Abstract
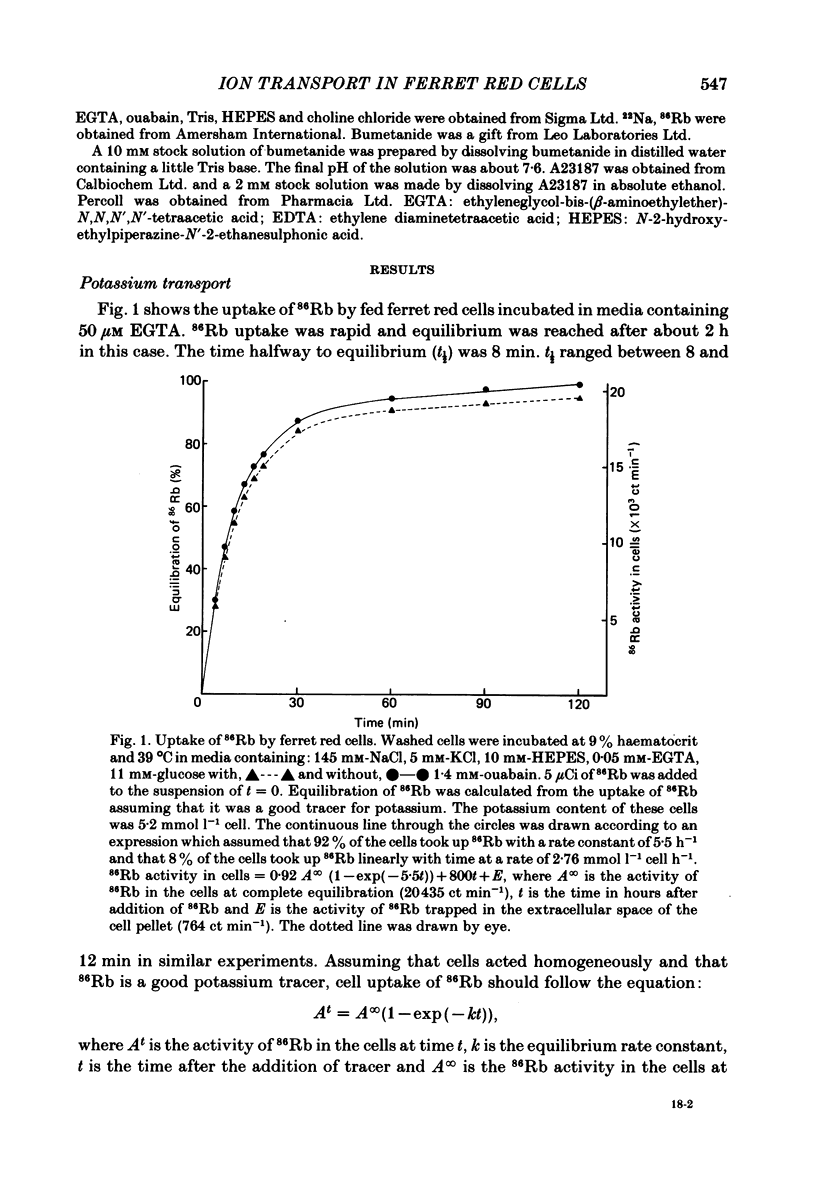
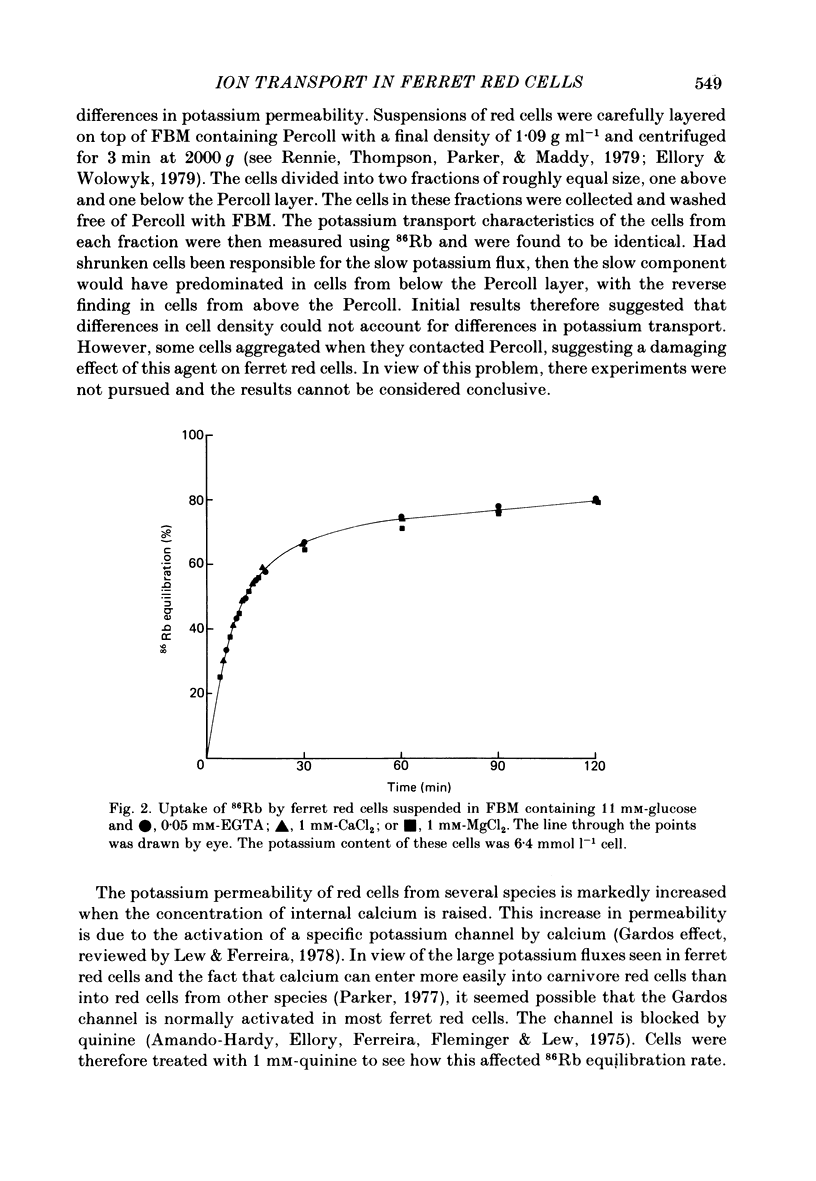
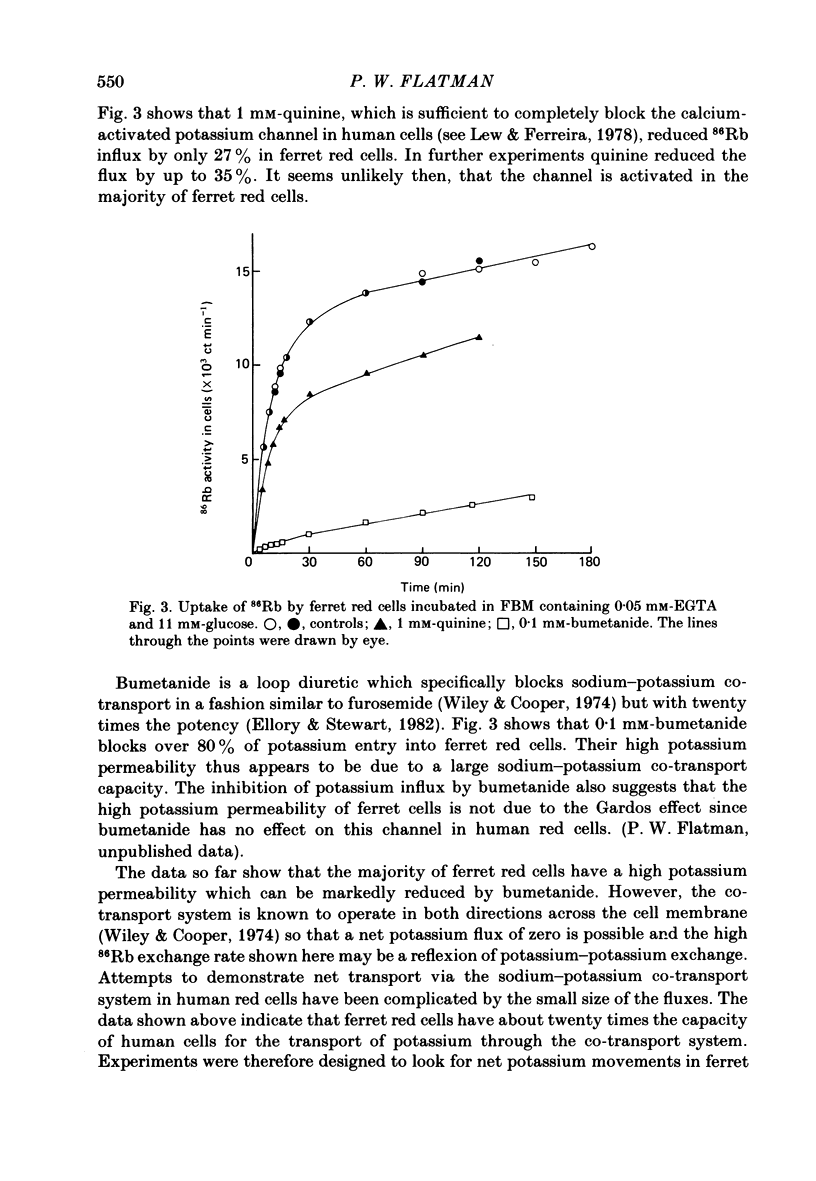
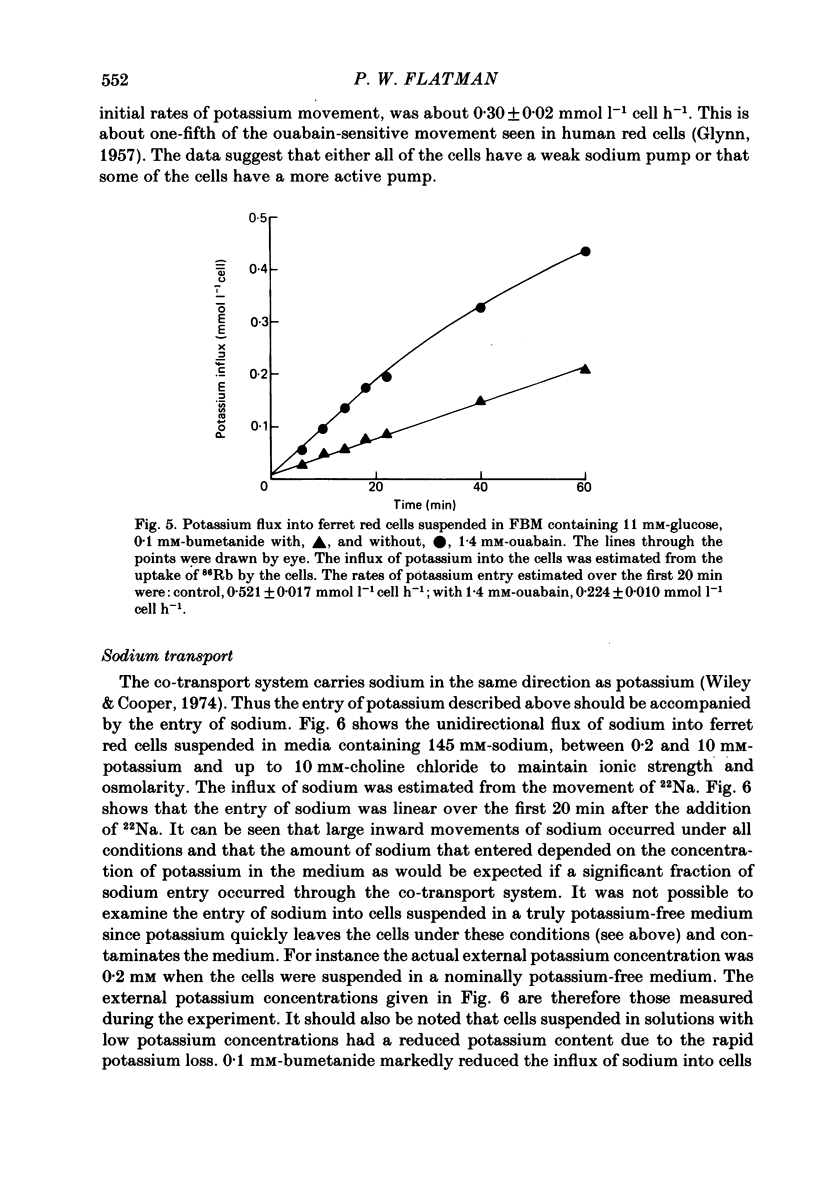
Potassium movements into ferret red cells were measured with the tracer 86Rb. Equilibration of 86Rb between medium and cells could be resolved into two components. 70-90% occurred rapidly with a rate constant of between 3.5-5.5 h-1. The remaining 10-30% occurred slowly. The slow movement was equivalent to a potassium influx of about 1.2-2.76 mmol l-1 cell h-1. Potassium influx was inhibited by 80-90% by 0.1 mM-bumetanide (a high-ceiling, loop diuretic). This suggests that the sodium-potassium co-transport system has a high capacity for carrying potassium (estimated at about 17-35 mmol l-1 cell h-1). After bumetanide (0.1 mM) remaining potassium movements (approximately 0.5 mmol l-1 cell h-1) are at a similar level to that found in red cells from other animals. The sodium pump makes a very small contribution to potassium flux into ferret red cells. Much of this pump activity may be attributed to reticulocytes present in cell samples. Sodium movements across the red cell membrane were measured with 22Na. Sodium equilibrated more slowly than potassium. 60-70% of the sodium influx was inhibited by 0.1 mM-bumetanide, indicating that most sodium influx in ferret red cells is also through the co-transport system. The co-transport system can transport up to 49 mmol sodium l-1 cell h-1 and is half maximally activated by 0.38 mM-potassium