Abstract
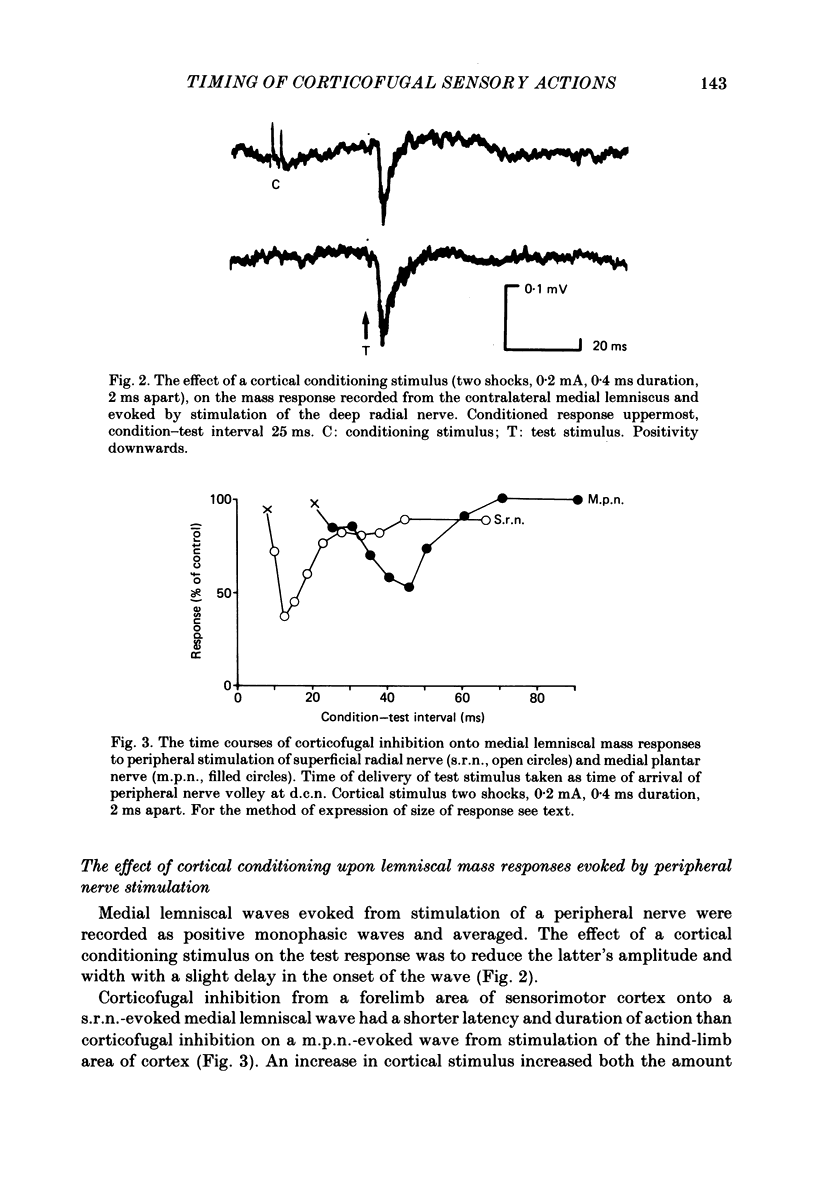
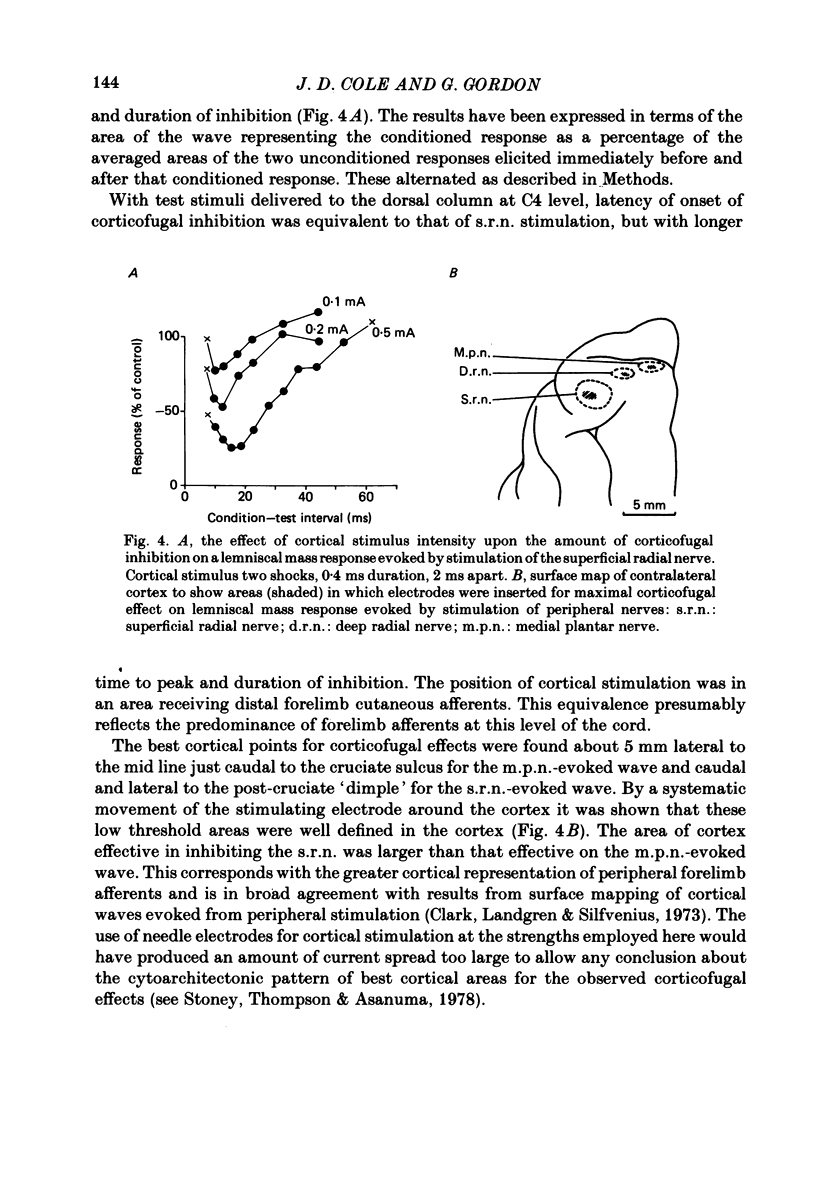
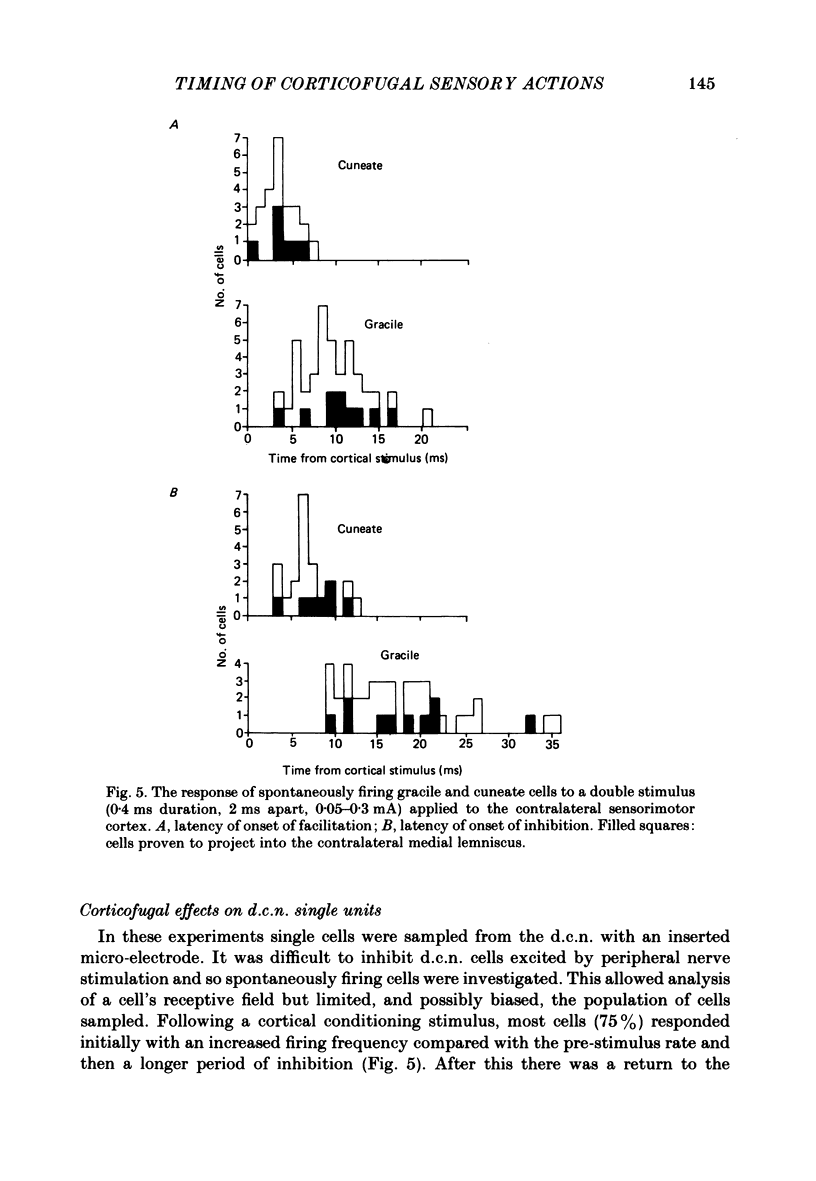
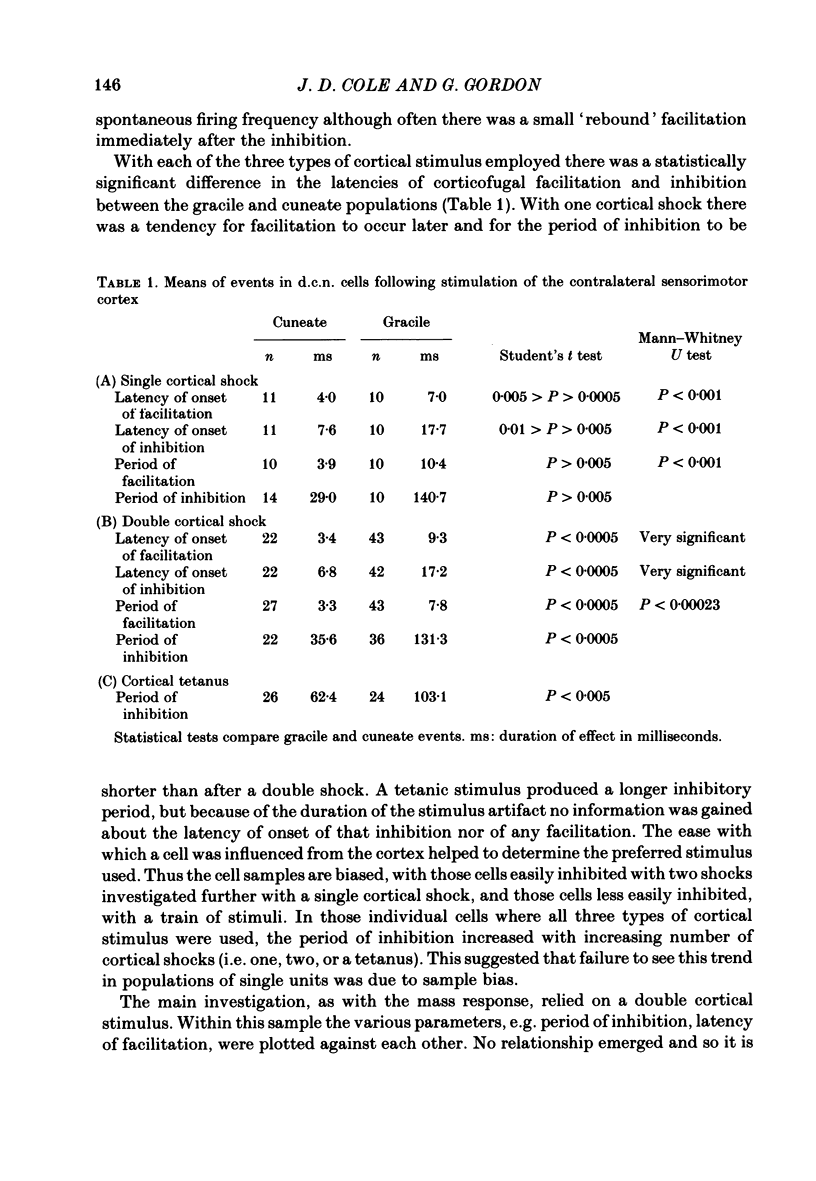
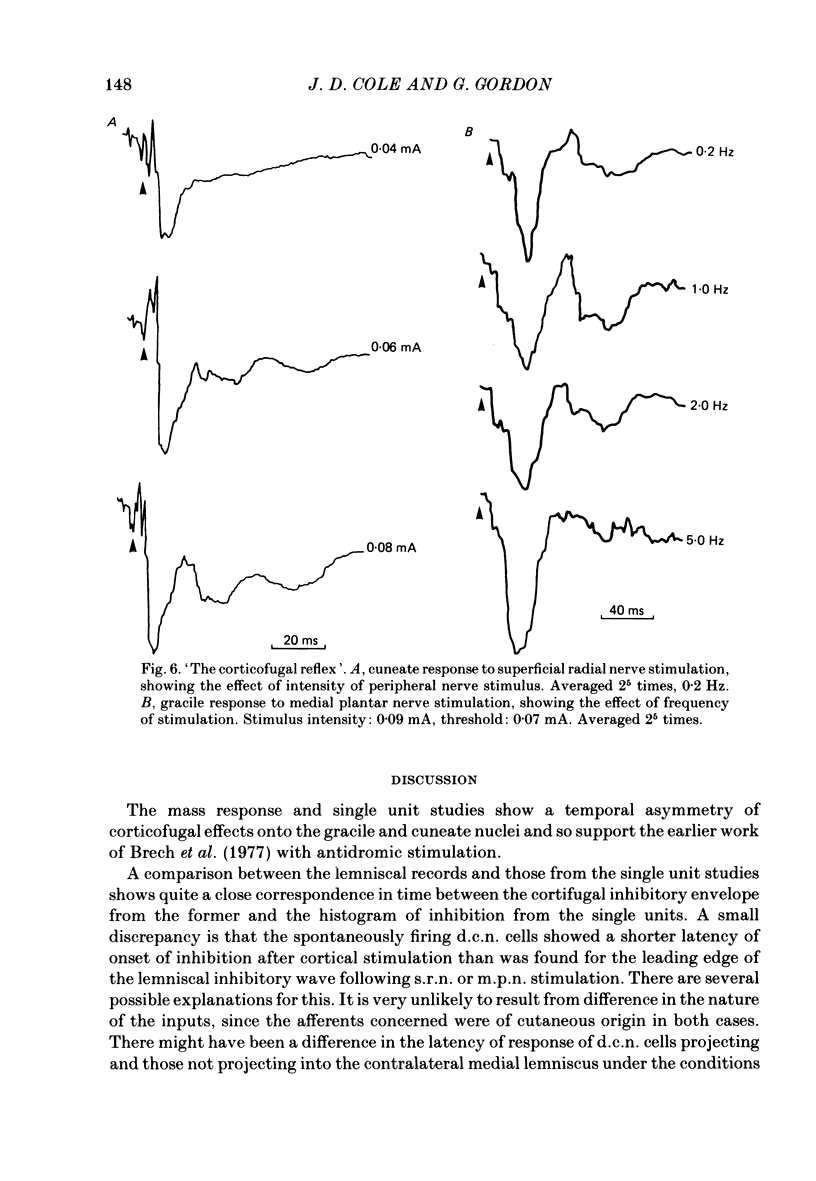
A comparison is presented of the latencies of corticofugal effects from the contralateral somatosensory cortex (SI) onto the cat's dorsal column nuclei (d.c.n.) under pentobarbitone anaesthesia. The latencies for transmission in the ascending pathway from d.c.n. to SI after stimulation within the gracile and cuneate nuclei were found to be 3.3 ms for the former and 2.8 ms for the latter. The time courses of inhibition of a medial lemniscal mass response following cortical conditioning and evoked by stimulation of peripheral nerves were measured. All latencies were corrected to exclude the different times taken for stimuli to reach the nuclei from the two limbs. The optimal condition-test interval was 12 ms with a duration of 14.3 ms for the superficial radial nerve (s.r.n.) and 45 ms and 30 ms respectively for the medial plantar nerve (m.p.n.). In each case cortical conditioning inhibited the wave by about 50%. The effect of cortical conditioning upon spontaneously firing