Abstract
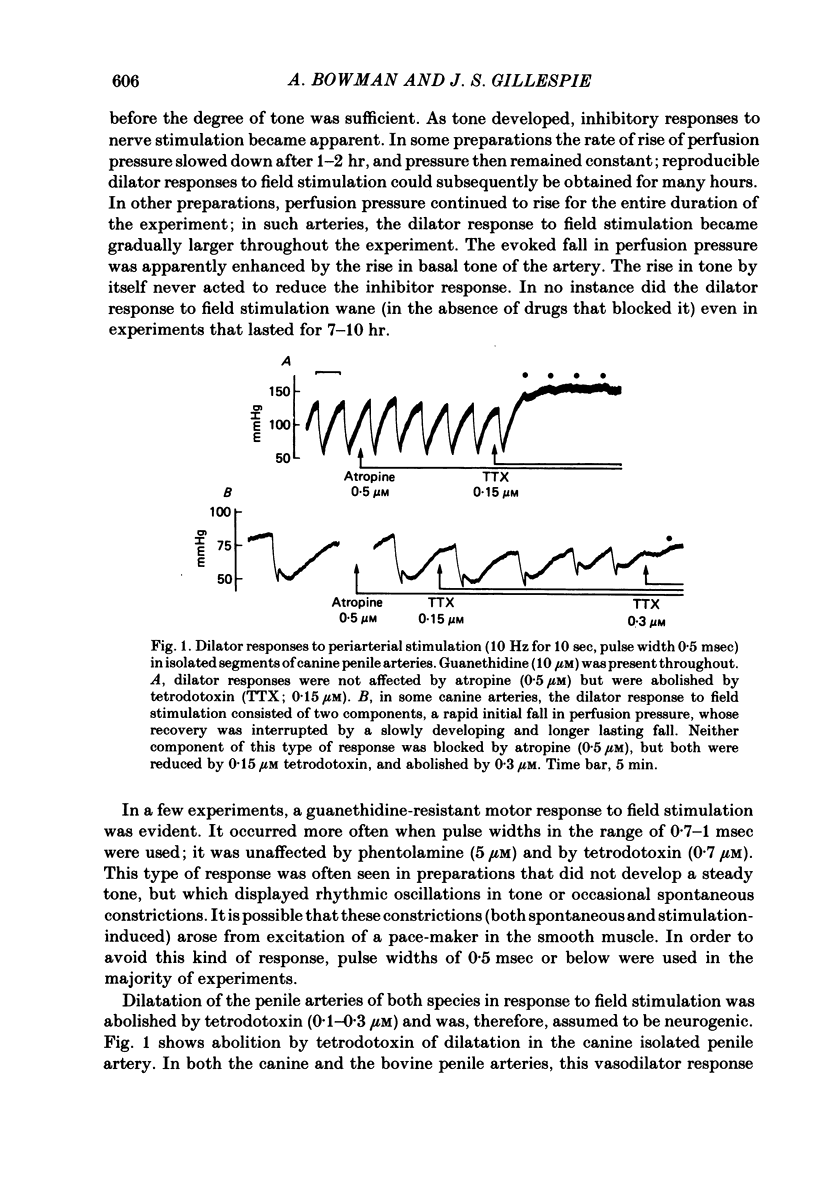
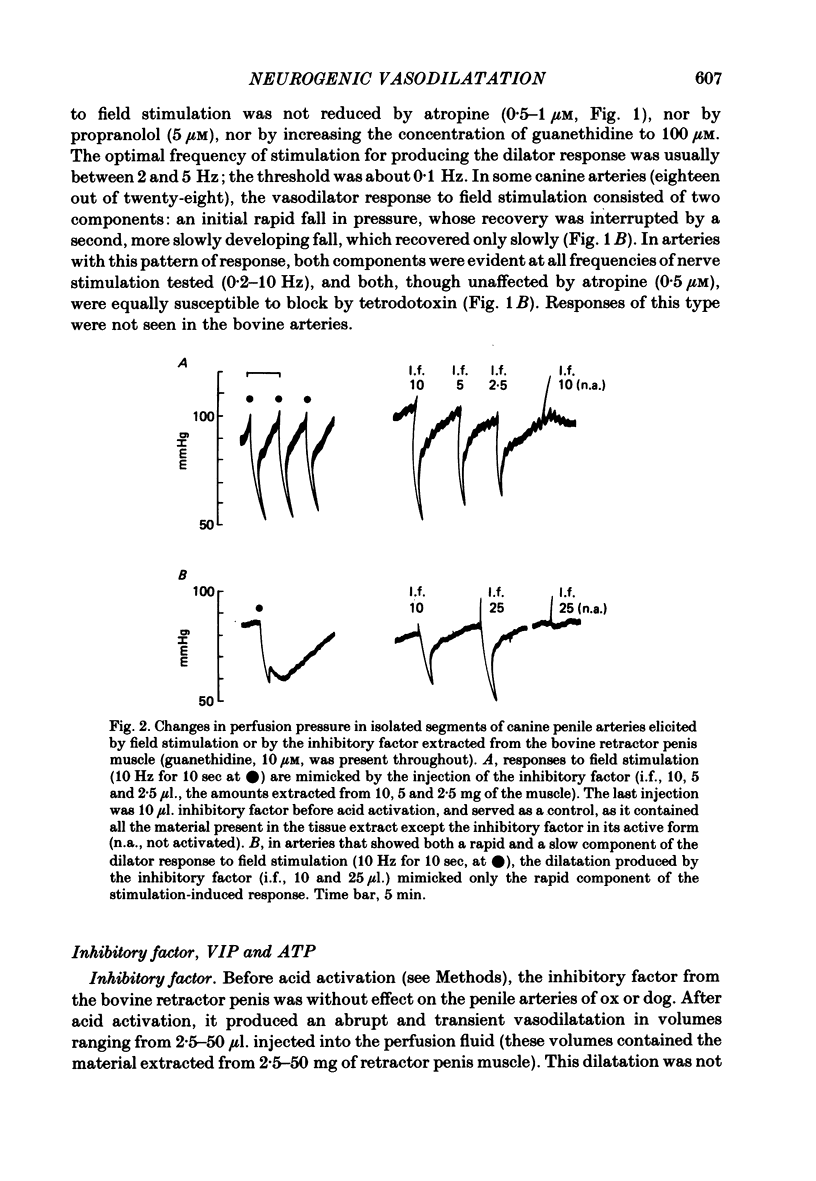
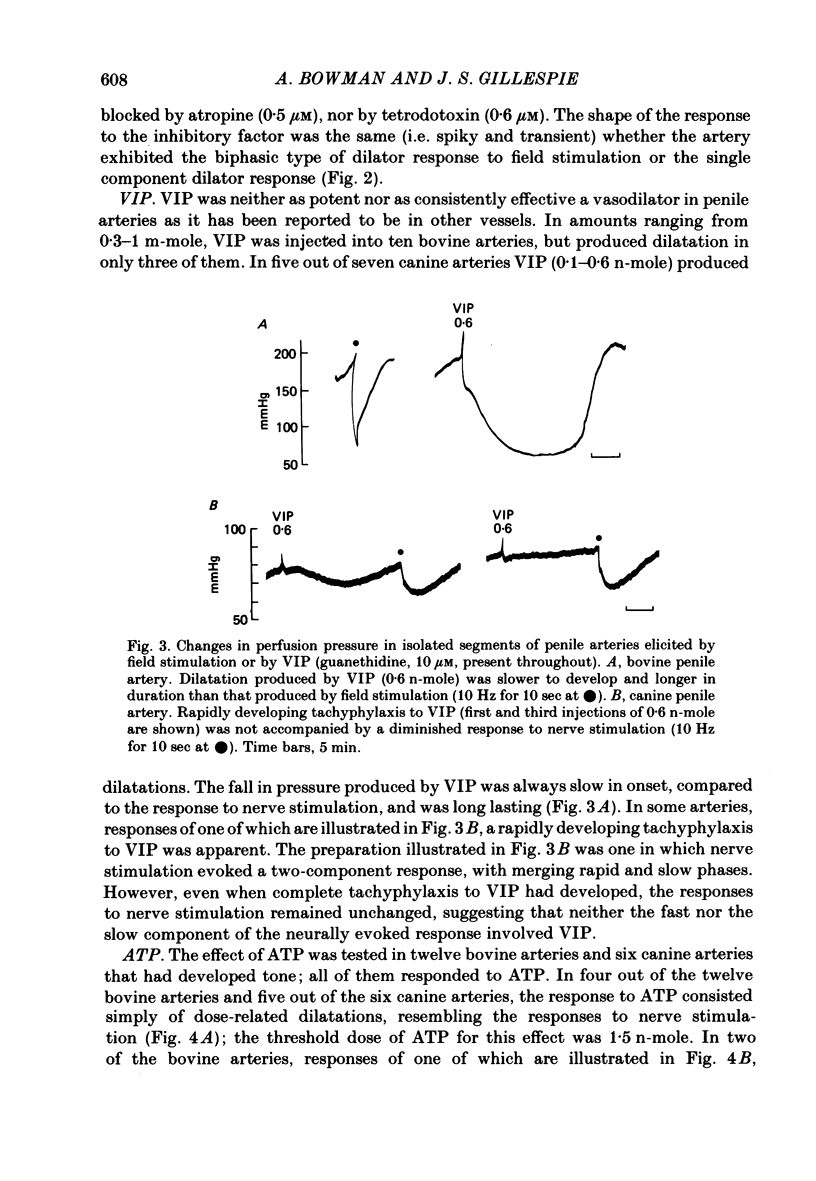
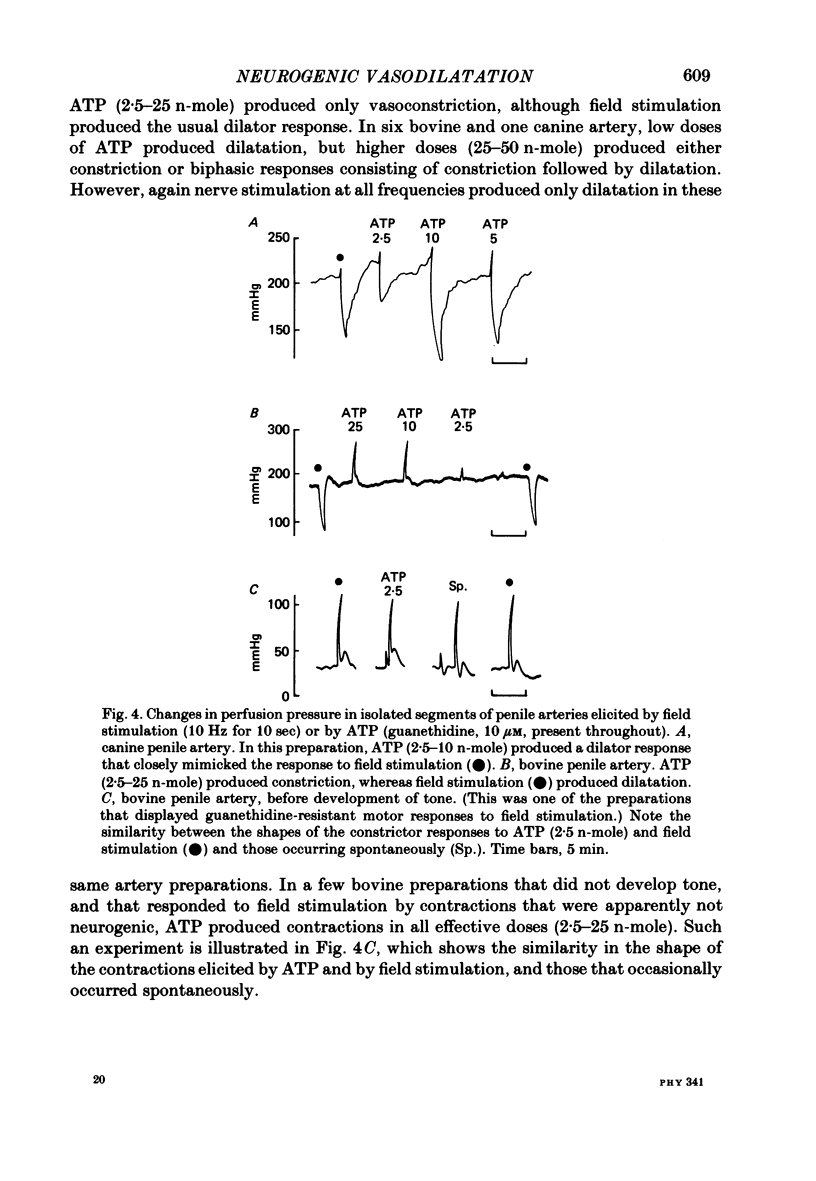
Field stimulation of isolated, perfused bovine or canine penile arteries produced dilatation, after the adrenergic motor component of the response had been blocked with guanethidine and the vessels had developed a background tone. The vasodilatation was blocked by tetrodotoxin but not by atropine. The vasodilator responses to field stimulation were compared with those produced by ATP, by vasoactive intestinal peptide (VIP), and by the inhibitory factor extracted from the bovine retractor penis muscle. Of the three putative transmitters, the inhibitory factor produced responses that most closely resembled those to field stimulation. Haemoglobin, which blocks non-adrenergic, non-cholinergic inhibitory transmission in the bovine and canine retractor penis muscles, did not impair the vasodilatations produced by ATP or VIP, but slowly reduced or abolished those produced by field stimulation or by the inhibitory factor. Haemoglobin itself produced a powerful constriction of the isolated penile arteries. The results are compatable with the possibility that the inhibitory factor from the bovine retractor penis muscle (which may be the inhibitory transmitter in that muscle) is, or closely resembles, the transmitter of non-adrenergic, non-cholinergic vasodilator fibres in the penile arteries of dog and ox.