in the external medium. In the presence of bumetanide, sodium influx (about 18 mmol l-1 cell h-1) is similar to that of other carnivore red cells. This is about five times greater than that of red cells from non-carnivores. The possibility that there are two populations of ferret red cells with different potassium transport characteristics is discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armando-Hardy M., Ellory J. C., Ferreira H. G., Fleminger S., Lew V. L. Inhibition of the calcium-induced increase in the potassium permeability of human red blood cells by quinine. J Physiol. 1975 Aug;250(1):32P–33P. [PubMed] [Google Scholar]
- Davson H. The haemolytic action of potassium salts. J Physiol. 1942 Nov 30;101(3):265–283. doi: 10.1113/jphysiol.1942.sp003981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M. J. Red blood cell calcium and magnesium: effects upon sodium and potassium transport and cellular morphology. Biochim Biophys Acta. 1974 May 30;352(1):97–116. doi: 10.1016/0005-2736(74)90182-5. [DOI] [PubMed] [Google Scholar]
- Elford B. C. Interactions between temperature and tonicity on cation transport in dog red cells. J Physiol. 1975 Mar;246(2):371–395. doi: 10.1113/jphysiol.1975.sp010895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellory J. C., Stewart G. W. The human erythrocyte Cl-dependent Na-K cotransport system as a possible model for studying the action of loop diuretics. Br J Pharmacol. 