Abstract
The limiting membrane of the lysosome contains a group of transmembrane glycoproteins named lysosome-associated membrane proteins (Lamps). These proteins are targeted to lysosomes by virtue of tyrosine-based sorting signals in their cytosolic tails. Four adaptor protein (AP) complexes, AP-1, AP-2, AP-3, and AP-4, interact with such signals and are therefore candidates for mediating sorting of the Lamps to lysosomes. However, the role of these complexes and of the coat protein, clathrin, in sorting of the Lamps in vivo has either not been addressed or remains controversial. We have used RNA interference to show that AP-2 and clathrin—and to a lesser extent the other AP complexes—are required for efficient delivery of the Lamps to lysosomes. Because AP-2 is exclusively associated with plasma membrane clathrin coats, our observations imply that a significant population of Lamps traffic via the plasma membrane en route to lysosomes.
INTRODUCTION
The limiting membrane of the lysosome is enriched in a group of highly glycosylated transmembrane proteins named lysosome-associated membrane proteins (Lamps; also known as LIMPs or lgps) (Kornfeld and Mellman, 1989; Hunziker and Geuze, 1996; Eskelinen et al., 2003). Among the most abundant proteins in this group are two type I transmembrane proteins, Lamp-1 and Lamp-2, and the tetraspanin Lamp-3 (most commonly known as CD63). These three proteins have short (10–11 amino acid residues) C-terminal cytosolic tails that end with a GYXXØ motif (G is glycine, Y is tyrosine, and Ø is a bulky hydrophobic amino acid). This motif corresponds to a subset of YXXØ-type, tyrosine-based sorting signals that mediate various sorting events in post-Golgi compartments (reviewed by Bonifacino and Traub, 2003). The G, Y, and Ø residues (Williams and Fukuda, 1990; Harter and Mellman, 1992; Höning and Hunziker, 1995; Höning et al., 1996; Hirst et al., 1999; Rous et al., 2002), as well as the exact placement of the motif relative to the transmembrane domain (Rohrer et al., 1996), are critical for efficient biosynthetic targeting of the Lamps to lysosomes; changes in any of these elements result in increased expression of the Lamps at the cell surface. Like other YXXØ-type signals, the lysosomal targeting motifs interact with the μ subunits (i.e., μ1, μ2, μ3, and μ4) of four heterotetrameric adaptor protein (AP) complexes (i.e., AP-1, AP-2, AP-3, and AP-4, respectively) (Ohno et al., 1995, 1996; Boll et al., 1996; Gough et al., 1999; Aguilar et al., 2001; Rous et al., 2002). These complexes are components of protein coats that participate in cargo selection and vesicle formation in endocytic and late secretory pathways (Kirchhausen, 1999; Bonifacino and Traub, 2003). AP-1, AP-2, and to some extent AP-3 are part of coats that contain the scaffolding protein clathrin, whereas AP-4 is part of a nonclathrin coat. The interaction of GYXXØ motifs with one or more of these AP complexes is thought to lead to incorporation of the Lamps into coated vesicles that mediate selective protein transport to lysosomes.
Two different pathways, referred to as “direct” and “indirect,” have been proposed to participate in the biosynthetic transport of Lamps to lysosomes (Kornfeld and Mellman, 1989; Hunziker and Geuze, 1996; Eskelinen et al., 2003). The direct pathway is a completely intracellular route that involves transport of newly synthesized Lamps from the trans-Golgi network (TGN) to either early or late endosomes and then to lysosomes (Green et al., 1987; Carlsson and Fukuda, 1992; Harter and Mellman, 1992; Höning and Hunziker, 1995). In the indirect pathway, in contrast, Lamps are first transported from the TGN to the plasma membrane, after which they are internalized and sequentially delivered to early endosomes, late endosomes, and lysosomes (Lippincott-Schwartz and Fambrough, 1986; Furuno et al., 1989a,b; Nabi et al., 1991; Mathews et al., 1992; Gough et al., 1999). There is consensus that both pathways contribute to the delivery of the Lamps to lysosomes and that the fraction of the Lamps expressed at the cell surface at steady state is low (e.g., 0–3% of the total Lamp-1; Lippincott-Schwartz and Fambrough, 1986; Lippincott-Schwartz and Fambrough, 1987; Harter and Mellman, 1992). However, estimates of the fraction of newly synthesized Lamps that traffic via the plasma membrane en route to lysosomes vary widely from 4 to 70% of the total (Nabi et al., 1991; Höning and Hunziker, 1995). Thus, the exact contribution of endocytic trafficking to the overall delivery of Lamps to lysosomes remains uncertain.
Equally uncertain are the physiological roles of the different AP complexes in the sorting of the Lamps to lysosomes. Although all four AP complexes bind GYXXØ signals in biochemical assays, to date it is not known which of these interactions are required for sorting of the Lamps in vivo. AP-1 is associated with the TGN and endosomes and was first proposed to mediate transport of the Lamps directly from the TGN to endosomes (Höning et al., 1996). However, embryonic fibroblasts from mice deficient in the μ1A subunit isoform of AP-1 exhibit normal localization of Lamps to lysosomes (Meyer et al., 2000). AP-2 is exclusively associated with the plasma membrane under normal conditions (Robinson, 1987) and thus could in principle only participate in the indirect pathway. Its role in the lysosomal targeting of the Lamps in cells, however, has not yet been assessed. AP-3 is mainly associated with an early endosomal tubular network (Dell'Angelica et al., 1998; Peden et al., 2004), and cells from humans and mice deficient in AP-3 subunits display increased trafficking of Lamps via the plasma membrane (Le Borgne et al., 1998; Dell'Angelica et al., 1999b, 2000; Peden et al., 2002, 2004; Rous et al., 2002). This has led to the proposal that AP-3 functions to sort Lamps from early to late endosomes in either the direct or indirect pathways (Dell'Angelica et al., 1999b; Peden et al., 2004). The steady-state localization of the Lamps to lysosomes, however, is only slightly altered or unaltered in AP-3-deficient cells (Le Borgne et al., 1998; Dell'Angelica et al., 1999b, 2000; Reusch et al., 2002). Moreover, fibroblasts deficient in both AP-1 and AP-3 also exhibit largely normal distribution of Lamps to lysosomes, confirming that the roles of these two complexes in lysosomal targeting are nonessential (Reusch et al., 2002). Finally, AP-4 is associated mainly with the TGN (Dell'Angelica et al., 1999a; Hirst et al., 1999; Simmen et al., 2002), but antisense-RNA-mediated depletion of AP-4 showed no effect on the lysosomal transport of Lamp-2 (Simmen et al., 2002). Adding to the puzzle, ablation of the gene encoding the clathrin heavy chain (CHC) in chicken DT40 cells was reported not to affect the cellular distribution of LEP100, the chicken ortholog of mammalian Lamp-1 (Wettey et al., 2002). Thus, even the long-assumed role of clathrin in Lamp sorting (Kornfeld and Mellman, 1989; Hunziker and Geuze, 1996; Eskelinen et al., 2003) has been questioned.
To address these issues, we have taken advantage of the ability to suppress expression of specific AP complexes or clathrin using small-interfering RNAs (siRNA) in HeLa cells. This approach allows comparison of the trafficking of the Lamps in cells deficient in the expression of one or more of these coat proteins in the same genetic background. The results of our study show that elimination of clathrin or AP-2, and to a lesser extent the other AP complexes, causes substantial accumulation of the Lamps at the plasma membrane and their decreased transport to lysosomes. These observations demonstrate a significant role for the endocytic machinery in the biosynthetic delivery of the Lamps to lysosomes.
