomized clinical trials and real-world studies. In the pivotal ADACCESS trial in patients diagnosed with PsO (21.1% had concomitant PsA), SDZ-ADL matched ref-ADL in terms of efficacy measured by PASI 75 and mean percentage change from baseline in PASI. Efficacy was equivalent between treatment groups who were switched multiple (four) times between SDZ-ADL and ref-ADL or continued treatment up to Week 51 [15, 16]. These data are consistent with real-world data from registries collecting effectiveness data in patients with PsO. The British BADBIR registry confirmed effectiveness of SDZ-ADL in biologic-naïve patients initiating treatment and observed stable disease activity in patients switching from other biologics [14]. In the Danish DERMBIO registry, disease activity and QoL measures following a switch from ref-ADL to an ADL biosimilar were unchanged (SDZ-ADL or SB5) [26]. This is in line with the comparable QoL and PROs between treatment groups and post-switch, as assessed in ADACCESS [16].
SDZ-ADL has been shown to be effective in patients with inflammatory rheumatic diseases based on two randomized phase III trials (ADMYRA, SURPASS) and real-world studies. In the ADMYRA trial, the efficacy of SDZ-ADL, measured by the change from baseline in DAS28-CRP, was comparable to ref-ADL in patients with RA and remained unchanged after a single switch between SDZ-ADL and ref-ADL [17]. In the SURPASS trial, the proportion of patients with no radiographic progression (change from baseline in mSASSS ≤ 0.5) was not significantly different between patients treated with SDZ-ADL versus those treated with secukinumab. After 2 years, the mean ± SE change from baseline in mSASSS was 0.72 ± 0.18 in the SDZ-ADL in SURPASS trial, in line with previously reported data for ref-ADL in patients with radiographic-axSpA (mean ± SD change from baseline in mSASSS: 0.8 ± 2.6 at 2 years) [39].
The clinical trial data concur with results from nationwide patient registries (DANBIO, SRQ), which reported effectiveness in rheumatic patients (RA, PsA, axSpA) initiating treatment with SDZ-ADL or unchanged disease activity in patients switching from ref-ADL to SDZ-ADL [12, 13]. Jyssum et al. confirmed in the observational NOR-DMARD study that no significant difference in treatment response was observed between rheumatic patients initiating ref-ADL or SDZ-ADL [28]. One single study center from Italy investigated the disease activity in rheumatic patients before and after switching from ref-ADL to SDZ-ADL and equally found no significant difference post-switch [27].
Several publications present pooled data for rheumatic patients switching to any ADL biosimilar. A Romanian registry reporting data in patients with RA treated with various ADL biosimilars, including SDZ-ADL, found that treatment initiation with ADL biosimilars (SDZ-ADL, SB5, FKB327, and MSB11022) or ref-ADL showed no significant differences after 6 months regarding Boolean (15.0% vs. 12.3%; p = 0.401), DAS28-CRP (32.4% vs. 34.2%; p = 0.686), and SDAI (16.4% vs. 14.0%; p = 0.483) remission rates [40]. A retrospective analysis using German BIKER-registry data described the experience of ADL biosimilar use in JIA in clinical practice. Disease activity parameters, including the 10-joint Juvenile Arthritis Disease Activity Score, Physician Global Assessment 0- to 100-mm VAS, Parent/Patient Global Assessment 0- to 100-cm VAS, active joint count 0–71, truncated at 10, erythrocyte sedimentation rate, and Childhood Health Assessment Questionnaire-Disability Index, were analyzed in 55 patients who switched to ADL biosimilars and in 44 patients who received an ADL biosimilar as first-line therapy. The results were comparable between ADL biosimilars and ref-ADL throughout the observation period, indicating no difference in effectiveness in patients with JIA exposed to ref-ADL or ADL biosimilars [41].
The regulatory approval of SDZ-ADL based on the biosimilar development package [15, 20, 42] and extrapolation of indications did not require a controlled clinical trial in IBD. Post-marketing clinical data exist for SDZ-ADL in patients with IBD, which demonstrated effectiveness at inducing remission in ADL-naïve patients with IBD, with efficacy comparable to that observed for ref-ADL [30, 31, 33]. Clinical remission rates were maintained after switching from ref-ADL to SDZ-ADL [29, 32, 33]. Lontai et al. assessed clinical outcomes in a biosimilar-to-biosimilar switch scenario in patients with IBD. No significant differences were found in clinical disease activity scores (median Crohn’s CDAI, pMayo) and in proportions of patients in clinical remission prior to and after the switch from another ADL biosimilar to SDZ-ADL [29], confirming that clinical benefit was maintained following the biosimilar-to-biosimilar switch.
Safety
In the ADACCESS and ADMYRA clinical trials, overall rates of AEs and safety profiles of SDZ-ADL were comparable to ref-ADL, and no new safety signals emerged. Safety profiles were also unaffected by a single switch (in patients with RA) or multiple switches (in patients with PsO) between SDZ-ADL and ref-ADL [15, 17]. In SURPASS, rates of AEs were similar between SDZ-ADL and secukinumab [18].
