Abstract
1. We have recorded electroretinograms (e.r.g.s) in normal subjects. Television monitors were used as stimulators. The screens were surrounded by brightly lit white reflecting surfaces to ensure that the responses were developed by defined retinal areas.
2. Various types of stimuli were employed. Either (i) a pattern of dark and bright squares was reversed, to evoke a pattern e.r.g. (p.e.r.g.), (ii) the luminance of the uniform screen was abruptly increased and decreased to evoke a focal on—off e.r.g. or (iii) a pattern was made to appear and disappear from a uniform background. In each of these cases, the sequence of changes of luminance at any one point could be made identical. The aim of the experiments was to determine whether the e.r.g. was modified by the spatial organization of the stimulus.
3. In other experiments a colour monitor was used so that (i) a red—green flicker, (ii) red—green pattern reversal or (iii) the appearance of a red—green pattern from a yellow background could be used as a stimulus. The responses were caused by the changes in hue, since all the colours were equiluminant.
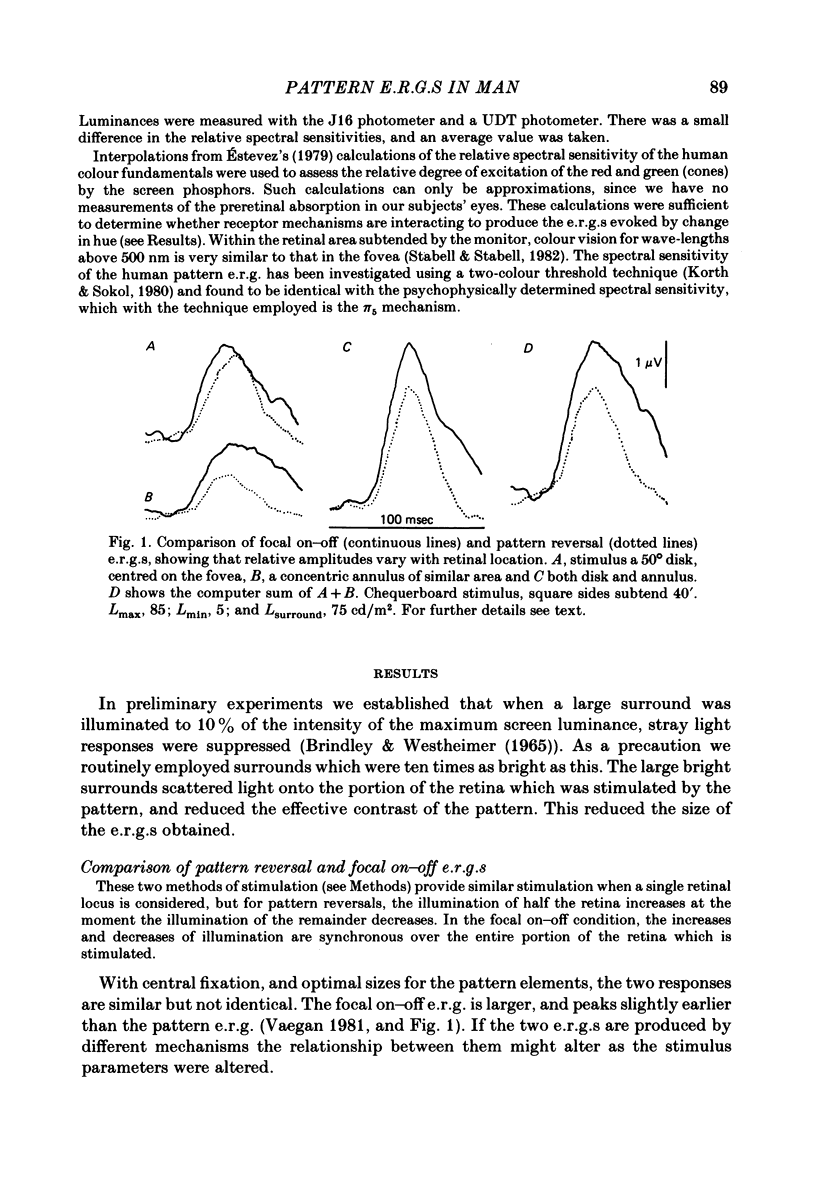
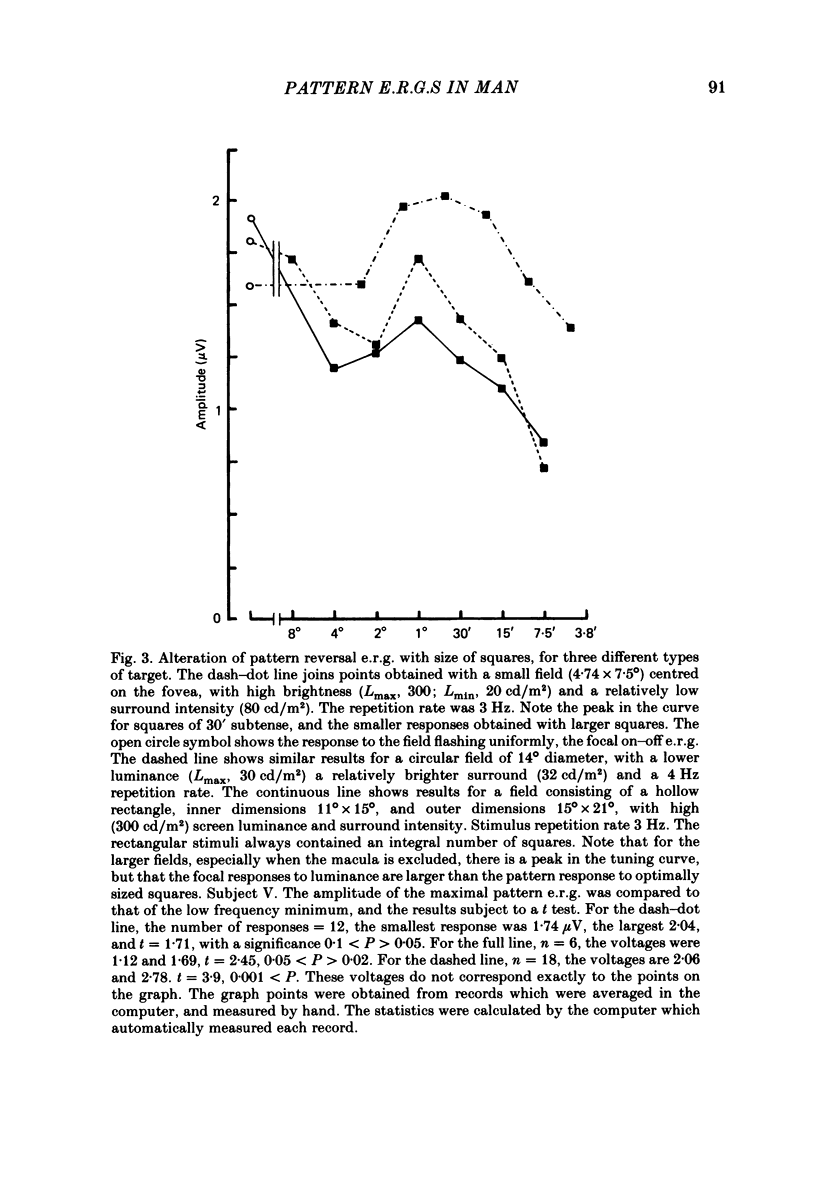
4. With black and white patterns the p.e.r.g. peaks 5 msec later than the focal on—off e.r.g. The largest response is produced by squares of 0.5-1° subtense.
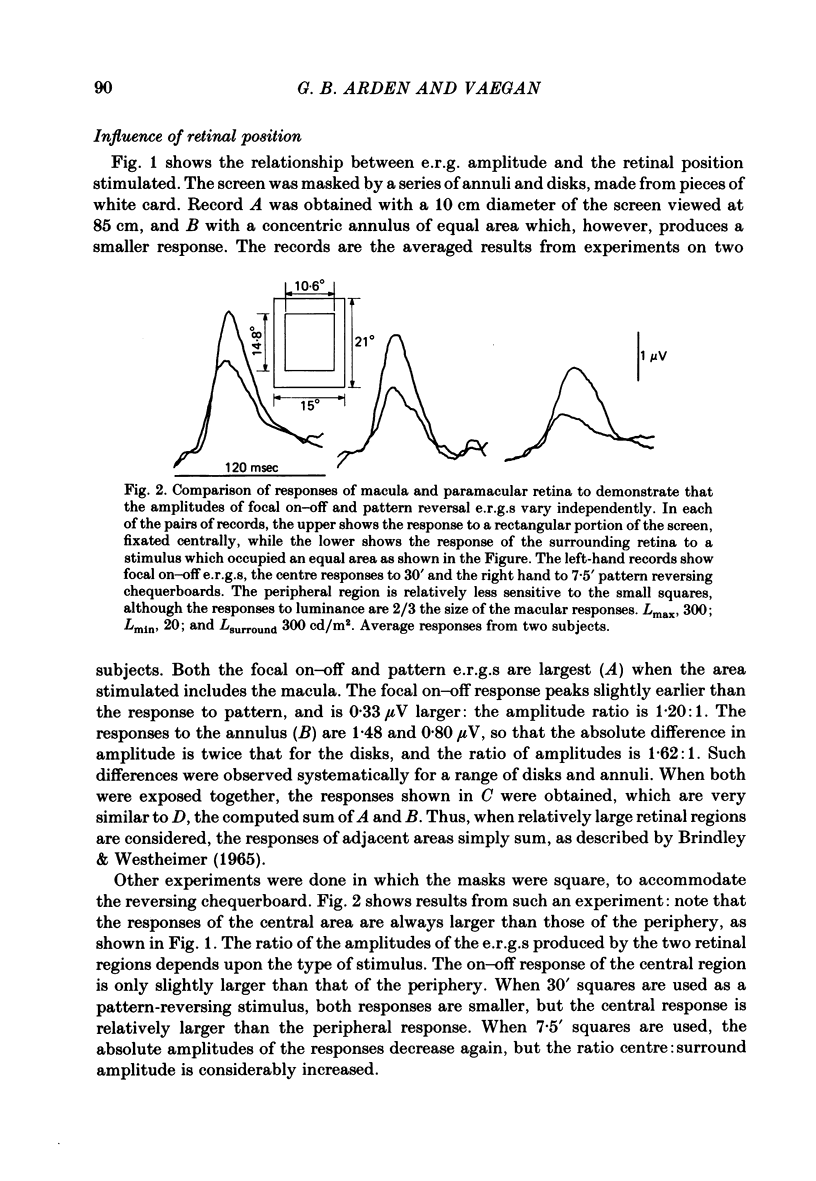
5. The ratio of the amplitudes of the p.e.r.g. to the focal on—off response is largest for stimuli confined to the macula and smallest for those projected onto peripheral retina.