MATERIALS AND METHODS
Antibodies
The following mouse monoclonal antibodies were used: H4A3 to Lamp-1, H4B4 to Lamp-2, H5C6 to CD63, and SA4 to Δ-adaptin (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA); CV-24 to LEP100 (gift of D. Fambrough, Johns Hopkins University, Baltimore, MD); DF 1513 to the transferrin receptor (TfR) (CD71), 100/3 to γ1-adaptin (Sigma-Aldrich, St. Louis, MO); AP-6 to α-adaptin (Affinity Bioreagents, Golden, CO); X22 (American Type Culture Collection, Manassas, VA) and clone 23 (BD Biosciences, San Diego, CA) to the CHC; W6/32 to human major histocompatibility complex (MHC-I) and 7G7.B6 to human Tac (American Type Culture Collection) and HA.11 to the hemagglutinin (HA) tag (Covance, Princeton, NJ). The following polyclonal antibodies were used: rabbit RY/1 to μ1 (gift of L. Traub, University of Pittsburgh, Pittsburgh, PA); rabbit anti-μ2 (Aguilar et al., 1997); rabbit anti-μ3 (Dell'Angelica et al., 1997); rabbit anti-β4 (Dell'Angelica et al., 1999); rabbit TB4 anti-human Tac (Aguilar et al., 2001); sheep anti-TGN46 (Serotec, Raleigh, NC); donkey Alexa 596-conjugated anti-mouse and anti-rabbit IgG (Molecular Probes, Eugene, OR); donkey Cy3-conjugated anti-sheep; phycoerythrin (PE)-conjugated anti-mouse IgG (Jackson ImmunoResearch Laboratories, West Grove, PA); and horseradish peroxidase-conjugated anti-mouse and anti-rabbit IgG (Amersham Biosciences, Piscataway, NJ).
Recombinant DNA Constructs
The HA-tagged CD63 construct was obtained by PCR amplification of CD63 cDNA and cloned in frame in the EcoRI-SalI sites of the pCI-HA3 vector described by Martina et al., 2003. LEP100 cDNA in pCB6 vector was a gift of D. Fambrough (Johns Hopkins University, Baltimore, MD). The green fluorescent protein (GFP)-tagged histone H2B was generated as described previously (Dey et al., 2000). The GFP-tagged Dynamin2 wild-type (WT) and K44A constructs were gifts from M. McNiven (Mayo Clinic, Rochester, MN).
Cell Culture and Transfections
HeLa cells (American Type Culture Collection) were grown in DMEM supplemented with 10% (vol/vol) fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Transfections were carried out using the Lipofectamine reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The cells were analyzed 20 h after transfection.
RNA-mediated Interference (RNAi)
RNAi of CHC and the μ subunits of the AP complexes was performed using siRNAs (QIAGEN, Valencia, CA) to the following human target sequences: GGCAUCAAGUAUCGGAAGA for μ1A, GUGGAUGCCUUUCGGGUCA for μ2, GGAGAACAGUUCUUGCGGC for μ3A, GUCUCGUUUCACAGCUCUG for μ4, GAGCAUGUGCACGCUGGCCA for α-adaptin, and UCCAAUUCGAAGACCAAUU for CHC. A nonfunctional siRNA for human Vps35p to the target sequence GGUCCAGUCAUUCCAAAUG was used as a control. Cells were transfected twice at 72-h intervals with the siRNAs using Oligofectamine (Invitrogen) according to the manufacturer's protocol. The cells were analyzed 48–72 h after the second round of transfection. Trypan blue exclusion assays showed the following percentages of viable cells for each RNAi treatment at the time of the experiments: mock, 90; μ1A, 87; μ2, 86; μ3A, 84; and μ4, 66.
RNA Purification and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA isolation was performed using the TRIzol reagent (Invitrogen). RNA concentration was quantified by spectrophotometry. The expression level of μ4 mRNA in mock- and μ4-siRNA-treated cells was determined by RT-PCR using appropriate primers and the SuperScript One Step RT-PCR with Platinum Taq kit (Invitrogen). PCR products were resolved on a 1% (wt/vol) agarose gel containing 5 μg/ml ethidium bromide.
Flow Cytofluorometry
For quantification of cell surface levels of TfR, MHC-I, Lamp-1, Lamp-2 and CD63, 106 transfected cells were harvested, washed in ice-cold 1% bovine serum albumin (BSA)-phosphate-buffered saline (PBS), and then labeled for 1 h at 4°C with the indicated antibody. After three washes in ice-cold BSA-phosphate-buffered saline, the cell surface-associated antibodies were revealed by incubation for 1 h at 4°C with PE-conjugated anti-IgG. The cells were washed three times, fixed in 1% formaldehyde in BSA-phosphate-buffered saline, and flow cytofluorometry was performed on a three-color FACSCalibur flow cytometer equipped with CellQuest prosoftware (BD Biosciences, San Jose, CA). Measurements of forward scatter were used to exclude dead cells and debris.
Antibody Uptake and Endocytosis Assays
Antibody uptake essays were carried out by incubation for 1 h at 37°C of HeLa cells grown on coverslips, in the presence of the indicated antibody diluted in DMEM, 1% BSA, and 25 mM HEPES, pH 7.4. The cells were washed three times in ice-cold PBS, fixed in 4% paraformaldehyde, and incubated for 1 h with Alexa 594-conjugated anti-mouse IgG diluted in 0.1% BSA, 0.1% saponin, PBS. The cells were then washed and mounted on glass slides using Fluoromount G. The rate of endocytosis of TfR and CD63 in mock- and siRNA-treated cells was analyzed using a fluorescence-activated cell sorter (FACS) assay (Schwartz et al., 1996). The percentage of endocytosis was calculated as follows: [(mo - mx)/mo] 100, where mo is the mean fluorescence at time 0 and mx the mean fluorescence at each time point.
Immunofluorescence Microscopy
Indirect immunofluorescence staining of fixed, permeabilized cells and surface staining of intact cells were performed as described previously (Dell'Angelica et al., 1997, 1999c). To analyze targeting to lysosomes of newly synthesized Lamps, mock- and μ2 siRNA-treated cells grown on coverslips were transfected with cDNAs encoding LEP100 or HA-CD63 for 6 h and then incubated with 2 μg/ml brefeldin A (BFA) (Sigma-Aldrich) for 12 h to accumulate the newly synthesized proteins in the endoplasmic reticulum (ER). The cells were then washed and incubated for different times at 37°C in regular culture medium. After fixation and permeabilization, the cells were stained using antibodies to LEP100 or HA. Images were acquired on a Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY) and processed with Adobe Photoshop.
Metabolic Labeling and Percoll Gradient Fractionation
Metabolic labeling of cells was carried out as described (Dell'Angelica et al., 1997). Herein, cells were pulse-labeled for 30 min using 0.1 mCi/ml [35S]methionine-cysteine (Express Protein Label; PerkinElmer Life and Analytical Sciences, Boston, MA) and chased for 6 h in regular culture medium. For Percoll gradient fractionation, confluent cells grown on a 150-cm2 plate were harvested, washed twice with ice-cold homogenization buffer (HB) (10 mM Tris-HCl, pH 7.4, 0.28 M sucrose, 2 mM EDTA, 10 μg/ml leupeptin, and 0.1 mM phenylmethylsulfonyl fluoride), homogenized in 850 μl of HB by 25 passages through a 25-gauge needle attached to a 1-ml syringe and then fractionated on an 18% Percoll density gradient as described previously (Barriocanal et al., 1986). Seven 1.4-ml fractions were collected from the top. Fractions were diluted in an equal volume of 10 mM Tris-HCl, pH 7.4, 2% Triton X-100, 200 mM NaCl, 2 mM EDTA, and protease inhibitors (Roche, Nutley, NJ), incubated on ice for 30 min, and centrifuged for 2 h at 20,000 × g to remove the Percoll and insoluble materials. The cleared supernatant was used for further analyses. Immunoprecipitation was performed overnight at 4°C as described previously (Bonifacino and Dell'Angelica, 1998). Immunoprecipitates were analyzed by SDS-PAGE and autoradiography. Quantification was performed on a Typhoon 9200 PhosphorImager (Amersham Biosciences) using ImageQuant analysis software. β-Hexosaminidase activity was measured on 50 μl of each fraction by incubation with 10 mM 4-methylumbelliferyl-2-acetamido-2-deoxy-β-d-glucopyranoside (Sigma-Aldrich) as a substrate (Arighi et al., 2004).