1982 Jan;75(1):183–188. doi: 10.1111/j.1476-5381.1982.tb08771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman P. W., Andrews P. L. Cation and ATP content of ferret red cells. Comp Biochem Physiol A Comp Physiol. 1983;74(4):939–943. doi: 10.1016/0300-9629(83)90373-0. [DOI] [PubMed] [Google Scholar]
- GLYNN I. M. Sodium and potassium movements in human red cells. J Physiol. 1956 Nov 28;134(2):278–310. doi: 10.1113/jphysiol.1956.sp005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLYNN I. M. The action of cardiac glycosides on sodium and potassium movements in human red cells. J Physiol. 1957 Apr 3;136(1):148–173. doi: 10.1113/jphysiol.1957.sp005749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay R. P., Ciccone J. Inhibition of the Na+/K+ cotransport system by cyclic AMP and intracellular Ca2+ in human red cells. Biochim Biophys Acta. 1982 Jun 28;688(3):786–792. doi: 10.1016/0005-2736(82)90292-9. [DOI] [PubMed] [Google Scholar]
- HOFFMAN J. F. Cation transport and structure of the red-cell plasma membrane. Circulation. 1962 Nov;26:1202–1213. doi: 10.1161/01.cir.26.5.1201. [DOI] [PubMed] [Google Scholar]
- Haas M., Schmidt W. F., 3rd, McManus T. J. Catecholamine-stimulated ion transport in duck red cells. Gradient effects in electrically neutral [Na + K + 2Cl] Co-transport. J Gen Physiol. 1982 Jul;80(1):125–147. doi: 10.1085/jgp.80.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie C. M., Thompson S., Parker A. C., Maddy A. Human erythrocyte fraction in "Percoll" density gradients. Clin Chim Acta. 1979 Oct 15;98(1-2):119–125. doi: 10.1016/0009-8981(79)90172-4. [DOI] [PubMed] [Google Scholar]