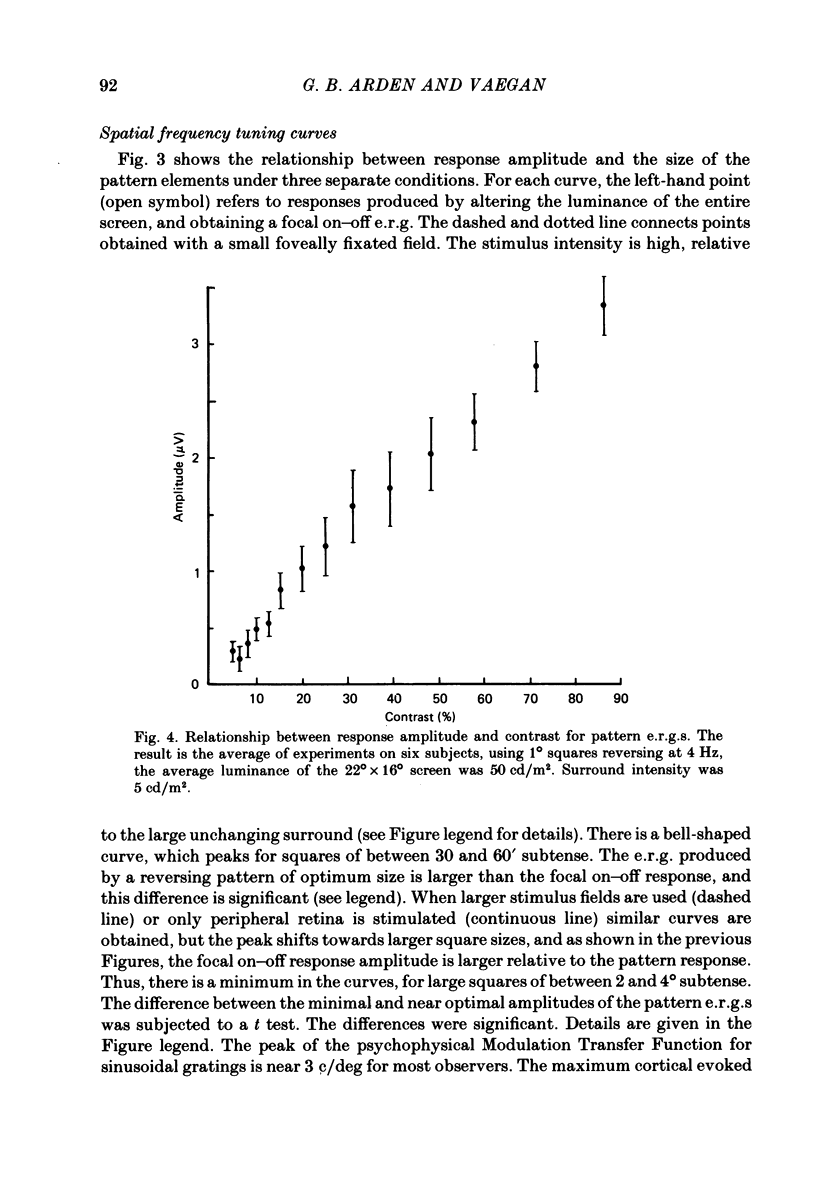
6. The amplitude of responses to chequerboard reversing patterns increases nearly linearly with contrast up to the maximum contrast available.