RESULTS
Expression of a Dynamin Dominant-Negative Mutant Increases the Levels of Lamps at the Cell Surface
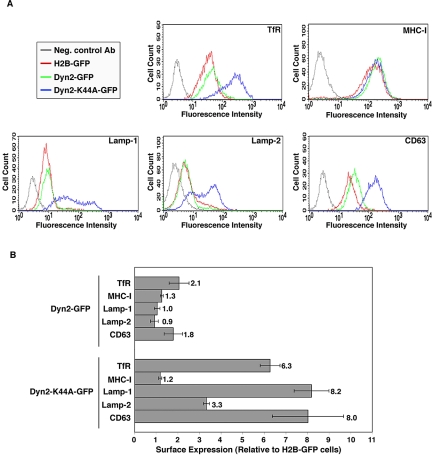
As a first step to assess the role of the endocytic machinery in the trafficking of the Lamps, we examined the effect of overexpressing a dominant-negative mutant of dynamin 2 (Dyn2) on the surface levels of Lamps. Dyn2 is a ubiquitously expressed GTPase that is required for the clathrin-dependent internalization of endocytic receptors (Damke et al., 1994; Altschuler et al., 1998). It is controversial, however, whether Dyn2 also is involved in other steps, such as the transport of proteins from the TGN or endosomes to the plasma membrane (Damke et al., 1994; Altschuler et al., 1998; Jones et al., 1998; Kasai et al., 1999; van Dam and Stoorvogel, 2002). We transfected HeLa cells with expression plasmids encoding GFP-tagged histone H2B (H2B-GFP; negative control), GFP-tagged Dyn2 (Dyn2-GFP), or a dominant-negative mutant Dyn2 tagged with GFP (Dyn2-K44A-GFP) and then measured the surface expression of the TfR, class I molecules of the MHC-I (these two proteins were used as controls), Lamp-1, Lamp-2, and CD63 by immunofluorescent staining and FACS analysis. We observed that expression of Dyn2-GFP had little or no effect on the surface levels of all the proteins examined (Figure 1, A and B). We also observed that expression of Dyn2-K44A-GFP increased the surface levels of the TfR but not those of MHC-I (Figure 1, A and B). This was consistent with the known involvement of clathrin in endocytosis of the TfR (Wettey et al., 2002; Hinrichsen et al., 2003; Motley et al., 2003; Huang et al., 2004) but not MHC-I (Naslavsky et al., 2003). Importantly, we observed that expression of Dyn2-K44A-GFP caused a three- to eight-fold increase in the levels of Lamp-1, Lamp-2, and CD63 at the cell surface (Figure 1, A and B). The fact that the surface levels of all of these proteins did not decrease but either remained unchanged (i.e., MHC-I) or increased (i.e., TfR, Lamp-1, Lamp-2 and CD63), made it unlikely that Dyn2-K44A-GFP expression caused a significant block in transport to the plasma membrane from either the TGN or endosomes in this system. Rather, the increased surface expression of TfR in the Dyn2-K44A-GFP-transfected cells indicated that inhibition of endocytosis was the prevalent phenotype elicited by this mutant protein in HeLa cells. These results thus suggested that significant fractions of the Lamps traffic via the cell surface and are internalized by a dynamin-dependent process.
Figure 1.
Overexpression of a Dynamin 2 dominant-negative mutant increases cell surface levels of Lamps. HeLa cells were transfected with expression vectors encoding GFP-tagged forms of histone 2B (H2B-GFP), Dynamin 2 (Dyn2-GFP), or Dynamin 2 K44A mutant (Dyn2-K44A-GFP). Twenty-two hours later, the cells were immunolabeled for TfR, MHC-I, Lamp-1, Lamp-2 and CD63, and analyzed by FACS. (A) FACS profiles of the cell surface expression of TfR, MHC-I, Lamp-1, Lamp-2, and CD63 in cells expressing Dyn2-GFP (green), Dyn2-K44A-GFP (blue), or H2B-GFP (red). The gray curve shows the fluorescence distribution of H2B-GFP-expressing cells stained with secondary antibody in the absence of primary antibody. (B) The cell surface levels of TfR, MHC-I, and Lamps in cells overexpressing Dyn2-GFP or Dyn2-K44A-GFP were normalized relative to those in the control cells expressing H2B-GFP. Values represent the mean ± SD from four independent experiments.
siRNA-mediated Depletion of Clathrin or AP-2 Increases Surface Levels of Lamps
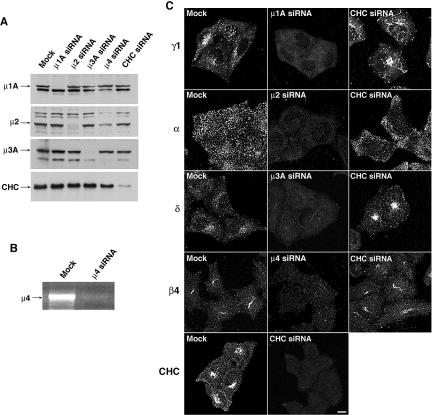
Because of the caveats concerning the use of dynamin dominant-negative mutants, we sought more specific means of interfering with the protein sorting machinery. To this end, we reduced expression of CHC, μ1A, μ2, μ3A, or μ4 in HeLa cells using siRNA oligonucleotides (μ1A and μ3A are ubiquitously expressed isoforms of these AP subunits). Immunoblot analysis revealed an 80–85% reduction in the levels of CHC and 90–95% reduction in the levels of μ1A, μ2, and μ3A in different experiments (Figure 2A). We could not test the effectiveness of the μ4 siRNA by immunoblotting because of the unavailability of suitable antibodies. However, RT-PCR analysis showed that the μ4 siRNA treatment resulted in >95% reduction in the level of μ4 mRNA (Figure 2B). For simplicity, we refer to these reductions as “depletion” of the subunits or complexes, with the understanding that they represent substantial although incomplete elimination of the target proteins. Immunofluorescence microscopy of mock- and siRNA-treated cells showed that depletion of the corresponding μ subunits caused almost complete disappearance of the staining for the γ1, α, δ, and β4 subunits of AP-1, AP-2, AP-3, and AP-4, respectively (Figure 2C) (γ1 is a ubiquitously expressed isoform of the γ subunit of AP-1). This could be due to degradation of the remaining AP subunits or to their failure to associate with the corresponding target membranes (Dell'Angelica et al., 1999b; Zhen et al., 1999; Hinrichsen et al., 2003; Motley et al., 2003). Depletion of clathrin did not change the intensity of staining for the different AP complexes, but it caused tubulation of the AP-1-containing compartment and juxtanuclear clustering of the AP-3-containing compartment (Figure 2C).
Figure 2.
Depletion of AP complexes and clathrin by RNAi. (A) HeLa cells were transfected twice with siRNAs directed to the μ1A, μ2, μ3A, or μ4 subunits of the corresponding AP complexes, or to CHC. Forty-eight hours after the second round of transfection, equivalent amounts of homogenates of mock- and siRNA-treated cells were subjected to SDS-PAGE and immunoblotting using antibodies to μ1A, μ2, or μ3A, or CHC (the amount of homogenate from μ4-siRNA-treated cells loaded on the gel was lower than that from other cells, thus the lower signal in all the lanes). (B) Total RNA was extracted from mock- and μ4-siRNA-treated cells, and RT-PCR was performed using specific primers for μ4 mRNA. The amplified fragments were resolved by agarose gel electrophoresis and visualized by ethidium bromide staining. (C) The effects of μ chain or CHC depletion on the expression and distribution of AP complexes and clathrin in HeLa cells were assessed by immunofluorescence microscopy. Mock- and siRNA-treated cells grown on coverslips were fixed, permeabilized, and stained with antibodies to the γ1 subunit of AP-1 (first row), the α-subunit of AP-2 (second row), the δ subunit of AP-3 (third row), the β4 subunit of AP-4 (fourth row), and CHC (fifth row). Bar, 5 μm.
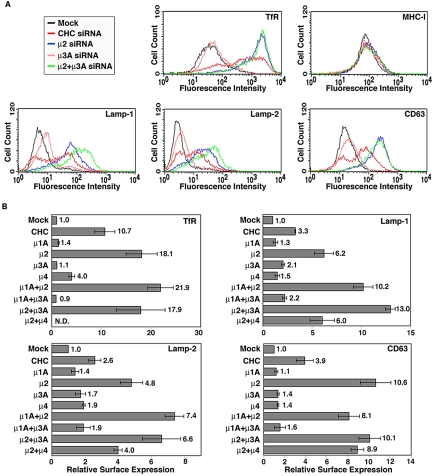
FACS analysis of the intact, siRNA-treated cells showed that depletion of clathrin caused a three to fourfold increase and depletion of AP-2 a 5- to 11-fold increase in the levels of Lamp-1, Lamp-2, and CD63 at the cell surface (Figure 3, A and B). For the AP-2-depleted cells, this corresponded to the accumulation of 25–45% of the Lamps at the cell surface, as measured by FACS analysis of surface versus intracellular levels (our unpublished data). Similar results were obtained upon siRNA-mediated depletion of another subunit of AP-2, α-adaptin, from HeLa cells (Supplemental Figure 1) as well as depletion of CHC or μ2 from the melanoma cell line Mel JuSo (Supplemental Figure 2). The effects of depleting each of the other AP complexes were smaller (up to 2-fold increase; Figure 3, A and B). Simultaneous depletion of AP-2 and AP-1, or AP-2 and AP-3, however, showed a trend toward greater increases in the surface expression of some of the Lamps relative to the depletion of AP-2 alone (although not all the differences were statistically significant) (Figure 3, A and B). As controls, we showed that depletion of clathrin or AP-2, but not AP-3, increased the surface expression of the TfR and that depletion of either of these proteins had no effect on the expression levels of MHC-I (Figure 3, A and B; our unpublished data). From these experiments, we concluded that clathrin and AP-2 are both required to maintain low levels of the Lamps at the cell surface, whereas the other AP complexes are less important.
