Abstract
Exosomes, nanosized extracellular vesicles released by various cell types, are intensively studied for the diagnosis and treatment of cancer and neurodegenerative diseases, and they also display high usability in regenerative medicine. Emphasizing their diagnostic potential, exosomes serve as carriers of disease-specific biomarkers, enabling non-invasive early detection and personalized medicine. The cargo loading of exosomes with therapeutic agents presents an innovative strategy for targeted drug delivery, minimizing off-target effects and optimizing therapeutic interventions. In regenerative medicine, exosomes play a crucial role in intercellular communication, facilitating tissue regeneration through the transmission of bioactive molecules. While acknowledging existing challenges in standardization and scalability, ongoing research efforts aim to refine methodologies and address regulatory considerations. In summary, this review underscores the transformative potential of exosomes in reshaping the landscape of medical interventions, with a particular emphasis on cancer, neurodegenerative diseases, and regenerative medicine.
Keywords: exosomes, regenerative medicine, biomarker, cancer, neurodegenerative diseases
Graphical abstract
1. Introduction
Extracellular vehicles (EVs) are cellular structures released by cells into the extracellular space and have recently become a focal point of research due to their multifunctional role in many biological processes (1, 2). According to the new classification, EVs are divided into several types based on their biogenesis (e.g., exosome, microvesicle, apoptosome, and autophagic EVs), concept (e.g., oncosome, matrix vesicle, stress EVs, and migrasome), and size (e.g., small EVs and large EVs) (3). In general, EVs were divided into three types including apoptotic bodies (apoptosomes), which are the largest EVs with a size range between 1 and 5 μm and are released during programmed cell death (apoptosis). They contain cellular organelles and fragmented DNA and are cleared by phagocytic cells. Microvesicles, typically 100–1,000 nm in diameter, are shed directly from the plasma membrane through outward budding, a process in which a portion of the cellular membrane protrudes outward from the cell surface. They contain proteins, lipids, polysaccharides, and nucleic acids and are involved in intercellular communication and signaling (2, 3). Exosomes (Figure 1), the smallest EVs ranging in size from 30 to 150 nm, are lipid bilayer vesicles and were discovered three decades ago by Pan and Johnstone during investigations of reticulocyte maturation (2–5). Recent studies discovered small exosomes (Exo-S) and large exosomes (Exo-L). Exo-S are in the size range 40–80 nm and contain exosomal tetraspanin marker CD63, while Exo-L (80–150 nm) contain CD9 (3). Initially perceived as cellular waste products responsible for eliminating unnecessary cellular components, our understanding of exosomes has undergone a paradigm shift over the years, revealing their multifaceted functions in cellular communication and signaling (1, 2).
Figure 1.
Structure of exosomes. From a structural perspective, exosomes can be defined as lipid nanoparticles characterized by a phospholipid bilayer membrane. The exosomal membrane is enriched with a diverse array of proteins and saccharide markers, including immunomodulatory molecules, such as PD-1 and PD-L1, and hormone receptors, such as EGFR, tetraspanins, and glypicans. The internal composition of exosomes comprises a variety of biomolecules, including intracellular and cytoskeletal proteins, nucleic acids, growth factors, and cytokines. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Exosomes not only represent a promising material for the diagnosis of serious pathological states but can also be effectively utilized for medicinal applications and drug transport. Given their significance, this review presents the medicinal potential of exosomes, encompassing areas such as regenerative medicine, early diagnosis, and drug treatment. In addition, to provide a more comprehensive understanding, the review rigorously assesses exosome biogenesis, isolation, and characterization.