From AEs summarized in real-world studies, it is evident that the safety profile of SDZ-ADL in clinical practice does not differ significantly from that observed in controlled clinical studies. As would be expected, frequencies of AEs and SAEs were generally lower in real-world studies versus clinical studies. In the BADBIR registry, the incidence rate of SAEs reported with SDZ-ADL in patients with PsO was 31.5 per 1000 patient-years [14]. This is comparable to the incidence rate of SAEs reported with ref-ADL in patients with PsO included in the ESPRIT registry (4.4 per 100 patient-years) [43]. In real-world studies in patients with inflammatory rheumatic diseases, safety data were sparse. The SRQ register reported similar rates of discontinuation due to AEs at 1 year in patients who switched to SDZ-ADL (20.0%) and matched controls who continued ref-ADL (16.7%) [13]. In the DANBIO register, a withdrawal rate of 8.5% for SDZ-ADL was observed over 1 year of follow-up [12]. Pooled safety analysis from various ADL biosimilars, including SDZ-ADL, resulted in a total of 107 AEs: 18.7% with ADL biosimilars compared to 81.3% with ref-ADL. The authors concluded that, due to the small sample size and short follow-up period, safety data did not significantly differ between the treatment groups and were also in line with literature reports [40].
In observational studies, incidence rates of AEs in patients with IBD treated with SDZ-ADL were generally low [29, 30, 33], and safety profiles were consistent with those reported in large registry studies of ref-ADL in patients with UC and CD [44, 45]. In an observational study in patients with IBD switched to SDZ-ADL, 13.8% of patients discontinued SDZ-ADL because of an AE [29], comparable to the 13.0% of ADL-naïve patients who discontinued ref-ADL because of TEAEs in a large registry study [45]. No new safety concerns were identified in patients with IBD who switched from ADL biosimilars to SDZ-ADL [29].
Immunogenicity
Overall, immunogenicity data confirm clinical efficacy and safety observations. Immunogenicity was assessed in clinical trials, with the incidence rate of ADAs and neutralizing ADAs found to be comparable between SDZ-ADL and ref-ADL, and unaffected by switching in patients with PsO or RA [15, 17]. Immunogenicity data in a real-world setting are lacking, as immunogenicity is not routinely assessed in clinical practice. One observational study from Norway found that patients with rheumatic diseases who initiated treatment with ref-ADL had significantly higher rates of ADA formation over 2 years compared with patients who received SDZ-ADL (14% vs. 4%; p = 0.004) [28]. Reasons for this difference were not speculated on, but clinical outcomes, including response rates and drug survival over 2 years, were not impacted, as per the authors’ conclusion [28].
Treatment Retention
Based on real-world data from European registries and single and multicenter studies, treatment retention rates with SDZ-ADL were high. The combined 1-year retention rate after a switch from ref-ADL to ADL biosimilars (SDZ-ADL and SB5) was reported at 89.5% in the DANBIO registry in patients with inflammatory rheumatic diseases. Lower risk of withdrawal and higher remission rates were observed for SDZ-ADL versus SB5, which were also observed after adjustments of baseline variables in both main and sensitivity analyses, indicating differences in effectiveness in routine care [12]. Nabi et al. hypothesized that these differences might be due to several factors, including (but not limited to) differences in devices, injection-site reactions (itching and burning at the injection site), pain (reported more often in SB5- vs. SDZ-ADL-treated patients), geographic clusters, and regional differences in transition and communication strategy. Similarly, high retention rates for SDZ-ADL were reported from the Swedish SRQ register in biologic-naïve (76%) and switched patients (90%) with rheumatic disease [13]. In an observational study from Hungary in patients with IBD, no significant differences in treatment retention were observed between patients switched from ref-ADL (91.6%) or another ADL biosimilar to SDZ-ADL (87.0%) after a median of 40 weeks’ follow-up [29]. In the DERMBIO registry, including patients with PsO, switching to ADL biosimilars (53.4% of patients switched to SDZ-ADL) was associated with a 92.0% treatment retention rate at 1 year, comparable to the rate observed for ref-ADL (92.1%) [26]. These data for SDZ-ADL are supported by pooled data for ADL biosimilars, including a retrospective analysis from France in patients with rheumatic diseases, which showed no differences in retention rate between ref-ADL (median retention 30 months) and ADL biosimilars [46].