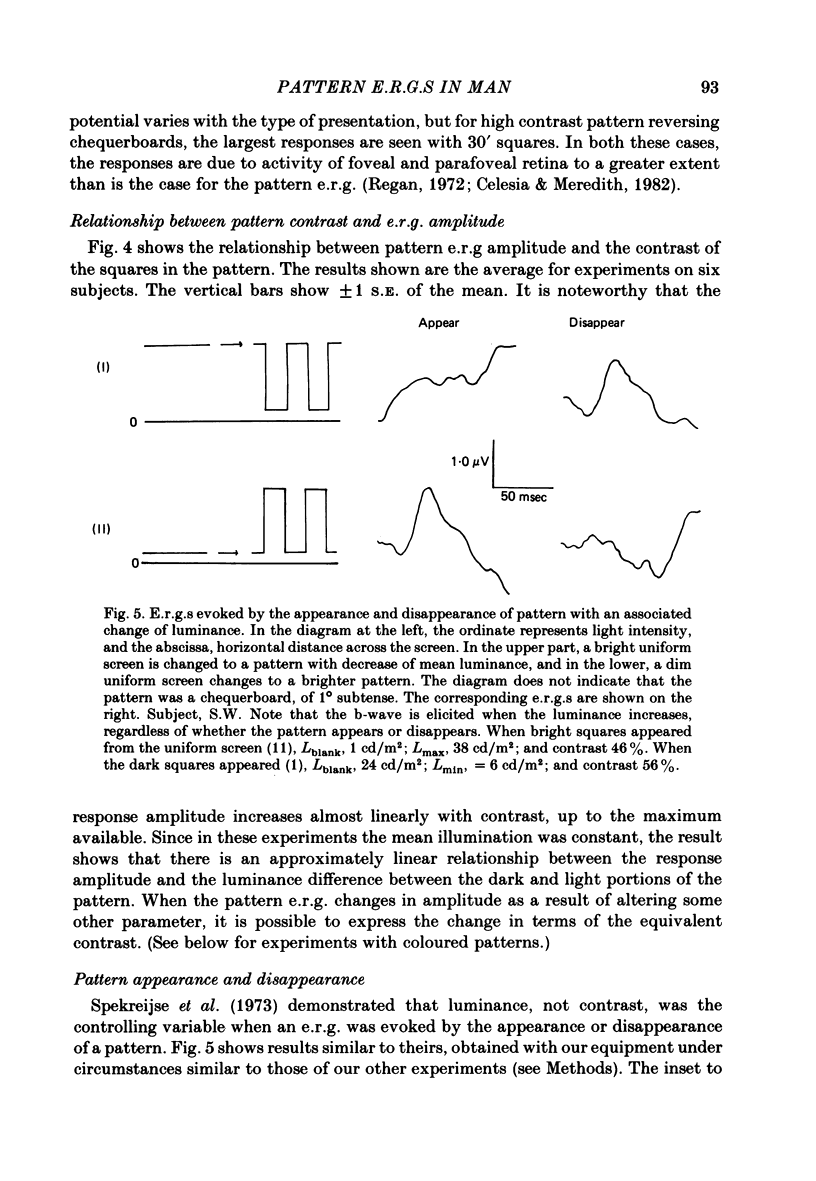
7. When patterns appear or disappear from a uniform screen, and there is an associated change in the quantity of light entering the eye, recognizable b-waves occur when the average screen luminance increases, independently of whether pattern contrast increases (appearance) or decreases (disappearance).
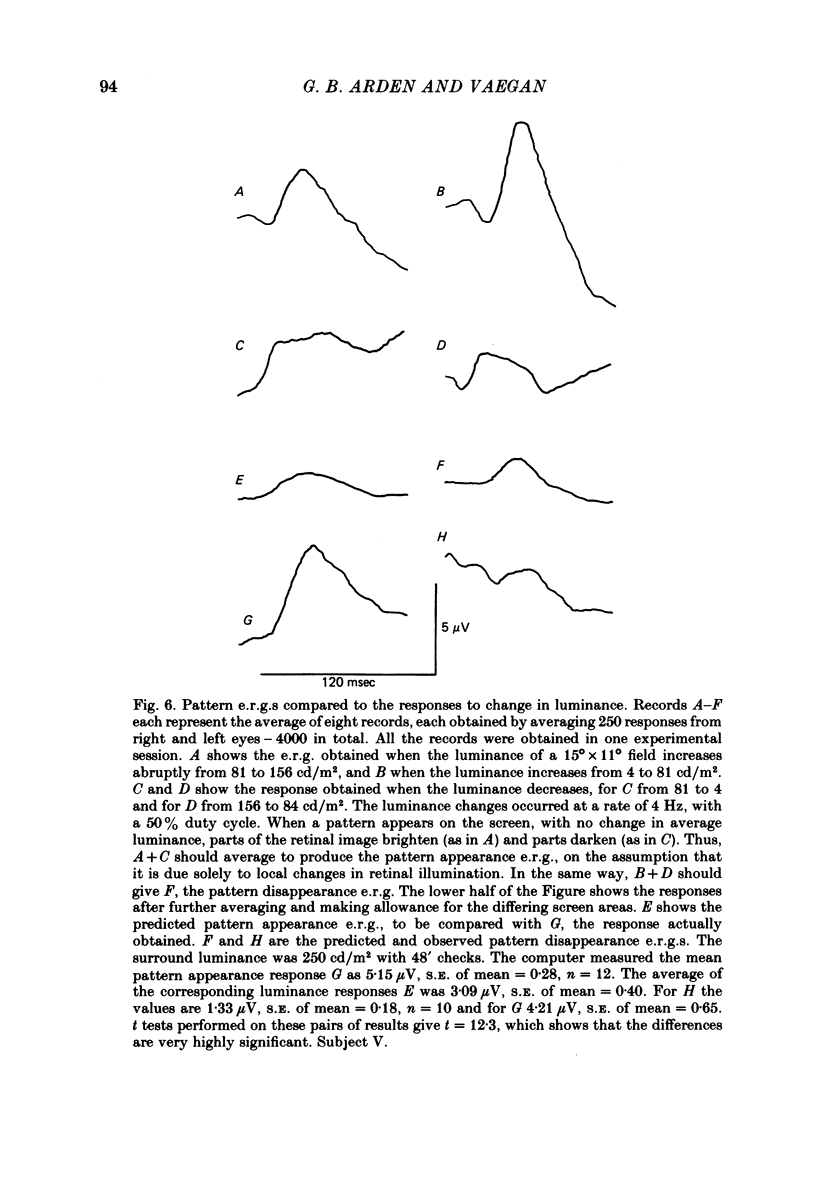