2. Biogenesis of exosomes
Understanding the biogenesis of exosomes is crucial for advancing the knowledge of their biological functions, their roles in diseases, and potential applications in therapeutics (1, 3). Their life cycle is a complex process involving three main steps: biogenesis, transport, and release (2, 3). The whole process is illustrated in Figure 2 and is initiated by an inward cell membrane budding (6). During this invagination of the plasma membrane, a portion of a cellular membrane undergoes inward folding, and a cup-shaped structure containing extracellular proteins, lipids, metabolites, and cell membrane proteins is formed. This leads to the formation of early endosomes (EEs), which subsequently mature and transform into late endosomes (LEs). Maturation involves the inward budding of the EEs membrane, leading to the sequestration of EE cytoplasmic contents and intraluminal vesicle (ILVs) formation within the endosomal lumen. During ILV formation, specific cargo molecules such as proteins, lipids, and nucleic acids are selectively sorted into the ILVs. LEs containing ILVs are called multi-vesicular bodies (MVBs). The fate of MVBs is determined by the specific proteins present on their surface, which in turn influence various intracellular pathways involved in cargo sorting and trafficking. The MVBs can either fuse with lysosomes or autophagosomes to be degraded or fuse with a plasma membrane to release the contained ILVs as exosomes (2, 6).
Figure 2.
Biogenesis of exosomes. Extracellular membranes are characterized by the presence of numerous transmembrane proteins, including various receptors. (1) Upon ligand binding, receptor-mediated endocytosis is initiated, facilitated by actin filaments, which are integral components of the cytoskeleton. This process results in the invagination of the membrane surrounding the receptor, leading to the formation of an early endosome within the cell. (2) In this early endosome, the bilayer phospholipid membrane exhibits an orientation that is opposite to that of the cytoplasmic membrane, causing the extracellular domains of the transmembrane proteins to be directed inward, toward the lumen of the endosome. (3) Within the cellular context, endosomes are integral components of the complex endosomal-lysosomal system that interacts in a parallel manner (in both directions) with various organelles (e.g., Golgi apparatus and endoplasmic reticulum). This interaction allows endosomes and exosomes to encapsulate biomolecules derived from diverse cellular compartment. (4) During this process, early endosomes undergo maturation into late endosomes, which possess the normal orientation of transmembrane proteins and (5) generate intraluminal vesicles that will ultimately become exosomes. (6) Following the fusion of multi-vesicular bodies containing these intraluminal vesicles with the cytoplasmic membrane, exosomes are released into the extracellular environment. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
3. Isolation and characterization methods of exosomes
Isolation of exosomes is challenging due to the complexity of biological fluids (7, 8). The most common isolation methods include ultracentrifugation, ultrafiltration, size exclusion chromatography, polymer precipitation, tangential flow filtration, and immunoaffinity approaches. A comparison of these methods is provided in Table 1. The optimal isolation strategy should be selected based on the application field, as well as the volume and number of biological samples (7–9). Furthermore, the application of exosomes directly influences the use of subsequent characterization methods (9, 10). This chapter does not aim to provide a detailed explanation of the principles behind individual isolation and characterization methods of exosomes but rather to offer a comprehensive overview that assists researchers in selecting the most appropriate approaches based on considering the specific requirements of different experimental and application contexts.
Table 1.
Advantages and disadvantages of the most common isolation techniques of exosomes.