Figure 3.
Effects of clathrin or AP complex depletion on the cell surface levels of Lamps. (A) FACS profiles of the cell surface expression of TfR, MHC-I, Lamp-1, Lamp-2, and CD63 in mock-treated HeLa cells (black), and HeLa cells depleted of CHC (red); μ2 (blue), μ3A (pink), and μ2 plus μ3A (green) using specific siRNAs. (B) The cell surface levels of TfR, Lamp-1, Lamp-2, and CD63 in cells depleted of the μ subunits indicated on the left were normalized to those in the mock-treated cells. Bar values represent the mean ± SD from four independent experiments. Numbers on the right indicate the mean fold increase. N.D., not determined.
Inhibition of Lamp Endocytosis in Cells Depleted of AP-2
To determine whether the depletion of AP-1, AP-2, or AP-3 affected the endocytosis of surface Lamps, we performed microscopic antibody uptake experiments. Depletion of AP-1 caused either no increase or a slight increase in the uptake of the antibodies to the three Lamps (Figure 4, compare first and second row), in agreement with previous work using mouse embryonic fibroblasts from μ1A-deficient mice (Meyer et al., 2000). Depletion of AP-3 caused a more appreciable increase in the uptake of anti-Lamp antibodies (Figure 4, fourth row), a finding that also was in accordance with previous studies on AP-3-deficient human and mouse cells (Le Borgne et al., 1998; Dell'Angelica et al., 1999b, 2000; Meyer et al., 2000; Peden et al., 2002, 2004; Rous et al., 2002). In contrast, depletion of AP-2 resulted in accumulation of bound antibody on the cell surface (Figure 4, third row). We also used a FACS-based endocytosis assay to examine the effect of depleting AP-2 on the internalization of antibody to CD63, the only of the three Lamps that is expressed at high enough levels on the surface of untreated cells for quantitative analyses (Dell'Angelica et al., 1999b; Peden et al., 2004). In line with the microscopic assays, we found that CD63 was rapidly internalized in the mock-treated cells, but not in the AP-2-depleted cells (Supplemental Figure 3). From these experiments, we concluded that the increased expression of Lamps at the surface of AP-2-depleted cells was due to decreased internalization. These experiments also suggested that the small additional effects of AP-1 or AP-3 depletion result from increased Lamp trafficking via the plasma membrane.
Figure 4.
Uptake of antibodies to Lamps in live, siRNA-treated cells. Live mock-, μ1A-, μ2-, or μ3A-siRNA-treated HeLa cells grown on coverslips were incubated for 1 h at 37°C with mouse monoclonal antibodies to Lamp-1, Lamp-2, or CD63, as indicated in the figure. Cells were then fixed, permeabilized, stained with Alexa 594-conjugated donkey anti-mouse IgG, and analyzed by confocal fluorescence microscopy. Bar, 5 μm.
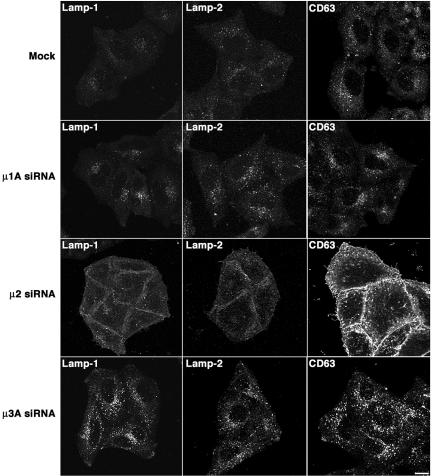
Altered Distribution of Lamps in Cells Depleted of Clathrin or AP-2
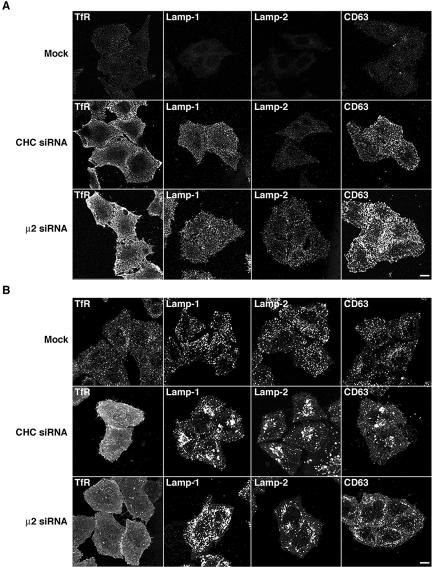
We next examined by immunofluorescence microscopy the distribution of the Lamps in mock-, clathrin- or AP-depleted cells. In agreement with the experiments described above, depletion of clathrin or AP-2 caused a visible increase in the surface staining for Lamp-1, Lamp-2, and CD63 in nonpermeabilized cells (Figure 5A). We also observed increased plasma membrane staining for the three Lamps in the corresponding permeabilized cells (evident as a hazy background; Figure 5B), although this was less apparent than in nonpermeabilized cells because of the partial extraction of the plasma membrane caused by permeabilization. These analyses also revealed that the intracellular Lamp-containing structures had a more swollen and clumped appearance in the clathrin-deficient, and to a lesser extent, AP-2-deficient cells (Figure 5B). Immunoelectron microscopy showed the presence of large (up to 2 μm) vacuoles containing Lamp-2 in clathrin-depleted cells; these structures were rarely seen in mock-treated cells (our unpublished data). No obvious changes in the distribution of the Lamps were observed in cells depleted, singly or in various combinations of AP-1, AP-3, and AP-4; the Lamp-containing organelles in these cells had a normal appearance and distribution, and for the most part did not contain the TGN marker TGN46, and the early endosomal markers TfR and EEA1 (Supplemental Figures 4–6; our unpublished data). Together, these observations indicate that the overall distribution of Lamps and the morphology of lysosomes are substantially altered upon depletion of clathrin or AP-2, but not of the other AP complexes. Nonetheless, much of the Lamps remain intracellular in clathrin- or AP-2-depleted cells, indicating that depletion of these proteins does not lead to complete redistribution of the Lamps to the plasma membrane over the time course of these experiments, but rather it may affect the delivery of a discrete population of Lamps to lysosomes.
Figure 5.
Alteration of the cellular distribution of Lamps by clathrin or AP-2 depletion. (A) Surface staining of TfR and Lamps in mock-, CHC- and μ2-siRNA-treated HeLa cells. Live cells grown on coverslips were immunostained for TfR, Lamp-1, Lamp-2, or CD63 at 4°C, fixed, permeabilized, stained with Alexa 594-conjugated donkey anti-mouse IgG, and analyzed by confocal fluorescence microscopy. (B) Staining for TfR and Lamps in mock- and siRNA-treated, permeabilized cells. HeLa cells were fixed, permeabilized, and then immunostained for TfR, Lamp-1, Lamp-2, and CD63 before confocal fluorescence microscopy. Bars, 5 μm.