Switch-back to Reference Medicine
In real-world studies identified in this review, switch-back rates to ref-ADL were low for SDZ-ADL, ranging between 0.4% and 6.8% [12, 13, 27, 29]. The DANBIO registry reported that 3% of all patients switched back to ref-ADL during follow-up, mainly because of lack of effect (47%) and AEs (37%) [12]. One study reported a switch-back rate of 6.8% (n = 6/88) in patients with rheumatic diseases; in five patients, causes of discontinuation were subjective reasons or nocebo effect [27]. Glintborg et al. reported the reasons for switching back to the reference product and treatment withdrawal of biosimilars may likely be a result of subjective perception of patients, also suggesting a nocebo effect [47]. The nocebo effect is an unexplainable, unfavorable therapeutic effect driven mostly by perceived negative expectations from patients or treating physicians on treatment outcomes; this may also be responsible for higher rates of AE reporting. Of note, mandatory switching may foster negative expectations and further increase the nocebo effect [48]. This effect can be minimized by managing negative expectations and treatment-associated anxiety through the use of tailored, open communication that addresses patients’ concerns and expectations. Changes in biosimilar treatment should be proactively addressed, and appropriate patient information and educational strategies should be employed by healthcare providers, pharmacists, nurses, or other trained individuals to ensure optimal clinical results are achieved [48–50]. The HYRISS study (NCT05633771), with an estimated completion date of December 2025, aims to assess retention with SDZ-ADL treatment at 6 months in patients with IBD and will investigate percentages of patients who discontinue SDZ-ADL due to the nocebo effect [51].
Switching
Switching from reference medicines to biosimilars has become a common clinical practice. A single switch describes the switch from a reference medicine to its biosimilar, or the reverse. A systematic review from 2020 including 178 studies (~ 21,000 patients) reporting outcomes of switching from a reference medicine to a biosimilar indicated that single switching was not associated with any major efficacy, safety, or immunogenicity issues [52]. These findings are supported by those of a systematic review by García-Beloso et al. which concluded that single switching from reference ADL to ADL biosimilars in patients with various chronic, immune-mediated inflammatory diseases (RA, PsO, and IBD) had no impact on efficacy, safety, and immunogenicity [53]. A recent network meta-analysis of 17 randomized trials involving 6562 patients with RA was carried out to assess the impact of single switching from a reference biologic to a biosimilar (including SDZ-ADL) on efficacy and safety. Clinical efficacy was found to be equivalent between patients who switched to a biosimilar versus those who remained on the reference biologic, and switching did not impact the safety or immunogenicity profile [54]. Conclusions reached from this robust network meta-analysis support the systematic review findings and further solidify the conclusion that patients can single switch from a reference biologic to a biosimilar without loss in efficacy or impact on safety.
Multiple switching, defined as patients alternating back and forth multiple times between a reference medicine and biosimilar, has also been investigated. In the large systematic review from 2020, six studies investigating multiple switching were identified, five of which were phase III clinical trials across several indications. There were no clinically meaningful differences in efficacy, safety, or immunogenicity reported between the continued and multiple switched arms in these studies [52].
Biosimilar-to-biosimilar switching has also been investigated, although controlled data from randomized clinical trials are lacking for this scenario. A systematic review from 2023 summarized the available evidence for biosimilar-to-biosimilar switching (23 studies with 3657 patients) [55]. While conclusions were limited by the fact that all studies were observational in nature, the data suggest that biosimilar-to-biosimilar switching is a safe practice, with no reduction in effectiveness and no increase in AEs [55]. A second systematic review reached the same conclusion, highlighting that in 19 studies (17 of which overlapped with the first review) including 3111 patients, no significant concerns were reported when switching between biosimilars [51]. The Perception study found that biosimilar-to-biosimilar switching had no negative impact on levels of satisfaction among patients and that outcomes after switching from SDZ-ADL to the ADL biosimilar MSB11022 did not worsen significantly [50].
Interchangeability
Interchangeable use of EU-licensed biosimilars is widely accepted in Europe. In a joint statement, the EMA and the Heads of Medicines Agencies confirmed that an approved biosimilar in the EU is interchangeable with its reference product (or vice versa) or with another biosimilar of the same reference product. With demonstrated efficacy, safety, and immunogenicity of biosimilars, EU experts consider that post-approval systematic switch studies for biosimilars are not required to prove interchangeability [56]. In the USA, due to concerns regarding the potential for increased or different immunogenicity with biosimilars upon switching, the Biologics Price Competition and Innovation Act of 2009 incorporated a unique legal designation of interchangeability that is distinct from biosimilarity. This designation allows product substitution at the pharmacy level without prescribers’ permission [57–59]. Subsequently, the FDA developed scientific guidance recommending that a multiple-switch study with a PK primary endpoint needs to be performed to obtain the additional, optional, US legal designation of “interchangeable biologic” for a given biosimilar. However, based on the available evidence to date from randomized controlled trials and the majority of real-world evidence, biosimilars do not elicit different immune responses associated with different efficacy or safety outcomes compared with their reference medicines, warranting the need to reconsider the requirement for this additional designation [58]. Indeed, the FDA has recently updated its biosimilar labeling recommendations for all biosimilars, including interchangeable biosimilars, to include one “biosimilarity statement,” with the reasoning that statements describing interchangeability are not necessary for informing safe and effective use of biosimilars among prescribing healthcare professionals [60]. However, it must also be noted that in the USA, although the FDA allows for pharmacy-level substitution of biosimilars, state-level regulations can differ, which can impact substitution decisions.