| Isolation technique | Advantages | Disadvantages | References |
|---|---|---|---|
| Ultracentrifugation | Low cost, separation of large volumes, low operating expenses, compatibility with a wide range of samples | Time consumption, low purity, inappropriateness for small volumes, diminishing biological activity of exosomes, high equipment cost | (8, 25, 26, 216, 217) |
| Ultrafiltration | Simplicity, fast, absence of special equipment | Potential deformation of exosomes, moderate purity, loss of exosomes, particularly problematic for isolating from small volumes, reduced purification efficiency due to clogging of membrane pores | (218–221) |
| Size exclusion chromatography | High purity, scalable, cost-effective for large-scale processing, availability of commercial kits, preservation of biological activity | Requirement of dedicated equipment, low yield, target product dilution | (25, 218, 220, 222, 223) |
| Precipitation techniques | High morphological and functional quality of exosomes, fast, simplicity, compatibility with low sample volumes, availability of commercial kits | Low purity, contamination of precipitating agent, not suitable for large sample volumes | (28, 29, 221, 224, 225) |
| Immunoaffinity techniques | High purity of specific exosomes | Loss of exosomes with lower expression levels, challenge separating exosomes from the bound antibodies, low capacity, low yields | (216, 226) |
| Tangential flow filtration | Processing of large sample volumes, high recovery rate of exosomes with minimal loss, preservation of exosome integrity, simultaneous concentration and buffer exchange (diafiltration), scalable for industrial production, reduction of processing time | High initial cost of equipment and consumables, require pre-filtration steps to remove large particles or debris, potential risk of membrane fouling leading to reduced efficiency over time | (52, 53, 55, 56) |
For diagnostic purposes, exosomes from various biological fluids, including blood (serum and plasma), saliva, and urine, are used in non-invasive diagnostics due to their ability to carry specific molecular information (9, 11). Blood-derived exosomes provide systemic insights, reflecting the physiological and pathological state of the entire body, making them ideal for detecting various diseases such as cancer, neurodegenerative, cardiovascular, and autoimmune diseases (12–16). Salivary exosomes, primarily originating from the salivary glands and the oral cavity, are valuable for diagnosing oral diseases and gastrointestinal tract disorders (17–19). Urinary exosomes, secreted by epithelial cells of the urinary tract, are effective in diagnosing renal diseases, bladder cancer, and prostate conditions (20–22).
When utilizing exosomes for diagnostic purposes, the primary goal is to identify disease-specific exosomal markers. To determine disease-specific markers, it is essential to identify markers that differ in presence or expression level between samples from diseased and healthy patients. This requires the comparison and processing of a large number (tens to hundreds) of biological samples for statistical relevance (23, 24). This procedure thus involves processing large numbers of samples with small volume, typically in the maximum of a few milliliters. For limited numbers of samples, typically in the range of lower tens, differential ultracentrifugation (DUC) is considered the gold standard and is the most widely used method. It usually involves several consecutive rounds of centrifugation with increasing centrifugal force and centrifugation time to remove cells, cell debris, and larger microvesicles. The final step at 100,000 g or higher serves to precipitate the exosomes (12, 25–27).
Because DUC is time-consuming, for handling tens to hundreds of samples, the final ultracentrifugation step is often replaced with commercial kits based on precipitation. Several commercial kits use a polyethylene glycol precipitation technology, including the Total Exosome Isolation Kit (Invitrogen), ExoQuick-TC Exosome Precipitation Solution (System Biosciences), miRCURY Exosome Kits (QIAGEN), Exo-Prep (HansaBioMed), PureExo Exosome Isolation Kit (101Bio), ExoGAG (NasasBiotech), Exosome Precipitation Solutions (Immunostep), and the miRCURY Exosome Isolation Kit (Exiqon) (28–32). These methods result in the isolation of exosomes referred to as total exosomes. In neurodegenerative diseases (NDs), specific subpopulations of exosomes are isolated from the total exosome pool using immunoaffinity methods with specific antibodies, such as anti-L1CAM, anti-NCAM, anti-MOG, or anti-GLAST. These antibodies bind specifically neural, oligodendrocyte, or astrocyte exosomes, which are the most relevant for identifying markers of NDs as Alzheimer disease (AD), multiple sclerosis (MS), dementia, or schizophrenia (33–36).
After the isolation of total exosomes or specific exosomal subpopulations, the characterization of these exosomes and the identification of specific disease markers are performed (10). The most common markers include specific proteins or RNAs. For protein marker identification, techniques such as tandem of liquid chromatography and mass spectrometry (LC–MS) are used, while next-generation sequencing (NGS) is used for RNA. Once specific disease markers are identified, it is crucial to determine whether their exosomal expression differs significantly between diseased and healthy patients in a statistically relevant manner. To quantify markers, techniques such as enzyme-linked immunosorbent assay (ELISA) or quantitative reverse transcription polymerase chain reaction (qRT-PCR) are commonly used (37–42).