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Killick S. W., Aboo Aar M. Extraction from ox retractor penis of an inhibitory substance which mimics its atropine-resistant neurogenic relaxation. Br J Pharmacol. 1975 Jul;54(3):409–410. doi: 10.1111/j.1476-5381.1975.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Bell C. Autonomic nervous control of reproduction: circulatory and other factors. Pharmacol Rev. 1972 Dec;24(4):657–736. [PubMed] [Google Scholar]
- Bell C., McLean J. R. The distribution of cholinergic and adrenergic nerve fibres in the retractor penis and vas deferens of the dog. Z Zellforsch Mikrosk Anat. 1970;106(4):516–522. doi: 10.1007/BF00340289. [DOI] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S. Block of some non-adrenergic inhibitory responses of smooth muscle by a substance from haemolysed erythrocytes. J Physiol. 1982 Jul;328:11–25. doi: 10.1113/jphysiol.1982.sp014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. Actions on the cardiovascular system of an inhibitory material extracted from the bovine retractor penis. Br J Pharmacol. 1981 Feb;72(2):365–372. doi: 10.1111/j.1476-5381.1981.tb09136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. The inhibitory material in extracts from the bovine retractor penis muscle is not an adenine nucleotide. Br J Pharmacol. 1979 Nov;67(3):327–328. doi: 10.1111/j.1476-5381.1979.tb08683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Pollock D. Oxyhaemoglobin blocks non-adrenergic non-cholinergic inhibition in the bovine retractor penis muscle. Eur J Pharmacol. 1982 Nov 19;85(2):221–224. doi: 10.1016/0014-2999(82)90470-8. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic nerves. Pharmacol Rev. 1972 Sep;24(3):509–581. [PubMed] [Google Scholar]