Depletion of AP-2 Impairs the Delivery of Newly Synthesized Lamps to Lysosomes
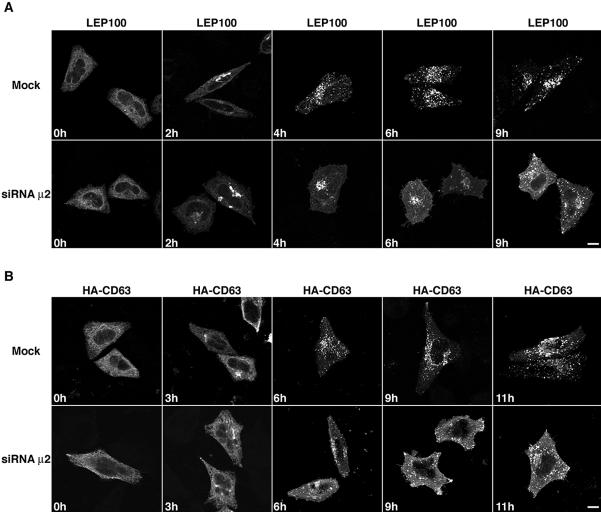
The experimental results described thus far demonstrate that the endocytic machinery plays a role in the trafficking of Lamps, but they do not provide a measure of the relative importance of this machinery for the biosynthetic delivery of the Lamps to lysosomes. This is because immunofluorescent staining does not distinguish the population of newly synthesized Lamps affected by RNAi from the preexisting pool of Lamps. To examine the effect of AP-2 depletion on a cohort of newly synthesized Lamps, we used two types of “pulse-chase” protocols, one morphological and the other biochemical. The morphological approach consisted of transfecting mock-treated or AP-2-depleted HeLa cells with plasmids encoding LEP100 (i.e., chicken Lamp-1) or human CD63 tagged at the N terminus with the HA epitope (HA-CD63). The expression of these heterologous proteins allowed distinction of the newly synthesized from the preexisting, endogenous pools of Lamps. At 6 h after transfection, the cells were treated with 2 μg/ml BFA for 12 h to arrest export from the ER (Doms et al., 1989; Lippincott-Schwartz et al., 1989), while new synthesis of LEP100 and HA-CD63 took place. Proteins were then released from ER retention by removal of the BFA and the transport of LEP100 and HA-CD63 was monitored after different times of chase (Figure 6). Using immunofluorescence microscopy of fixed permeabilized cells, we observed that at the start of the chase both LEP100 and HA-CD63 were in the ER of the mock-treated cells (Figure 6). By 2 h, the presence of LEP100 and HA-CD63 in the Golgi complex was apparent, and from 4 to 11 h they were all in lysosomes (Figure 6). The pattern of staining was the same in the AP-2-depleted cells for up to 2 h; however, staining of the plasma membrane could be observed beginning at 4 h and lasting for up to 9–11 h (Figure 6). Intracellular vesicular staining also was apparent at 6–11 h of chase (Figure 6), suggesting that the block in the delivery of the newly synthesized Lamps to lysosomes was not complete.
Figure 6.
Immunofluorescence microscopy analysis of the transport of newly synthesized Lamps to lysosomes. Mock- and μ2-siRNA-treated cells were transfected with expression vectors encoding LEP100 (A) or HA-CD63 (B). Six hours after transfection, the cells were incubated for an additional 12 h in 2 μg/ml BFA in regular culture medium at 37°C (“pulse”). The BFA was then removed and the cells incubated for the indicated periods at 37°C (“chase”). The cells were fixed, permeabilized, and the distribution of LEP100 or HA-CD63 revealed by indirect immunofluorescence staining and confocal microscopy. Bars, 5 μm.
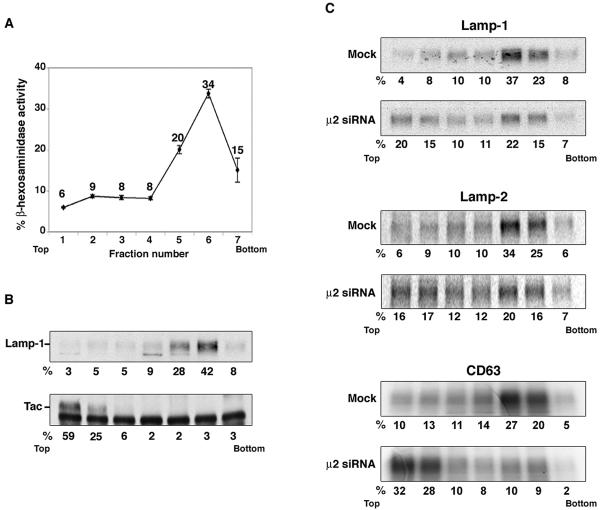
We also used a biochemical approach to quantify the extent of the block in lysosomal targeting in AP-2-depleted cells. In this approach, mock-treated and AP-2-depleted HeLa cells were pulse-labeled for 30 min with [35S]methionine-cysteine and then chased for 6 h in complete medium. The cells were disrupted under conditions that maintain the integrity of lysosomes, and total membranes were centrifuged in a self-forming Percoll gradient (Barriocanal et al., 1986; Green et al., 1987; Rohrer et al., 1996). The gradients were divided into seven fractions, and lysosomes were located to fractions 5 and 6 from the top by measurement of β-hexosaminidase activity (Figure 7A) and immunoblotting for Lamp-1 (Figure 7B). The plasma membrane was localized to fractions 1 and 2 by immunoblotting for the Tac antigen (Figure 7B). Other organelles such as the ER, Golgi, and endosomes also are known to band in these lighter fractions (Brown and Swank, 1983). Immunoprecipitation of the labeled Lamps revealed that in the mock-treated cells, 60% of Lamp-1, 59% of Lamp-2 and 47% of CD63 sedimented in the lysosomal fractions after 6 h of chase (Figure 7C). In contrast, in the AP-2-depleted cells, 37% of Lamp-1, 36% of Lamp-2, and 19% of CD63 were in lysosomes at 6 h of chase (Figure 7C). From this analysis, we estimated that depletion of AP-2 caused 40–60% reductions in the biosynthetic targeting of these three Lamps to lysosomes.
Figure 7.
Analysis of the transport of newly synthesized Lamps to lysosomes by centrifugation on Percoll gradients. (A) Distribution of β-hexosaminidase activity on Percoll gradients. HeLa cells were homogenized by passage through a 25-gauge needle and the postnuclear supernatant was fractionated by centrifugation on a self-forming 18% Percoll gradient. Seven fractions were collected from the top (fraction 1), and an aliquot of each fraction was assayed for β-hexosaminidase activity. Values are the mean ± SD from three independent experiments. Numbers next to each point indicate the percent of total β-hexosaminidase activity. (B) Distribution of Lamp-1 and Tac on Percoll gradients. Untransfected HeLa cells or HeLa cells transfected with an expression vector encoding human Tac (a plasma membrane marker) were fractionated as described above. The distribution of Lamp-1 was analyzed by SDS-PAGE and immunoblotting. Tac was first immunoprecipitated from Triton X-100-solubilized fractions and then resolved by SDS-PAGE and immunoblotting. The lower band present in all the lanes of the Tac immunoblot corresponds to the immunoglobulin heavy chain. The numbers under each lane indicate the percentage of the total Lamp-1 or Tac present in each fraction. (C) Mock- and μ2-siRNA-treated cells were metabolically labeled for 30 min with [35S]methionine-cysteine and chased for 6 h to follow the lysosomal targeting of newly synthesized Lamps. Cell homogenates were then fractionated on Percoll gradients as described above, and the fractions were solubilized and subjected to immunoprecipitation for Lamp-1 (top two panels), Lamp-2 (middle two panels), and CD63 (bottom two panels). The immunoprecipitates were analyzed by SDS-PAGE and autoradiography. The numbers under each lane indicate the percentage of the total radiolabeled Lamps present in each fraction.
DISCUSSION
The use of RNAi technology has allowed us to address two outstanding questions concerning the biosynthetic sorting of the Lamps to lysosomes: do clathrin and AP complexes participate in this sorting, and, by inference, does sorting involve transport via the plasma membrane? The results of our study show that, at least in HeLa and Mel JuSo cells, clathrin and AP-2 play important roles in this process, supporting the notion that a significant pool of Lamps traffic via the plasma membrane on their way to lysosomes.
Requirement of Clathrin and AP-2 for Delivery of the Lamps to Lysosomes
Depletion of clathrin caused a three- to fourfold increase in the levels of Lamps at the cell surface and altered the morphology of lysosomes. This role of clathrin is consistent with the localization of Lamp-1 to clathrin-coated areas of the TGN (Höning et al., 1996) and the plasma membrane (Lippincott-Schwartz and Fambrough, 1987). It contrasts, however, with a previous report that elimination of CHC expression in a chicken DT40 B cell clone does not alter the distribution of LEP100 (Wettey et al., 2002). We think that this latter result may be explained by the selection of a rare DT40 clone that adapted to life without clathrin due to evolution of compensatory mechanisms, as is the case for yeast (Seeger and Payne, 1992).