d.c.n. single units was investigated. For cuneate cells the mean latency was 6.8 ms and the mean duration 36.8 ms. For gracile cells the latency of onset of inhibition was 17.2 ms and its duration 129 ms. In 75% of cells mixed effects were seen with facilitation preceding inhibition. The latencies of 'corticofugal reflex' action on the gracile and cuneate nuclei after stimulation of the s.r.n. and m.p.n. were determined. The gracile response had a latency approximately 4 times that for the cuneate response. The temporal asymmetry of these corticofugal effects suggests that the pathway is not purely a simple feed-back loop, but may be concerned in other physiological contexts, some of which are discussed.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., OSHIMA T., SCHMIDT R. F. MECHANISMS OF SYNAPTIC TRANSMISSION IN THE CUNEATE NUCLEUS. J Neurophysiol. 1964 Nov;27:1096–1116. doi: 10.1152/jn.1964.27.6.1096. [DOI] [PubMed] [Google Scholar]
- Brech A. J., Gordon G., Powell T. P. Corticofugal cells responding antidromically to stimulation of the cuneate or gracile nuclei of the cat. Brain Res. 1977 Jun 3;128(1):29–52. doi: 10.1016/0006-8993(77)90234-7. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Gordon G., Kay R. H. A study of single axons in the cat's medial lemniscus. J Physiol. 1974 Jan;236(1):225–246. doi: 10.1113/jphysiol.1974.sp010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesa-Bianchi M. G., Sotgiu M. L. Control by brain stem reticular formation of sensory transmission in Burdach nucleus. Analysis of single units. Brain Res. 1969 Mar;13(1):129–139. doi: 10.1016/0006-8993(69)90147-4. [DOI] [PubMed] [Google Scholar]
- Clark F. J., Landgren S., Silfvenius H. Projections to the cat's cerebral cortex from low threshold joint afferents. Acta Physiol Scand. 1973 Dec;89(4):504–521. doi: 10.1111/j.1748-1716.1973.tb05544.x. [DOI] [PubMed] [Google Scholar]
- Cole J. D., Gordon G. Proceedings: Dissimilar timing of corticofugal inhibition of the gracile and cuneate nuclei in cats anaesthetized with pentobarbitone. J Physiol. 1976 Mar;256(1):38P–39P. [PubMed] [Google Scholar]
- Coulter J. D. Sensory transmission through lemniscal pathway during voluntary movement in the cat. J Neurophysiol. 1974 Sep;37(5):831–845. doi: 10.1152/jn.1974.37.5.831. [DOI] [PubMed] [Google Scholar]