Access
Biosimilar medicines are cost-effective versions of reference medicines and can reduce healthcare spendings. Based on experience in Europe, cost savings generated from biosimilars can broaden access to biologic treatment, enabling more patients to be treated, as well as enabling treatment initiation in earlier phase of disease, resulting in improved health outcomes [61]. Biosimilar entries in Europe resulted in significantly increased utilization (expressed in defined daily doses per 1000 inhabitants per day) of anti-TNFs in most countries by an average of 88.9%, 14.6% and 22.4% for infliximab, etanercept and adalimumab, respectively. For example, in Poland, utilization of infliximab increased by 246% following biosimilar entry, with increases of > 100% also seen in Austria (110.9%), Ireland (160.6%), Portugal (146.4%), Croatia (186.7%), Latvia (115.0%), Bosnia (170.6%), Serbia (161.9%), and Norway (167.2%) [1, 61]. In Sweden, the proportion of patients with IBD treated with anti-TNFs/targeted therapies increased from 5% to 26% in CD and from 1% to 10% in UC between 2007 and 2020 [7]. In the USA, ADL biosimilars have only recently become commercially available, and their true uptake remains to be seen.
Limitations
The evidence summary compiled here is comprehensive, with most data coming from peer-reviewed journals. There are several limitations of this review, including the non-systematic approach, which may have led to a selection bias. In addition, the studies included here vary in study design, participant characteristics, interventions, and outcome measures; this heterogeneity presents a challenge in comparing results across studies.
Conclusion
Substantial clinical evidence exists on using SDZ-ADL, which was one of the first approved subcutaneous anti-TNF biosimilars. Data across several immune-mediated inflammatory diseases show SDZ-ADL matches its reference product in terms of efficacy, safety and tolerability, immunogenicity, and QoL outcomes. Efficacy and safety results for SDZ-ADL from phase III clinical trials are substantiated in real-world studies, confirming the reliability of switching to, or initiating therapy with, SDZ-ADL in clinical practice. The wealth of supportive data should enhance confidence in SDZ-ADL use by both clinicians and patients and may lead to increased patient access.
Acknowledgements
We thank the participants of all studies included in this review.
Medical Writing/Editorial Assistance
The authors thank Mohammad Fahad Haroon and Ramji Narayanan of Novartis Healthcare Pvt. Ltd., Hyderabad, India, for providing medical writing and editorial assistance in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022). Additional medical writing support was provided by Eve Blumson, PhD, of Rhea, OPEN Health Communications, and was funded by Sandoz in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022).
Author Contributions
Piotr Wiland, Norman B. Gaylis, Russell D. Cohen, and Andrew Blauvelt contributed to writing—review and editing. Charlotte Both contributed to conceptualization; writing—original draft preparation; writing—review and editing; visualization. Jonas Halfvarson contributed to conceptualization; writing—review and editing. Lena Lemke and Oliver von Richter contributed to conceptualization; writing—review and editing; supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript was funded by Sandoz, who also funded the journal’s Rapid Service and Open Access Fees.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of interest
Piotr Wiland has served as a speaker and/or advisory board member for AbbVie, AstraZeneca, Bristol Myers Squibb (BMS), Novartis, Pierre Fabre, Pfizer, Sandoz, Sobi, and UCB. Norman B. Gaylis has no conflicts of interest to declare. Charlotte Both was an employee of Hexal AG at the time of manuscript development and is a current employee of Grünenthal. Russell D. Cohen has served on the speakers’ bureau for AbbVie, BMS/Celgene, and Takeda; is a consultant for AbbVie, BMS/Celgene, Eli Lilly and Company, Genentech, Gilead Sciences, Hoffmann La-Roche, Janssen, Pfizer, Takeda, and UCB Pharma; and is a clinical trials investigator for AbbVie, BMS/Celgene, Boehringer Ingelheim, Crohn’s & Colitis Foundation of America, Genentech, Gilead Sciences, Hollister, MedImmune, Mesoblast Ltd., Osiris Therapeutics, Pfizer, Receptos, RedHill Biopharma, Sanofi-Aventis, Schwarz Pharma, Seres Therapeutics, Takeda Pharma, and UCB Pharma. Jonas Halfvarson has served as a speaker and/or advisory board member for AbbVie, Aqilion, BMS, Celgene, Celltrion, Dr Falk Pharma and the Falk Foundation, Ferring, Galapagos, Gilead, Hospira, Index Pharma, Janssen, MEDA, Medivir, Medtronic, MSD, Novartis, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, Takeda, Thermo Fisher Scientific, Tillotts Pharma, Vifor Pharma, and UCB; and has received grant support from Janssen, MSD, and Takeda. Lena Lemke was an employee of Hexal AG at the time of manuscript development. Oliver von Richter is an employee of Hexal AG. Andrew Blauvelt has served as a speaker (received honoraria) for AbbVie, Eli Lilly and Company, Pfizer, and UCB; has served as a scientific advisor (received honoraria) for AbbVie, Abcentra, Aclaris, Affibody, Aligos, Almirall, Alumis, Amgen, Anaptysbio, Apogee, Arcutis, Arena, Aslan, Athenex, Bluefin Biomedicine, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, CTI BioPharma, Dermavant, EcoR1, Eli Lilly and Company, Escient, Evelo, Evommune, Forte, Galderma, HighlightII Pharma, Incyte, InnoventBio, Janssen, Landos, Leo, Lipidio, Microbion, Merck, Monte Rosa Therapeutics, Nektar, Novartis, Overtone Therapeutics, Paragon, Pfizer, Q32 Bio, Rani, Rapt, Regeneron, Sanofi Genzyme, Spherix Global Insights, Sun Pharma, Takeda, TLL Pharmaceutical, TrialSpark, UCB Pharma, Union, Ventyx, Vibliome, and Xencor; and has acted as a clinical study investigator (institution has received clinical study funds) for AbbVie, Acelyrin, Allakos, Almirall, Alumis, Amgen, Arcutis, Athenex, Boehringer Ingelheim, Bristol Myers Squibb, Concert, Dermavant, Eli Lilly and Company, Evelo, Evommune, Galderma, Incyte, Janssen, Leo, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma, UCB Pharma, and Ventyx.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
The original online version of this article was revised due to retrospective open access.