- Dorr L. D., Brody M. J. Hemodynamic mechanisms of erection in the canine penis. Am J Physiol. 1967 Dec;213(6):1526–1531. doi: 10.1152/ajplegacy.1967.213.6.1526. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Hunter J. C., Martin W. Some physical and chemical properties of the smooth muscle inhibitory factor in extracts of the bovine retractor penis muscle. J Physiol. 1981 Jun;315:111–125. doi: 10.1113/jphysiol.1981.sp013736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material extracted from the bovine retractor penis and rat anococcygeus muscles [proceedings]. J Physiol. 1978 Jul;280:45P–46P. [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material from the bovine retractor penis and rat anococcygeus muscles. J Physiol. 1980 Dec;309:55–64. doi: 10.1113/jphysiol.1980.sp013493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge E., Sjöstrand N. O. Comparative study of some isolated mammalian smooth muscle effectors of penile erection. Acta Physiol Scand. 1977 Jul;100(3):354–367. doi: 10.1111/j.1748-1716.1977.tb05961.x. [DOI] [PubMed] [Google Scholar]
- Klinge E., Sjöstrand N. O. Contraction and relaxation of the retractor penis muscle and the penile artery of the bull. Acta Physiol Scand Suppl. 1974;420:1–88. [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part II. The Bladder. Part III. The External Generative Organs. Part IV. The Internal Generative Organs. Part V. Position of the Nerve Cells on the Course of the Efferent Nerve Fibres. J Physiol. 