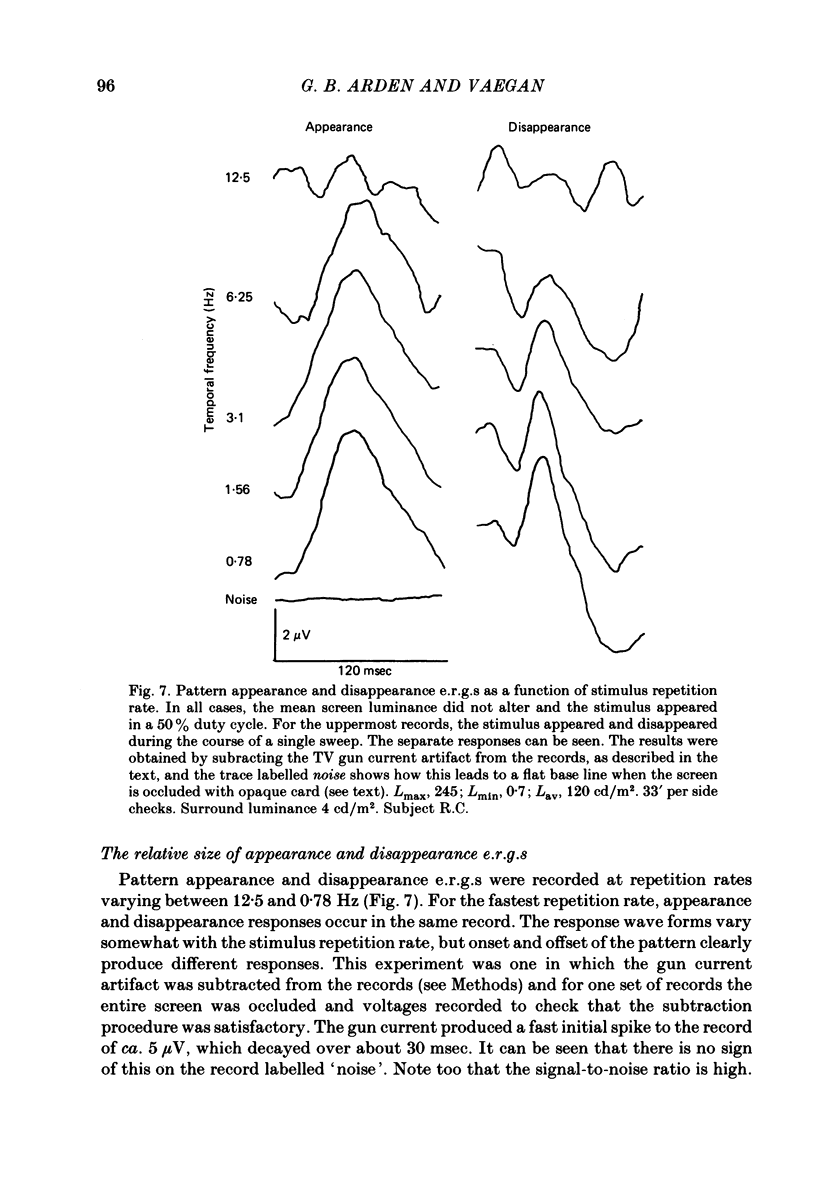
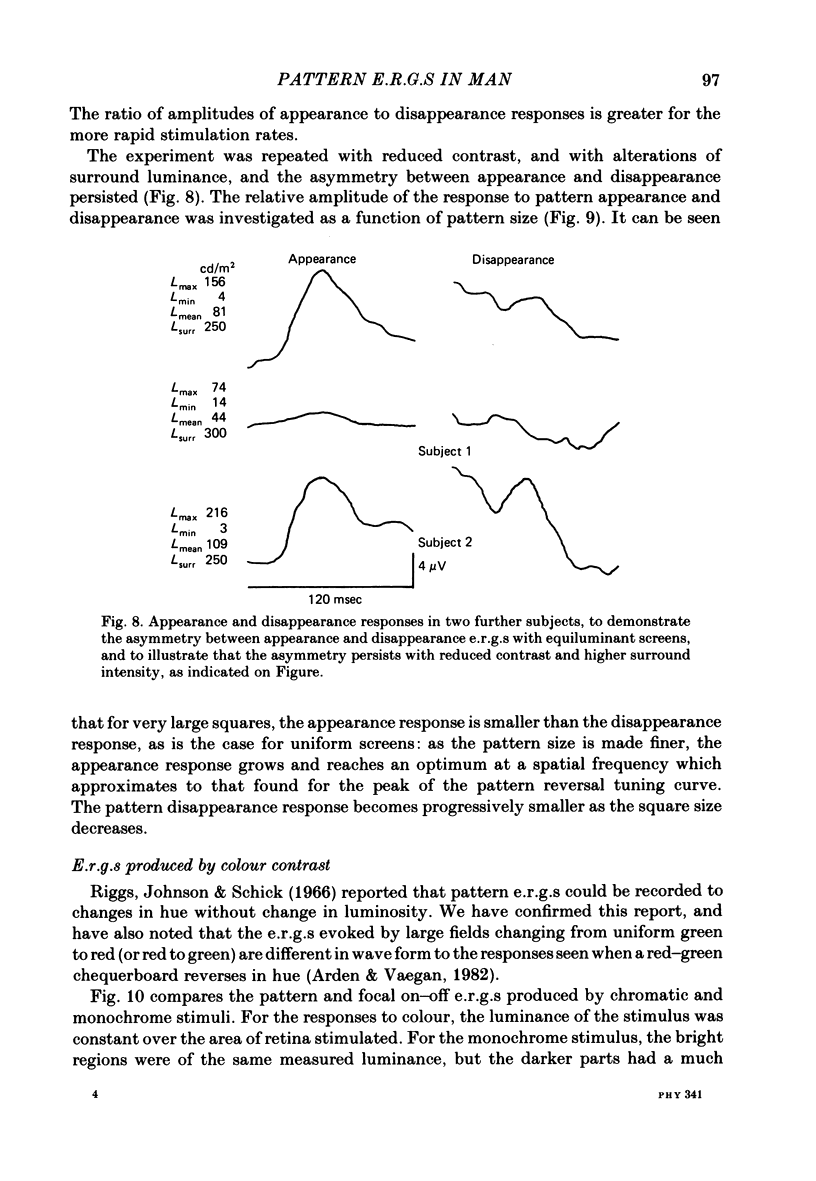
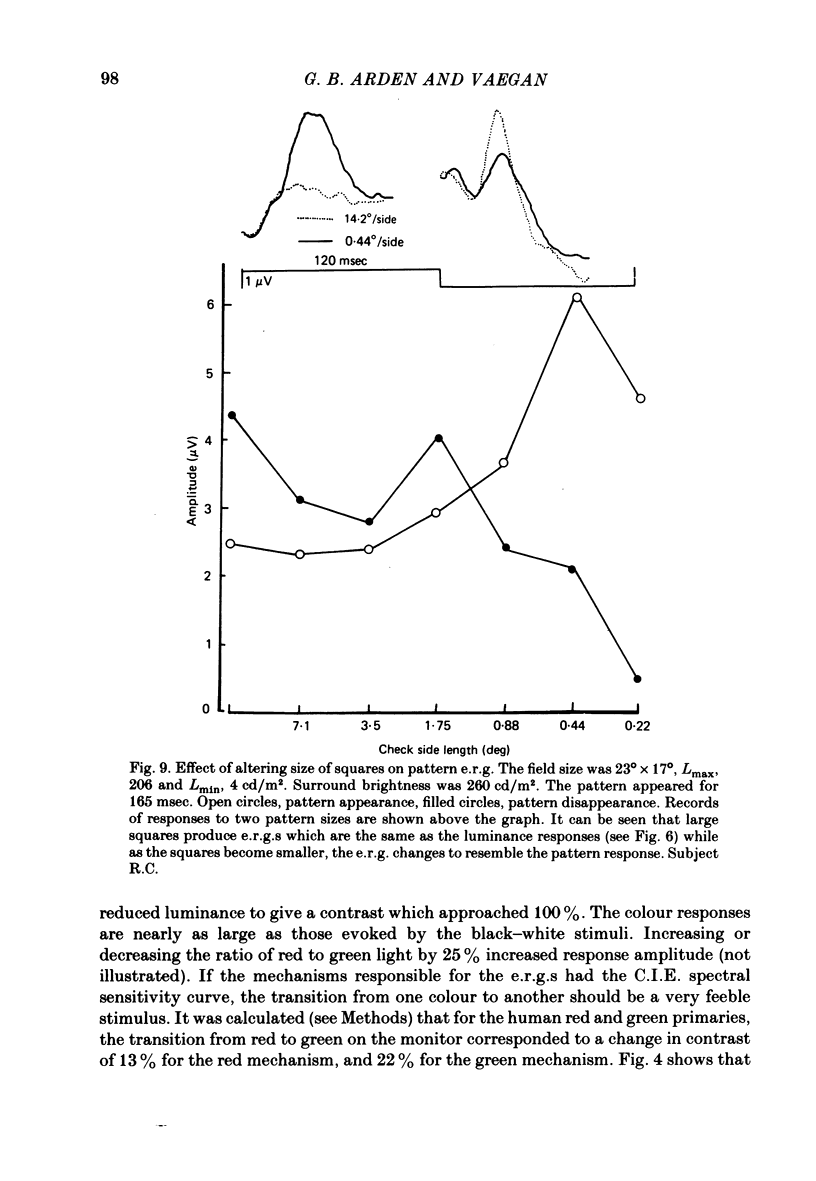
8. When a pattern appears or disappears with no change in luminance, e.r.g.s are evoked at both `on' and `off'. The disappearance of the dark parts of the pattern causes the largest logarithmic increase in local retinal illumination. For patterns of square size > 4° the pattern disappearance response is larger than for pattern appearance. As the square size is reduced, the appearance response grows and the disappearance response decreases. The e.r.g.s evoked by the appropriate changes in luminance of a uniform screen are no longer the same as those caused by the appearance and disappearance of the pattern.