Change history
3/20/2025
The original online version of this article was revised due to retrospective open access.
Change history
4/28/2025
A Correction to this paper has been published: 10.1007/s12325-025-03181-z
References
- 1.Kvien TK, Patel K, Strand V. The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin Arthritis Rheum. 2022;52: 151939. 10.1016/j.semarthrit.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 2.European Medicines Agency. Biosimilars in the EU: information guide for healthcare professionals. 2019. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Accessed 14 Dec 2023.
- 3.US Food and Drug Administration. Questions and answers on biosimilar development and the BPCI act: GUIDANCE for industry. 2021. https://www.fda.gov/media/119258/download. Accessed 14 Dec 2023.
- 4.World Health Organization. Guidelines on evaluation of similar biological products. 2021. https://cdn.who.int/media/docs/default-source/biologicals/ecbs/who-sbps_22-april-2021.pdf?sfvrsn=f283c924_5. Accessed 14 Dec 2023.
- 5.Bain B, Brazil M. Adalimumab. Nat Rev Drug Discov. 2003;2(9):693–4. 10.1038/nrd1182. [DOI] [PubMed] [Google Scholar]
- 6.Coghlan J, He H, Schwendeman AS. Overview of Humira® biosimilars: current European landscape and future implications. J Pharm Sci. 2021;110(4):1572–82. 10.1016/j.xphs.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everhov ÅH, Söderling J, Befrits G, et al. Increasing healthcare costs in inflammatory bowel disease 2007–2020 in Sweden. Aliment Pharmacol Ther. 2023;58(7):692–703. 10.1111/apt.17675. [DOI] [PubMed] [Google Scholar]
- 8.European Medicines Agency. Summary of product characteristics: Humira. 2009. https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf. Accessed 14 Dec 2023.
- 9.European Medicines Agency. Summary of product characteristics: Hyrimoz. 2018. https://www.ema.europa.eu/en/documents/product-information/hyrimoz-epar-product-information_en.pdf. Accessed 14 Dec 2023.
- 10.US Food and Drug Administration. HYRIMOZ (adalimumab-adaz) injection, for subcutaneous use. 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761071s015lbl.pdf. Accessed 14 Dec 2023.