Because the function of clathrin in protein sorting relies on adaptor proteins that bind to signals in the cytosolic tails of transmembrane proteins, it was expected that a clathrin adaptor also would be required for biosynthetic targeting of the Lamps. Among the various clathrin adaptors that have now been identified (Traub, 2003; Robinson, 2004), the heterotetrameric AP complexes were the best candidates because of their ability to bind the YXXØ-type, lysosomal-targeting signals of the Lamps (Bonifacino and Traub, 2003). The μ2 subunit of AP-2, in particular, binds this type of signal with the highest avidity and broadest specificity (Ohno et al., 1998). Indeed, we found that depletion of AP-2 caused a 5- to 11-fold increase in the surface expression at steady state and a 40–60% decrease in the biosynthetic targeting of three different Lamps to lysosomes. These numbers are likely underestimates given that the siRNA-treated cells still contained ∼5% of the normal levels of μ2. We do not know why depletion of AP-2 had more of an effect than depletion of clathrin. One possibility is that the residual amount of CHC (10–15%) in the siRNA-treated cells may still be sufficient to support substantial targeting. Unlike depletion of AP-2, depletion of AP-1 or AP-3, or the nonclathrin-associated AP-4, singly or in combinations, had only modest effects on the expression of Lamps at the cell surface (≤2-fold) and on the overall intracellular distribution of the Lamps. Moreover, in no case did the depletion of these intracellular AP complexes result in localization of the Lamps to the TGN or early endosomes, the two compartments where these complexes are normally located. These findings agree with previous observations made using other approaches to deplete cells of AP-1, AP-3, and/or AP-4 (Le Borgne et al., 1998; Dell'Angelica et al., 1999b, 2000; Meyer et al., 2000; Peden et al., 2002, 2004; Rous et al., 2002; Simmen et al., 2002). Together, these observations indicate that, of the four AP complexes, AP-2 is the singly most important one for targeting of the Lamps to lysosomes.
Transport of the Lamps via the Plasma Membrane
The requirement for AP-2 shown here lends support to the notion that a substantial fraction of newly synthesized Lamps are delivered to lysosomes via the plasma membrane. This is because, under normal conditions, AP-2 is exclusively associated with plasma membrane clathrin-coated pits and plasma-membrane-derived clathrin-coated vesicles (Robinson, 1987), where it plays critical roles in the internalization of endocytic receptors (Conner and Schmid, 2003; Hinrichsen et al., 2003; Motley et al., 2003; Huang et al., 2004). Only under experimental conditions involving pharmacological perturbation of cells (Wang et al., 1993) or in vitro recruitment assays (Seaman et al., 1993; Traub et al., 1996; West et al., 1997) has AP-2 been found to associate with other organelles of the endosomal-lysosomal system. It also has been reported that internalized epidermal growth factor (EGF) receptor in A431 cells recruits AP-2 to endosomes in vivo (Sorkina et al., 1999), but this could be due to the massive wave of internalization elicited by activation of the high number of EGF receptors on these cells (i.e., 2–4 million/cell) (Wiley, 1988) and/or the ability of EGF to recruit the endocytic machinery through signaling (Wilde et al., 1999). We considered the possibility that AP-2 remained associated with the internalized Lamps in endosomes, but immunofluorescence microscopy analyses failed to show any colocalization of AP-2 with an internalized Tac-Lamp-1 chimera in HeLa cells (our unpublished data). Thus, it is unlikely that the requirement of AP-2 for sorting of the Lamps reflects a role for this adaptor on endosomes or other intracellular compartments.
The above-mentioned considerations lead us to conclude that 40–60% of Lamps pass through the plasma membrane at some point in their itinerary to lysosomes. This could correspond to the population of Lamps that follow the conventional indirect pathway (Lippincott-Schwartz and Fambrough, 1986; Furuno et al., 1989a,b; Nabi et al., 1991; Mathews et al., 1992; Gough et al., 1999). However, our results are equally consistent with other models that combine elements of the indirect and direct pathways. For example, the appearance of newly synthesized Lamps at the cell surface could be preceded by passage through an endosomal compartment, as shown previously for some plasma membrane proteins (Futter et al., 1995; Leitinger et al., 1995; Ang et al., 2004). In addition, newly synthesized Lamps could undergo several rounds of cycling between the plasma membrane and endosomes before their delivery to lysosomes, as is the case for lysosomal acid phosphatase (Braun et al., 1989; Prill et al., 1993). Finally, exocytic fusion of lysosomes with the plasma membrane could generate a pool of surface Lamps that need to be retrieved to lysosomes (Reddy et al., 2001). Any of these processes would lead to the accumulation of Lamps at the cell surface in the absence of AP-2.
Postendocytic Transport of the Lamps
Once internalized, how do Lamps proceed to lysosomes? AP-3 likely facilitates their transport from early to late endosomes, because AP-3 mutant cells exhibit enhanced recycling to the plasma membrane (Peden et al., 2004). This role probably explains the additive effect caused by AP-3 depletion on top of AP-2 depletion. In any event, this function of AP-3 does not seem to be essential for the localization of the bulk of the Lamps to lysosomes at steady state (Dell'Angelica et al., 1999b; Reusch et al., 2002; Rous et al., 2002). Similarly, depletion of AP-1 —alone or in combination with AP-3 — or of AP-4 has little or no effect on the overall distribution of the Lamps (Reusch et al., 2002; Simmen et al., 2002; Suppl. Figures 4 and 5). This also rules out these complexes as essential players in the sorting of the Lamps from early to late endosomes and lysosomes. What then accounts for the efficiency of this sorting? Several possibilities can be entertained. First, there could be other adaptor molecules distinct from the AP complexes that recognize GYXXØ signals in endosomes. In fact, about two dozen proteins are now known or presumed to function as clathrin adaptors (Traub, 2003; Robinson, 2004). This explanation would be consistent with the proposal that early-to-late endosomal transport of the Lamps is signal mediated (Rohrer et al., 1996). Second, the extracellular or membrane spanning domains of the Lamps could participate in endosomal sorting. In this regard, the luminal domain of lgp120 (rat Lamp-1) has been shown to contribute to sorting (Reaves et al., 1998). It also is intriguing that the transmembrane domain of Lamp-1 and Lamp-2 is the domain that exhibits the highest sequence conservation from chicken to mammals (our unpublished observations). Moreover, mutations in the transmembrane domain of the TfR divert this receptor to lysosomes (Zaliauskiene et al., 2000). Finally, transport of the Lamps from early to late endosomes could occur by default, whereas recycling of endocytic receptors to the plasma membrane could be facilitated by coat proteins. In this regard, clathrin, dynamin, and AP-1 have all been proposed to increase the efficiency of recycling of internalized TfR to the plasma membrane (van Dam and Stoorvogel, 2002).
Alternative Transport Pathways
Notwithstanding the significant impact of depleting AP-2, roughly half of the Lamps still reach lysosomes in AP-2-depleted cells. Some of this transport could be due to the small amount of AP-2 left in the siRNA-treated cells. It is more likely, however, that this residual transport occurs by a direct pathway (Green et al., 1987; Carlsson and Fukuda, 1992; Harter and Mellman, 1992; Höning and Hunziker, 1995), involving AP-1, AP-3, and/or AP-4. Indeed, the levels of Lamps at the cell surface increased between 10 and 110% upon depletion of each of these complexes. Moreover, at least for Lamp-1 and Lamp-2, depletion of AP-3 caused additional increases over those elicited by depletion of AP-2 alone. Therefore, intracellular sorting events mediated by these complexes do contribute to the overall delivery of Lamps to lysosomes, perhaps through their participation in the direct pathway. The limited effects caused by depletion of these complexes, however, raises the possibility that the AP-2-independent component of Lamp targeting is also independent of the other AP complexes. In this regard, it is noteworthy that mutation of the critical tyrosine residue in the cytosolic tail of lgp120 results in only 39% of the protein being displayed at the cell surface (Harter and Mellman, 1992), which suggests the existence of a pathway that is GYXXØ-signal independent, and hence, AP-independent sorting. As discussed in the previous paragraph for sorting from early to late endosomes, AP-independent sorting could involve another type of adaptor or coat or could be dependent on luminal or transmembrane determinants.