- GORDON G., JUKES M. G. DESCENDING INFLUENCES ON THE EXTEROCEPTIVE ORGANIZATIONS OF THE CAT'S GRACILE NUCLEUS. J Physiol. 1964 Sep;173:291–319. doi: 10.1113/jphysiol.1964.sp007457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C., Lenzi G. L. Modulation of afferent transmission in the lemniscal system during voluntary movement in cat. Brain Res. 1970 Dec 18;24(3):542–542. doi: 10.1016/0006-8993(70)90195-2. [DOI] [PubMed] [Google Scholar]
- Gordon G., Miller R. Identification of cortical cells projecting to the dorsal column nuclei of the cat. Q J Exp Physiol Cogn Med Sci. 1969 Jan;54(1):85–98. doi: 10.1113/expphysiol.1969.sp002009. [DOI] [PubMed] [Google Scholar]
- Groos W. P., Ewing L. K., Carter C. M., Coulter J. D. Organization of corticospinal neurons in the cat. Brain Res. 1978 Mar 31;143(3):393–419. doi: 10.1016/0006-8993(78)90353-0. [DOI] [PubMed] [Google Scholar]
- Hulliger M., Nordh E., Thelin A. E., Vallbo A. B. The responses of afferent fibres from the glabrous skin of the hand during voluntary finger movements in man. J Physiol. 1979 Jun;291:233–249. doi: 10.1113/jphysiol.1979.sp012809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JABBUR S. J., TOWE A. L. Cortical excitation of neurons in dorsal column nuclei of cat, including an analysis of pathways. J Neurophysiol. 1961 Sep;24:499–509. doi: 10.1152/jn.1961.24.5.499. [DOI] [PubMed] [Google Scholar]
- LEVITT M., CARRERAS M., LIU C. N., CHAMBERS W. W. PYRAMIDAL AND EXTRAPYRAMIDAL MODULATION OF SOMATOSENSORY ACTIVITY IN GRACILE AND CUNEATE NUCLEI. Arch Ital Biol. 1964 Apr 18;102:197–229. [PubMed] [Google Scholar]
- Rosén I. Afferent connexions to group I activated cells in the main cuneate nucleus of the cat. J Physiol. 1969 Nov;205(1):209–236. doi: 10.1113/jphysiol.1969.sp008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustioni A., Hayes N. L. Corticospinal tract collaterals to the dorsal column nuclei of cats. An anatomical single and double retrograde tracer study. Exp Brain Res. 1981;43(3-4):237–245. doi: 10.1007/BF00238364. [DOI] [PubMed] [Google Scholar]
- Sotgiu M. L., Marini G. Reticulo-cuneate projections as revealed by horseradish peroxidase axonal transport. Brain Res. 1977 Jun 10;128(2):341–345. doi: 10.1016/0006-8993(77)90999-4. [DOI] [PubMed] [Google Scholar]
- Stoney S. D., Jr, Thompson W. D., Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol. 1968 Sep;31(5):659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]