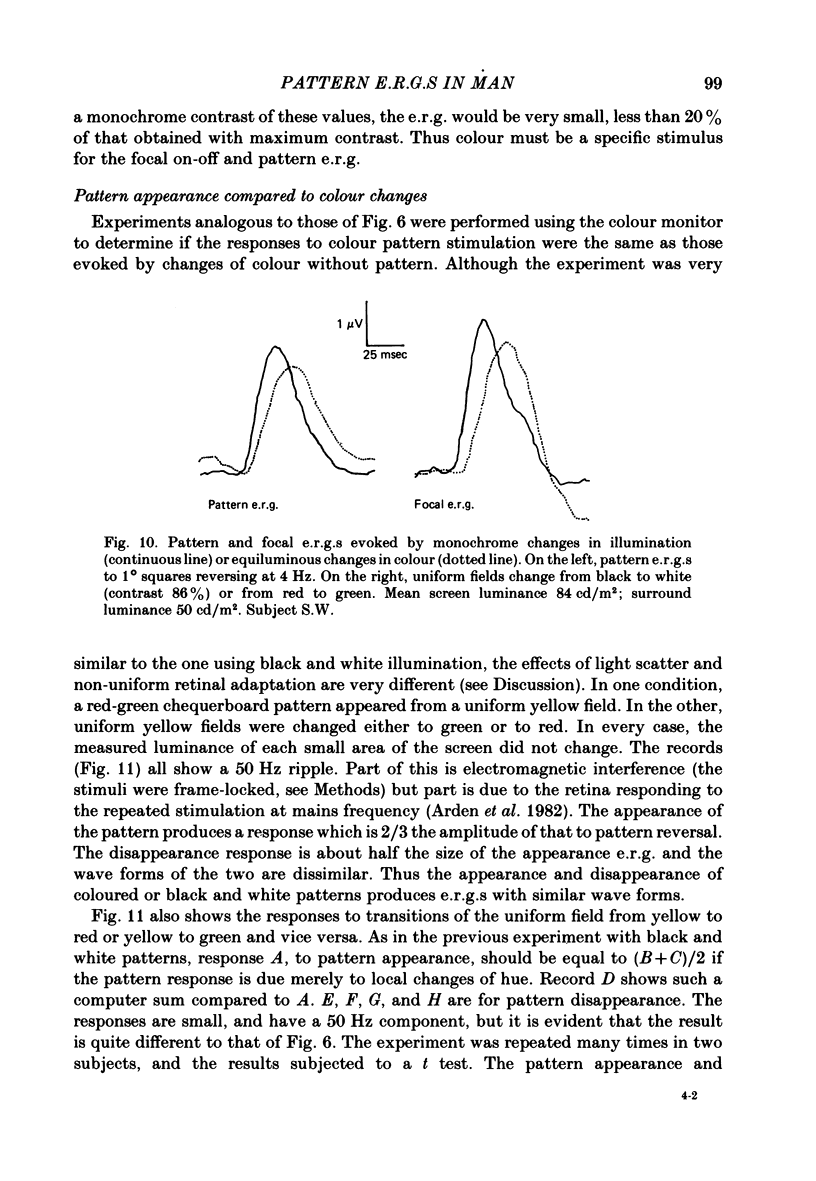
9. The responses to change of hue are 70% as large as those produced by black and white patterns. The same ratio occurs for pattern and focal on—off e.r.g.s.
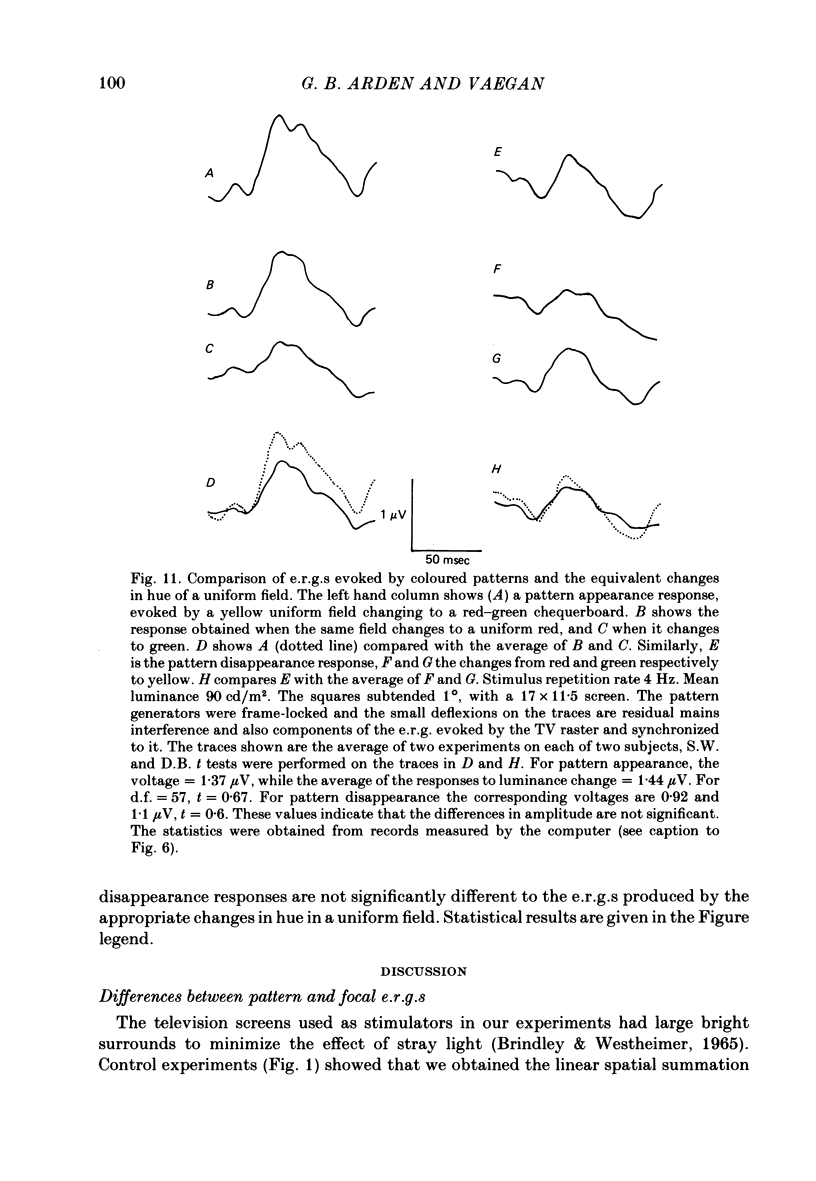
10. When coloured patterns appear from and disappear to a uniform field, the e.r.g.s. evoked are very similar to those recorded when the appropriate changes of hue occur in a uniform field. This result is quite different to the findings for black and white patterns (see 8 above).