- 11.von Richter O, O’Reilly T, Guerrieri D, et al. GP2017-HCF, a high concentration formulation, demonstrates similar pharmacokinetics, immunogenicity and safety to GP2017, an approved adalimumab biosimilar. Expert Opin Biol Ther. 2023;23:749–58. 10.1080/14712598.2022.2117546. [DOI] [PubMed] [Google Scholar]
- 12.Nabi H, Georgiadis S, Loft AG, et al. Comparative effectiveness of two adalimumab biosimilars in 1318 real-world patients with inflammatory rheumatic disease mandated to switch from originator adalimumab: nationwide observational study emulating a randomised clinical trial. Ann Rheum Dis. 2021;80(11):1400–9. 10.1136/annrheumdis-2021-219951. [DOI] [PubMed] [Google Scholar]
- 13.Di Giuseppe D, Lindstrom U, Bower H, et al. Comparison of treatment retention of originator vs biosimilar products in clinical rheumatology practice in Sweden. Rheumatology (Oxford). 2022;61(9):3596–605. 10.1093/rheumatology/keab933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puig L, Shams K, Furlan F, et al. Real-world effectiveness and safety of SDZ-ADL (adalimumab biosimilar) in patients with psoriasis from the British Association of Dermatologists Biologic and Immunomodulators Register (BADBIR). Biologics. 2022;2(4):213–25. 10.3390/biologics2040017. [Google Scholar]
- 15.Blauvelt A, Lacour JP, Fowler JF, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol. 2018;179(3):623–31. 10.1111/bjd.16890. [DOI] [PubMed] [Google Scholar]
- 16.Blauvelt A, Leonardi CL, Gaylis N, et al. Treatment with SDZ-ADL, an adalimumab biosimilar, in patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis: results of patient-reported outcome measures from two phase III studies (ADMYRA and ADACCESS). BioDrugs. 2021;35(2):229–38. 10.1007/s40259-021-00470-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wiland P, Jeka S, Dokoupilová E, et al. Switching to biosimilar SDZ-ADL in patients with moderate-to-severe active rheumatoid arthritis: 48-week efficacy, safety and immunogenicity results from the phase III, randomized, double-blind ADMYRA study. BioDrugs. 2020;34(6):809–23. 10.1007/s40259-020-00447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baraliakos X, Østergaard M, Poddubnyy D, et al. Effect of secukinumab versus adalimumab biosimilar on radiographic progression in patients with radiographic axial spondyloarthritis: a randomized phase IIIb study [abstract]. Arthritis Rheumatol. 2022;74(suppl 9):L15. [DOI] [PubMed] [Google Scholar]
- 19.von Richter O, Lemke L, Haliduola H, et al. Differences in immunogenicity associated with non-product related variability: insights from two pharmacokinetic studies using GP2017, an adalimumab biosimilar. Expert Opinion Biol Ther. 2019;19(10):1057–64. 10.1080/14712598.2019.1603959. [DOI] [PubMed] [Google Scholar]
- 20.von Richter O, Lemke L, Haliduola H, et al. GP2017, an adalimumab biosimilar: pharmacokinetic similarity to its reference medicine and pharmacokinetics comparison of different administration methods. Expert Opinion Biol Ther. 2019;19(10):1075–83. 10.1080/14712598.2019.1571580. [DOI] [PubMed] [Google Scholar]
- 21.Baraliakos X, Østergaard M, Gensler LS, et al. Comparison of the effects of secukinumab and adalimumab biosimilar on radiographic progression in patients with ankylosing spondylitis: design of a randomized, phase IIIb study (SURPASS). Clin Drug Investig. 2020;40(3):269–78. 10.1007/s40261-020-00886-7. [DOI] [PubMed] [Google Scholar]
- 22.Baraliakos X, Østergaard M, Poddubnyy D, et al. OP0059 Effect of secukinumab versus adalimumab biosimilar on radiographic progression in patients with radiographic axial spondyloarthritis: a randomised phase IIIB study. Ann Rheum Dis. 2023;82(Suppl 1):38–40. 10.1136/annrheumdis-2023-eular.301. [Google Scholar]
- 23.Novartis. Sandoz receives US FDA approval for biosimilar Hyrimoz® (adalimumab-adaz) high-concentration formulation. 2023. https://www.novartis.com/news/media-releases/sandoz-receives-us-fda-approval-biosimilar-hyrimoz-adalimumab-adaz-high-concentration-formulation. Accessed 14 Dec 2023.
- 24.Novartis. Sandoz receives approval by European Commission for Hyrimoz® (adalimumab) high-concentration formulation. 2023. https://www.novartis.com/news/media-releases/sandoz-receives-approval-european-commission-hyrimoz-adalimumab-high-concentration-formulation. Accessed 14 Dec 2023.
- 25.Bader LI, Solberg SM, Kaada SH, et al. Assays for infliximab drug levels and antibodies: a matter of scales and categories. Scand J Immunol. 2017;86(3):165–70. 10.1111/sji.12572. [DOI] [PubMed] [Google Scholar]
- 26.Loft N, Egeberg A, Rasmussen MK, et al. Outcomes following a mandatory nonmedical switch from adalimumab originator to adalimumab biosimilars in patients with psoriasis. JAMA Dermatol. 2021;157(6):676–83. 10.1001/jamadermatol.2021.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colaci M, Aprile ML, La Rosa A, Di Maggio A, Malatino L. AB0218 non-medical switch from adalimumab originator to the biosimilar GP2017 in patients affected by chronic arthritis. Ann Rheum Dis. 2021;80(Suppl 1):1135. 10.1136/annrheumdis-2021-eular.1563. [Google Scholar]