Concluding Remarks
Because the depletion of AP complexes by RNAi takes place over several days in culture, our conclusions are subject to three important caveats. First, the effects of depleting clathrin or AP-2 on Lamp trafficking could be indirect, for instance by trapping at the cell surface a key protein (e.g., a SNARE) that is required for Lamp sorting at an intracellular site. The fact that the GYXXØ-signals from the Lamps bind directly and with highest avidity to AP-2, however, makes it likely that the effects are direct. Second, as discussed above for clathrin mutant cells, RNAi-treated cells could adapt to the loss of AP-1, AP-3, or AP-4, by up-regulating compensatory mechanisms. Simultaneous depletion of these complexes would address the question of whether adaptation to the loss of one AP complex involves enhancement of a pathway mediated by another AP complex. Unfortunately we have been unable to attain complete elimination of all three complexes without significant loss of cell viability, so addressing their potentially redundant role in Lamp trafficking will require more controlled approaches to interfere with their functions. Finally, depletion of AP-1, AP-3, or AP-4 could affect the kinetics of delivery of Lamps to lysosomes while having minimal effects on their steady-state distribution. Despite these caveats, our results do support the main conclusion of our study that clathrin and AP-2 play critical roles in Lamp trafficking, which emphasizes the importance of the endocytic machinery for efficient targeting of the Lamps to lysosomes.
Supplementary Material
Acknowledgments
We thank X. Zhu for expert technical assistance; M. McNiven, L. Traub, and D. Fambrough for kind gifts of reagents; and J. Lippincott-Schwartz, G. Patterson, P. McCormick, R. Puertollano, and R. Mattera for helpful discussions and critical review of the manuscript.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05-03-0213) on June 29, 2005.tj;2
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Agular, R. C., Ohno, H., Roche, K. W., Bonifacino, J. S. (1997). Functional domain mapping of the clathrin-associated adaptor medium chains mu1 and mu2. J. Biol. Chem. 272. 27160-27166. [DOI] [PubMed] [Google Scholar]
- Aguilar, R. C., Boehm, M., Gorshkova, I., Crouch, R. J., Tomita, K., Saito, T., Ohno, H., and Bonifacino, J. S. (2001). Signal-binding specificity of the μ4 subunit of the adaptor protein complex, AP-4. J. Biol. Chem. 276, 13145-13152. [DOI] [PubMed] [Google Scholar]
- Altschuler, Y., Barbas, S. M., Terlecky, L. J., Tang, K., Hardy, S., Mostov, K. E., and Schmid, S. L. (1998). Redundant and distinct functions for dynamin-1 and dynamin-2 isoforms. J. Cell Biol. 143, 1871-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang, A. L., Taguchi, T., Francis, S., Fölsch, H., Murrells, L. J., Pypaert, M., Warren, G., and Mellman, I. (2004). Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 167, 531-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arighi, C. N., Hartnell, L. M., Aguilar, R. C., Haft, C. R., and Bonifacino, J. S. (2004). Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 165, 123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriocanal, J. G., Bonifacino, J. S., Yuan, L., and Sandoval, I. V. (1986). Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J. Biol. Chem. 261, 16755-16763. [PubMed] [Google Scholar]
- Boll, W., Ohno, H., Songyang, Z., Rapoport, I., Cantley, L. C., Bonifacino, J. S., and Kirchhausen, T. (1996). Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 15, 5789-5795. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J. S., and Dell'Angelica, E. C. (1998). Immunoprecipitation. In: Current Protocols in Cell Biology, ed. J. S. Bonifacino, M. Dasso, J. B. Harford, J. Lippincott-Schwartz, and K. Yamada, New York: John Wiley & Sons.
- Bonifacino, J. S., and Traub, L. M. (2003). Signals for Sorting of Transmembrane Proteins to Endosomes and Lysosomes. Annu. Rev. Biochem. 72, 395-447. [DOI] [PubMed] [Google Scholar]
- Braun, M., Waheed, A., and von Figura, K. (1989). Lysosomal acid phosphatase is transported to lysosomes via the cell surface. EMBO J. 8, 3633-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J. A., and Swank, R. T. (1983). Subcellular redistribution of newly synthesized macrophage lysosomal enzymes. Correlation between delivery to the lysosomes and maturation. J. Biol. Chem. 258, 15323-15328. [PubMed] [Google Scholar]
- Carlsson, S. R., and Fukuda, M. (1992). The lysosomal membrane glycoprotein lamp-1 is transported to lysosomes by two alternative pathways. Arch. Biochem. Biophys. 296, 630-639. [DOI] [PubMed] [Google Scholar]
- Conner, S. D., and Schmid, S. L. (2003). Differential requirements for AP-2 in clathrin-mediated endocytosis. J. Cell Biol. 162, 773-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damke, H., Baba, T., Warnock, D. E., and Schmid, S. L. (1994). Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127, 915-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica, E. C., Aguilar, R. C., Wolins, N., Hazelwood, S., Gahl, W. A., and Bonifacino, J. S. (2000). Molecular characterization of the protein encoded by the Hermansky-Pudlak syndrome type 1 gene. J. Biol. Chem. 275, 1300-1308. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica, E. C., Klumperman, J., Stoorvogel, W., and Bonifacino, J. S. (1998). Association of the AP-3 adaptor complex with clathrin. Science 280, 431-434. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica, E. C., Mullins, C., and Bonifacino, J. S. (1999a). AP-4, a novel protein complex related to clathrin adaptors. J. Biol. Chem. 274, 7278-7285. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica, E. C., Ohno, H., Ooi, C. E., Rabinovich, E., Roche, K. W., and Bonifacino, J. S. (1997). AP-3, an adaptor-like protein complex with ubiquitous expression. EMBO J. 15, 917-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica, E. C., Shotelersuk, V., Aguilar, R. C., Gahl, W. A., and Bonifacino, J. S. (1999b). Altered trafficking of lysosomal membrane proteins in Hermansky-Pudlak syndrome due to mutations in the β3A subunit of the AP-3 adaptor complex. Mol. Cell 3, 11-21. [DOI] [PubMed] [Google Scholar]
- Dey, A., Ellenberg, J., Farina, A., Coleman, A. E., Maruyama, T., Sciortino, S., Lippincott-Schwartz, J., and Ozato, K. (2000). A bromodomain protein, MCAP, associates with mitotic chromosomes and affects G(2)-to-M transition. Mol. Cell. Biol. 20, 6537-6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms, R. W., Russ, G., and Yewdell, J. W. (1989). Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell Biol. 109, 61-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen, E. L., Tanaka, Y., and Saftig, P. (2003). At the acidic edge: emerging functions for lysosomal membrane proteins. Trends Cell Biol. 13, 137-145. [DOI] [PubMed] [Google Scholar]
- Furuno, K., Ishikawa, T., Akasaki, K., Yano, S., Tanaka, Y., Yamaguchi, Y., Tsuji, H., Himeno, M., and Kato, K. (1989a). Morphological localization of a major lysosomal membrane glycoprotein in the endocytic membrane system. J. Biochem. 106, 708-716. [DOI] [PubMed] [Google Scholar]
- Furuno, K., Yano, S., Akasaki, K., Tanaka, Y., Yamaguchi, Y., Tsuji, H., Himeno, M., and Kato, K. (1989b). Biochemical analysis of the movement of a major lysosomal membrane glycoprotein in the endocytic membrane system. J. Biochem. 106, 717-722. [DOI] [PubMed] [Google Scholar]
- Futter, C. E., Connolly, C. N., Cutler, D. F., and Hopkins, C. R. (1995). Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J. Biol. Chem. 270, 10999-11003. [DOI] [PubMed] [Google Scholar]
- Gough, N. R., Zweifel, M. E., Martinez-Augustin, O., Aguilar, R. C., Bonifacino, J. S., and Fambrough, D. M. (1999). Utilization of the indirect lysosome targeting pathway by lysosome-associated membrane proteins (LAMPs) is influenced largely by the C-terminal residue of their GYXXØ targeting signals. J. Cell Sci. 112, 4257-4269. [DOI] [PubMed] [Google Scholar]
- Green, S. A., Zimmer, K. P., Griffiths, G., and Mellman, I. (1987). Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J. Cell Biol. 105, 1227-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter, C., and Mellman, I. (1992). Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J. Cell Biol. 117, 311-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen, L., Harborth, J., Andrees, L., Weber, K., and Ungewickell, E. J. (2003). Effect of clathrin heavy chain- and α-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J. Biol. Chem. 278, 45160-45170. [DOI] [PubMed] [Google Scholar]
- Hirst, J., Bright, N. A., Rous, B., and Robinson, M. S. (1999). Characterization of a fourth adaptor-related protein complex. Mol. Biol. Cell 10, 2787-2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höning, S., Griffith, J., Geuze, H. J., and Hunziker, W. (1996). The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 15, 5230-5239. [PMC free article] [PubMed] [Google Scholar]