11. The results suggest that it is the change in local adaptation caused by the black and white patterns which modifies the e.r.g. and not the presence of contrasting borders.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARDEN G. B., BROWN K. T. SOME PROPERTIES OF COMPONENTS OF THE CAT ELECTRORETINOGRAM REVEALED BY LOCAL RECORDING UNDER OIL. J Physiol. 1965 Feb;176:429–461. doi: 10.1113/jphysiol.1965.sp007560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arden G. B., Carter R. M., Hogg C., Siegel I. M., Margolis S. A gold foil electrode: extending the horizons for clinical electroretinography. Invest Ophthalmol Vis Sci. 1979 Apr;18(4):421–426. [PubMed] [Google Scholar]
- Arden G. B., Vaegan, Hogg C. R., Powell D. J., Carter R. M. Pattern ERGs are abnormal in many amblyopes. Trans Ophthalmol Soc U K. 1980;100(4):453–460. [PubMed] [Google Scholar]
- Armington J. C., Corwin T. R., Marsetta R. Simultaneously recorded retinal and cortical responses to patterned stimuli. J Opt Soc Am. 1971 Nov;61(11):1514–1521. doi: 10.1364/josa.61.001514. [DOI] [PubMed] [Google Scholar]
- Armington J. C. Simultaneous electroretinograms and evoked potentials. Ann N Y Acad Sci. 1982;388:572–579. doi: 10.1111/j.1749-6632.1982.tb50817.x. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindley G. S., Westheimer G. The spatial properties of the human electroretinogram. J Physiol. 1965 Aug;179(3):518–537. doi: 10.1113/jphysiol.1965.sp007678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celesia G. G., Meredith J. T. Visual evoked responses and retinal eccentricity. Ann N Y Acad Sci. 1982;388:648–650. doi: 10.1111/j.1749-6632.1982.tb50829.x. [DOI] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P. Functional properties of ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):167–195. doi: 10.1113/jphysiol.1975.sp011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monasterio F. M., Gouras P., Tolhurst D. J. Trichromatic colour opponency in ganglion cells of the rhesus monkey retina. J Physiol. 1975 Sep;251(1):197–216. doi: 10.1113/jphysiol.1975.sp011087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner D. J. A new television visual stimulator for contrast sensitivity and evoked response testing [proceedings]. J Physiol. 1978 Feb;275:7P–8P. [PubMed] [Google Scholar]
- Fiorentini A., Maffei L., Pirchio M., Spinelli D., Porciatti V. The ERG in response to alternating gratings in patients with diseases of the peripheral visual pathway. Invest Ophthalmol Vis Sci. 1981 Sep;21(3):490–493. [PubMed] [Google Scholar]
- Gouras P., Zrenner E. Enchancement of luminance flicker by color-opponent mechanisms. Science. 1979 Aug 10;205(4406):587–589. doi: 10.1126/science.109925. [DOI] [PubMed] [Google Scholar]
- Kaneko A. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J Physiol. 1970 May;207(3):623–633. doi: 10.1113/jphysiol.1970.sp009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth M. Human fast retinal potentials and the spatial properties of a visual stimulus. Vision Res. 1981;21(5):627–630. doi: 10.1016/0042-6989(81)90070-5. [DOI] [PubMed] [Google Scholar]
- Korth M., Sokol S. Electroretinographic and pyschophysical measures of cone spectral mechanisms using the two-color threshold technique. Vision Res. 1980;20(3):205–212. doi: 10.1016/0042-6989(80)90104-2. [DOI] [PubMed] [Google Scholar]
- Mafei L., Fiorentini A. Electroretinographic responses to alternating gratings before and after section of the optic nerve. Science. 1981 Feb 27;211(4485):953–955. doi: 10.1126/science.7466369. [DOI] [PubMed] [Google Scholar]
- May J. G., Ralston J. V., Reed J. L., Van Dyk H. J. Loss in pattern-elicited electroretinograms in optic nerve dysfunction. Am J Ophthalmol. 1982 Apr;93(4):418–422. doi: 10.1016/0002-9394(82)90131-3. [DOI] [PubMed] [Google Scholar]
- Stabell U., Stabell B. Color vision in the peripheral retina under photopic conditions. Vision Res. 1982;22(7):839–844. doi: 10.1016/0042-6989(82)90017-7. [DOI] [PubMed] [Google Scholar]