- 28.Jyssum I, Gehin JE, Kristianslund E, et al. POS0252 Differences in serum drug levels and anti-drug antibodies in patients with inflammatory arthritis treatment with originator vs biosimilar adalimumab. Ann Rheum Dis. 2023;82(Suppl 1):POS0252. 10.1136/annrheumdis-2023-eular.1373. [Google Scholar]
- 29.Lontai L, Gonczi L, Balogh F, et al. Non-medical switch from the originator to biosimilar and between biosimilars of adalimumab in inflammatory bowel disease – a prospective, multicentre study. Dig Liver Dis. 2022;54(12):1639–45. 10.1016/j.dld.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Mocci G, Bodini G, Allegretta L, et al. Adalimumab biosimilar GP2017 versus adalimumab originator in treating patients with inflammatory bowel diseases: a real-life, multicenter, observational study. Biomedicines. 2022;10(8):1799. 10.3390/biomedicines10081799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wasserbauer M, Hlava S, Drabek J, et al. Adalimumab biosimilars in the therapy of Crohn’s disease and ulcerative colitis: prospective multicentric clinical monitoring. PLoS ONE. 2022;17(8): e0271299. 10.1371/journal.pone.0271299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gros B, Soto Escribano P, Marín Pedrosa S, Medina Medina R, Benítez JM, Iglesias-Flores E. P534 Effectiveness and safety of Adalimumab switch from originator to biosimilar in patients with inflammatory bowel disease in clinical remission. J Crohns Colitis. 2022;16(Suppl 1): i486. 10.1093/ecco-jcc/jjab232.661. [Google Scholar]
- 33.Tursi A, Mocci G, Allegretta L, et al. Comparison of performances of adalimumab biosimilars SB5, ABP501, GP2017, and MSB11022 in treating patients with inflammatory bowel diseases: a real-life, multicenter, observational study. Inflamm Bowel Dis. 2023;29(3):376–83. 10.1093/ibd/izac092. [DOI] [PubMed] [Google Scholar]
- 34.Tursi A, Mocci G, Cuomo A, et al. Replacement of adalimumab originator to adalimumab biosimilar for a non-medical reason in patients with inflammatory bowel disease: a real-life comparison of adalimumab biosimilars currently available in Italy. J Gastrointest Liver Dis. 2022;31(4):411–6. 10.15403/jgld-4608. [DOI] [PubMed] [Google Scholar]
- 35.Alnaqbi DKA, Alsennani F, Aloqaili DL, et al. EE516 Budget-impact model of adalimumab-biosimilar Sandoz in the management of autoimmune diseases in the Middle East. Value Health. 2022;25(Suppl 12):S157. 10.1016/j.jval.2022.09.758. [Google Scholar]
- 36.Bustos Morell C, Gonzalez Puyuelo JM, Monforte Gasque MP, Labrador Andujar N, Provencio Del Olmo M, Lazaro Gallardo EM. 1ISG-025 Budget impact analysis of biosimilar adalimumab versus reference adalimumab. Eur J Hosp Pharm. 2021;28(Suppl 1):A3. 10.1136/ejhpharm-2021-eahpconf.6. [Google Scholar]
- 37.Chaplin S, van Stiphout J, Chen A, Li E. M5: budget impact analysis of including biosimilar adalimumab on formulary: a United States payer perspective. J Manag Care Spec Pharm. 2023;29(10-a):M5. 10.18553/jmcp.2024.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nabi H, Ibsen R, Ibsen M, Kjellberg J, Hetland ML, Glintborg B. POSO376. Does a mandatory switch from originator adalimumab to biosimilar GP2017 or SB5 lead to increased hospital costs? A DANBIO study of > 1300 patients with inflammatory arthritis. Ann Rheum Dis. 2023;82:441–2. 10.1136/annrheumdis-2023-eular.812. [Google Scholar]
- 39.van der Heijde D, Salonen D, Weissman BN, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up to 2 years. Arthritis Res Ther. 2009;11(4):R127. 10.1186/ar2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Popescu CC, Mogoșan CD, Enache L, Codreanu C. Comparison of efficacy and safety of original and biosimilar adalimumab in active rheumatoid arthritis in a real-world national cohort. Medicina. 2022;58(12):1851. 10.3390/medicina58121851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horneff G, Dressler F, Ruhlmann M, Geikowski T, Mrusek S, Klein A. Experience with adalimumab biosimilar use in clinical practice: data from the German Biker-Registry. Ann Rheum Dis. 2021;80:933–4. 10.1136/annrheumdis-2021-eular.1433. [Google Scholar]
- 42.Kronthaler U, Fritsch C, Hainzl O, Seidl A, da Silva A. Comparative functional and pharmacological characterization of Sandoz proposed biosimilar adalimumab (GP2017): rationale for extrapolation across indications. Expert Opin Biol Ther. 2018;18(8):921–30. 10.1080/14712598.2018.1495193. [DOI] [PubMed] [Google Scholar]
- 43.Menter A, Thaçi D, Wu JJ, et al. Long-term safety and effectiveness of adalimumab for moderate to severe psoriasis: results from 7-year interim analysis of the ESPRIT registry. Dermatol Ther (Heidelb). 2017;7(3):365–81. 10.1007/s13555-017-0198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bossuyt P, Atreya R, Taxonera C, et al. DOP004 Long-term safety of adalimumab in patients with moderate-to-severe ulcerative colitis: interim results of a non-interventional registry, LEGACY. J Crohns Colitis. 2018;12(Suppl 1):S032. 10.1093/ecco-jcc/jjx180.041. [Google Scholar]