- Romualdez A., Sha'afi R. I., Lange Y., Solomon A. K. Cation transport in dog red cells. J Gen Physiol. 1972 Jul;60(1):46–57. doi: 10.1085/jgp.60.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. F., 3rd, McManus T. J. Ouabain-insensitive salt and water movements in duck red cells. II. Norepinephrine stimulation of sodium plus potassium cotransport. J Gen Physiol. 1977 Jul;70(1):81–97. doi: 10.1085/jgp.70.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha'afi R. I., Hajjar J. J. Sodium movement in high sodium feline red cells. J Gen Physiol. 1971 Jun;57(6):684–696. doi: 10.1085/jgp.57.6.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha'afi R. I., Lieb W. R. Cation movements in the high sodium erythrocyte of the cat. J Gen Physiol. 1967 Jul;50(6):1751–1764. doi: 10.1085/jgp.50.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha'afi R. I., Naccache P. Sodium and calcium transport in cat red cells. J Cell Physiol. 1975 Jun;85(3):655–664. doi: 10.1002/jcp.1040850318. [DOI] [PubMed] [Google Scholar]
- Thornton P. C., Wright P. A., Sacra P. J., Goodier T. E. The ferret, Mustela putorius furo, as a new species in toxicology. Lab Anim. 1979 Apr;13(2):119–124. doi: 10.1258/002367779780943422. [DOI] [PubMed] [Google Scholar]
- Wiley J. S., Cooper R. A. A furosemide-sensitive cotransport of sodium plus potassium in the human red cell. J Clin Invest. 1974 Mar;53(3):745–755. doi: 10.1172/JCI107613. [DOI] [PMC free article] [PubMed] [Google Scholar]