- Höning, S., and Hunziker, W. (1995). Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J. Cell Biol. 128, 321-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, F., Khvorova, A., Marshall, W., and Sorkin, A. (2004). Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J. Biol. Chem. 279, 16657-16661. [DOI] [PubMed] [Google Scholar]
- Hunziker, W., and Geuze, H. J. (1996). Intracellular trafficking of lysosomal membrane proteins. Bioessays 18, 379-389. [DOI] [PubMed] [Google Scholar]
- Jones, S. M., Howell, K. E., Henley, J. R., Cao, H., and McNiven, M. A. (1998). Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science 279, 573-577. [DOI] [PubMed] [Google Scholar]
- Kasai, K., Shin, H. W., Shinotsuka, C., Murakami, K., and Nakayama, K. (1999). Dynamin II is involved in endocytosis but not in the formation of transport vesicles from the trans-Golgi network. J. Biochem. 125, 780-789. [DOI] [PubMed] [Google Scholar]
- Kirchhausen, T. (1999). Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 15, 705-732. [DOI] [PubMed] [Google Scholar]
- Kornfeld, S., and Mellman, I. (1989). The biogenesis of lysosomes. Annu. Rev. Cell Biol. 5, 483-525. [DOI] [PubMed] [Google Scholar]
- Le Borgne, R., Alconada, A., Bauer, U., and Hoflack, B. (1998). The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J. Biol. Chem. 273, 29451-29461. [DOI] [PubMed] [Google Scholar]
- Leitinger, B., Hille-Rehfeld, A., and Spiess, M. (1995). Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc. Natl. Acad. Sci. USA 92, 10109-10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., and Fambrough, D. M. (1986). Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J. Cell Biol. 102, 1593-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., and Fambrough, D. M. (1987). Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell 49, 669-677. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz, J., Yuan, L. C., Bonifacino, J. S., and Klausner, R. D. (1989). Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell 56, 801-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina, J. A., Moriyama, K., and Bonifacino, J. S. (2003). BLOC-3, a protein complex containing the Hermansky-Pudlak syndrome gene products HPS1 and HPS4. J. Biol. Chem. 278, 29376-29384. [DOI] [PubMed] [Google Scholar]
- Mathews, P. M., Martinie, J. B., and Fambrough, D. M. (1992). The pathway and targeting signal for delivery of the integral membrane glycoprotein LEP100 to lysosomes. J. Cell Biol. 118, 1027-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, C., Zizioli, D., Lausmann, S., Eskelinen, E. L., Hamann, J., Saftig, P., von Figura, K., and Schu, P. (2000). mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 19, 2193-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motley, A., Bright, N. A., Seaman, M. N., and Robinson, M. S. (2003). Clathrin-mediated endocytosis in AP-2-depleted cells. J. Cell Biol. 162, 909-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi, I. R., Le Bivic, A., Fambrough, D., and Rodriguez-Boulan, E. (1991). An endogenous MDCK lysosomal membrane glycoprotein is targeted basolaterally before delivery to lysosomes. J. Cell Biol. 115, 1573-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naslavsky, N., Weigert, R., and Donaldson, J. G. (2003). Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol. Biol. Cell 14, 417-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, H., Aguilar, R. C., Yeh, D., Taura, D., Saito, T., and Bonifacino, J. S. (1998). The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 273, 25915-25921. [DOI] [PubMed] [Google Scholar]
- Ohno, H., Fournier, M. C., Poy, G., and Bonifacino, J. S. (1996). Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 271, 29009-29015. [DOI] [PubMed] [Google Scholar]
- Ohno, H., Stewart, J., Fournier, M. C., Bosshart, H., Rhee, I., Miyatake, S., Saito, T., Gallusser, A., Kirchhausen, T., and Bonifacino, J. S. (1995). Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269, 1872-1875. [DOI] [PubMed] [Google Scholar]
- Peden, A. A., Oorschot, V., Hesser, B. A., Austin, C. D., Scheller, R. H., and Klumperman, J. (2004). Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J. Cell Biol. 164, 1065-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden, A. A., Rudge, R. E., Lui, W. W., and Robinson, M. S. (2002). Assembly and function of AP-3 complexes in cells expressing mutant subunits. J. Cell Biol. 156, 327-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prill, V., Lehmann, L., von Figura, K., and Peters, C. (1993). The cytoplasmic tail of lysosomal acid phosphatase contains overlapping but distinct signals for basolateral sorting and rapid internalization in polarized MDCK cells. EMBO J. 12, 2181-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves, B. J., Banting, G., and Luzio, J. P. (1998). Lumenal and transmembrane domains play a role in sorting type I membrane proteins on endocytic pathways. Mol. Biol. Cell 9, 1107-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, A., Caler, E. V., and Andrews, N. W. (2001). Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell 106, 157-169. [DOI] [PubMed] [Google Scholar]
- Reusch, U., Bernhard, O., Koszinowski, U., and Schu, P. (2002). AP-1A and AP-3A lysosomal sorting functions. Traffic 3, 752-761. [DOI] [PubMed] [Google Scholar]
- Robinson, M. S. (1987). 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J. Cell Biol. 104, 887-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, M. S. (2004). Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167-174. [DOI] [PubMed] [Google Scholar]
- Rohrer, J., Schweizer, A., Russell, D., and Kornfeld, S. (1996). The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J. Cell Biol. 132, 565-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rous, B. A., Reaves, B. J., Ihrke, G., Briggs, J. A., Gray, S. R., Stephens, D. J., Banting, G., and Luzio, J. P. (2002). Role of adaptor complex AP-3 in targeting wild-type and mutated CD63 to lysosomes. Mol. Biol. Cell 13, 1071-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, O., Marechal, V., Le Gall, S., Lemonnier, F., and Heard, J. M. (1996). Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2, 338-342. [DOI] [PubMed] [Google Scholar]
- Seaman, M. N., Ball, C. L., and Robinson, M. S. (1993). Targeting and mistargeting of plasma membrane adaptors in vitro. J. Cell Biol. 123, 1093-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger, M., and Payne, G. S. (1992). A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 11, 2811-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen, T., Höning, S., Icking, A., Tikkanen, R., and Hunziker, W. (2002). AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat. Cell Biol. 4, 154-159. [DOI] [PubMed] [Google Scholar]
- Sorkina, T., Bild, A., Tebar, F., and Sorkin, A. (1999). Clathrin, adaptors and eps15 in endosomes containing activated EGF receptors. J. Cell Sci. 112, 317-327. [DOI] [PubMed] [Google Scholar]
- Traub, L. M. (2003). Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J. Cell Biol. 163, 203-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub, L. M., Bannykh, S. I., Rodel, J. E., Aridor, M., Balch, W. E., and Kornfeld, S. (1996). AP-2-containing clathrin coats assemble on mature lysosomes. J. Cell Biol. 135, 1801-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dam, E. M., and Stoorvogel, W. (2002). Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles. Mol. Biol. Cell 13, 169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L.-H., Rothberg, K. G., and Anderson, R.G.W. (1993). Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123, 1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, M. A., Bright, N. A., and Robinson, M. S. (1997). The role of ADP-ribosylation factor and phospholipase D in adaptor recruitment. J. Cell Biol. 138, 1239-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettey, F. R., Hawkins, S. F., Stewart, A., Luzio, J. P., Howard, J. C., and Jackson, A. P. (2002). Controlled elimination of clathrin heavy-chain expression in DT40 lymphocytes. Science 297, 1521-1525. [DOI] [PubMed] [Google Scholar]
- Wilde, A., Beattie, E. C., Lem, L., Riethof, D. A., Liu, S. H., Mobley, W. C., Soriano, P., and Brodsky, F. M. (1999). EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell 96, 677-687. [DOI] [PubMed] [Google Scholar]
- Wiley, H. S. (1988). Anomalous binding of EGF to A431 cells is due to the effect of high receptor densities and a saturable endocytic system. J. Cell Biol. 107, 801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, M. A., and Fukuda, M. (1990). Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J. Cell Biol. 111, 955-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaliauskiene, L., Kang, S., Brouillette, C. G., Lebowitz, J., Arani, R. B., and Collawn, J. F. (2000). Down-regulation of cell surface receptors is modulated by polar residues within the transmembrane domain. Mol. Biol. Cell 11, 2643-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen, L., et al. (1999). Abnormal expression and subcellular distribution of subunit proteins of the AP-3 adaptor complex lead to platelet storage pool deficiency in the pearl mouse. Blood 94, 146-155. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.