- 45.Loftus EV, Reinisch W, Panaccione R, et al. Adalimumab effectiveness up to six years in adalimumab-naïve patients with Crohn’s disease: results of the PYRAMID registry. Inflamm Bowel Dis. 2019;25(9):1522–31. 10.1093/ibd/izz008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larid G, Baudens G, Dandurand A, et al. Differential retention of adalimumab and etanercept biosimilars compared to originator treatments: results of a retrospective French multicenter study. Front Med (Lausanne). 2022;9:989514. 10.3389/fmed.2022.989514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glintborg B, Loft AG, Omerovic E, et al. To switch or not to switch: results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. One-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann Rheum Dis. 2019;78(2):192–200. 10.1136/annrheumdis-2018-213474. [DOI] [PubMed] [Google Scholar]
- 48.Sarlos P, Bikar A, Farkas N, et al. Self-reported efficacy and safety of infliximab and adalimumab biosimilars after non-medical switch in patients with inflammatory bowel disease: results of a multicenter survey. Expert Opin Biol Ther. 2023;23:827–32. 10.1080/14712598.2023.2211204. [DOI] [PubMed] [Google Scholar]
- 49.Fleischmann R, Jairath V, Mysler E, Nicholls D, Declerck P. Nonmedical switching from originators to biosimilars: does the nocebo effect explain treatment failures and adverse events in rheumatology and gastroenterology? Rheumatol Ther. 2020;7(1):35–64. 10.1007/s40744-019-00190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gall S, Kiltz U, Kobylinski T, et al. Patient knowledge about biosimilars and satisfaction with the education provided by rheumatologists or nurse specialists in a biosimilar multiswitch scenario - the perception study. Semin Arthritis Rheum. 2022;57:152119. 10.1016/j.semarthrit.2022.152119. [DOI] [PubMed] [Google Scholar]
- 51.Allocati E, Godman B, Gobbi M, Garattini S, Banzi R. Switching among biosimilars: a review of clinical evidence. Front Pharmacol. 2022;13:917814. 10.3389/fphar.2022.917814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barbier L, Ebbers HC, Declerck P, Simoens S, Vulto AG, Huys I. The efficacy, safety, and immunogenicity of switching between reference biopharmaceuticals and biosimilars: a systematic review. Clin Pharmacol Ther. 2020;108(4):734–55. 10.1002/cpt.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.García-Beloso N, Altabás-González I, Samartín-Ucha M, et al. Switching between reference adalimumab and biosimilars in chronic immune-mediated inflammatory diseases: a systematic literature review. Br J Clin Pharmacol. 2022;88(4):1529–50. 10.1111/bcp.15101. [DOI] [PubMed] [Google Scholar]
- 54.de Oliveira AB, Almeida MO, de Medeiros-Ribeiro AC, de Oliveira Andrade DC, de Oliveira Junior HA, de Soárez PC. Impact of switching between reference biologics and biosimilars of tumour necrosis factor inhibitors for rheumatoid arthritis: a systematic review and network meta-analysis. Sci Rep. 2023;13(1):13699. 10.1038/s41598-023-40222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cohen HP, Hachaichi S, Bodenmueller W, Kvien TK, Danese S, Blauvelt A. Switching from one biosimilar to another biosimilar of the same reference biologic: a systematic review of studies. BioDrugs. 2022;36(5):625–37. 10.1007/s40259-022-00546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.European Medicines Agency and Heads of Medicines Agencies. Statement on the scientific rationale supporting interchangeability of biosimilar medicines in the EU. 2023. https://www.ema.europa.eu/en/documents/public-statement/statement-scientific-rationale-supporting-interchangeability-biosimilar-medicines-eu_en.pdf. Accessed 14 Dec 2023. [DOI] [PMC free article] [PubMed]
- 57.Park JP, Jung B, Park HK, et al. Interchangeability for biologics is a legal distinction in the USA, not a clinical one. BioDrugs. 2022;36(4):431–6. 10.1007/s40259-022-00538-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cohen HP, Turner M, McCabe D, Woollett GR. Future evolution of biosimilar development by application of current science and available evidence: the developer’s perspective. BioDrugs. 2023;37:583–93. 10.1007/s40259-023-00619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.US Food and Drug Administration. Considerations in demonstrating interchangeability with a reference product guidance for industry 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-demonstrating-interchangeability-reference-product-guidance-industry. Accessed 14 Dec 2023.
- 60.US Food and Drug Administration. Updated FDA labeling recommendations for biosimilar and interchangeable biosimilar products. 2023. https://www.fda.gov/drugs/our-perspective/updated-fda-labeling-recommendations-biosimilar-and-interchangeable-biosimilar-products. Accessed 19 Dec 2023.
- 61.Car E, Vulto AG, Houdenhoven MV, Huys I, Simoens S. Biosimilar competition in European markets of TNF-alpha inhibitors: a comparative analysis of pricing, market share and utilization trends. Front Pharmacol. 2023;14:1151764. 10.3389/fphar.2023.1151764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.