1895 Dec 30;19(1-2):71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. J., Hume W. R., Su C., Bevan J. A. Neurogenic vasodilation of cat cerebral arteries. Circ Res. 1978 Apr;42(4):535–542. doi: 10.1161/01.res.42.4.535. [DOI] [PubMed] [Google Scholar]
- Luduena F. P., Grigas E. O. Effect of some biological substances on the dog retractor penis in vitro. Arch Int Pharmacodyn Ther. 1972 Apr;196(2):269–274. [PubMed] [Google Scholar]
- Luduena F. P., Grigas E. O. Pharmacological study of autonomic innervation of dog retractor penis. Am J Physiol. 1966 Mar;210(3):435–444. doi: 10.1152/ajplegacy.1966.210.3.435. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Gu J., Mina S., Bloom S. R. Vipergic nerves in the penis. Lancet. 1981 Aug 1;2(8240):217–219. doi: 10.1016/s0140-6736(81)90471-2. [DOI] [PubMed] [Google Scholar]
- Sjöstrand N. O., Klinge E., Himberg J. J. Effects of VIP and other putative neurotransmitters on smooth muscle effectors of penile erection. Acta Physiol Scand. 1981;113(3):403–405. doi: 10.1111/j.1748-1716.1981.tb06914.x. [DOI] [PubMed] [Google Scholar]
- Tanishima T. Cerebral vasospasm: contractile activity of hemoglobin in isolated canine basilar arteries. J Neurosurg. 1980 Dec;53(6):787–793. doi: 10.3171/jns.1980.53.6.0787. [DOI] [PubMed] [Google Scholar]
- Toda N. Non-adrenergic, non-cholinergic innervation in monkey and human cerebral arteries. Br J Pharmacol. 1981 Feb;72(2):281–283. doi: 10.1111/j.1476-5381.1981.tb09126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