Currently, exosomes are being extensively investigated as drug delivery systems. Due to their nanoscale dimensions, they can deliver therapeutic agents specifically to tumor sites (43). Tumor tissues are characterized by a high absorption capacity and poor drainage (44), which facilitates the selective accumulation of exosomes with prolonged circulation times within these tissues. In addition, it is noteworthy that exosomes exhibit favorable permeability across BBB (45). Furthermore, the surface of exosomes can be effectively modified with various ligands that are specific to target cells, thereby significantly enhancing the selectivity of exosomes for these cells (46). As cell-derived products, exosomes are generally considered to be safer than conventional nanoparticles, particularly metal-based nanoparticles (47). Their unique structure, comprising a hydrophilic core and a lipid bilayer, allows them to transport a wide range of drug types (46). However, a significant limitation in the clinical application of exosomes is that cells produce only small quantities of various types of exosomes, which restricts their usability in therapeutic contexts (48).
In regenerative medicine and therapeutic applications, exosomes are isolated from cell culture media. The most commonly used cell sources are mesenchymal stem cells. Similar to diagnostic applications, where DUC is a preferred method for smaller sample quantities, DUC is also commonly employed for isolating exosomes from cell culture media for volumes ranging from tens to hundreds of milliliters. However, a drawback of the final ultracentrifugation step is that the high speeds lead to exosomal damage and the sedimentation of impurities, which diminishes exosomal therapeutic activity (12, 49, 50). Therefore, the final ultracentrifugation step is often replaced by density gradient ultracentrifugation (DGUC), utilizing sucrose or iodixanol gradients (OptiPrep™), where the exosomal fraction is not only less damaged but also better purified (49–51).
For larger volumes, in the range of hundreds of milliliters to liters, a combination of other techniques is employed due to the limited capacity of centrifuges. These primarily include ultrafiltration (UF), utilizing polymer filters of various pore sizes to remove cells, cell debris, and microvesicles, followed by tangential flow filtration (TFF) to remove contaminating proteins, to concentrate the sample, and to perform diafiltration of exosomes into the desired buffer (52–54). For final exosome purification, size exclusion chromatography (SEC) is employed, followed by a final TFF step to concentrate the sample and transfer it into suitable application buffers, most commonly PBS (55–57). The advantage of the combination of techniques such as UF, TFF, and SEC is that they are suitable for practical use on an industrial scale (55, 56). In addition, the isolated exosomes exhibit high purity and preserved therapeutic activity, which is usually subsequently confirmed by in vitro tests such as scratch or transwell assays (54, 58).
According to the recommendations of the International Society for Extracellular Vesicles (ISEV), exosomal samples should generally be characterized for size, concentration, and the presence of exosomes (59, 60). The presence of exosomes is typically confirmed by detecting at least one transmembrane protein (commonly tetraspanins: CD9, CD63, and CD81) or a GPI-anchored protein (e.g., integrins), along with one cytoplasmic lipid (e.g., sphingolipids, ceramides, and cholesterol) or cytoplasmic protein (e.g., ALIX, TSG101, and HSP70) (59, 60). These specific markers can be assayed using methods such as Western blot or ELISA (61, 62). In addition, electron microscopy is often employed to provide images of typical exosomal morphology, further confirming the presence of exosomes in the sample (63, 64).
The size and concentration of exosomes, expressed as particles per milliliter, are commonly determined using techniques such as dynamic light scattering (DLS), nanoparticle tracking analysis (NTA), and resistive pulse sensing (RPS). However, these techniques are not specific to exosomes and may overestimate their concentration (65, 66). Alternatively, exosomes can be labeled with specific dyes, where the measured concentration corresponds only to the positively stained population. This approach allows for more accurate quantification of exosomes and can be performed using nanoflow cytometry (nanoFCM). Nevertheless, underestimation of concentration may occur if the sample is not properly titrated (67, 68). In some cases, the concentration of exosomes is determined based on the total protein concentration per milliliter (μg/mL) using the Bradford assay (10). All these parameters must be thoroughly assessed to ensure sufficient sample purity and demonstrate that the observed therapeutic effect is primarily induced by exosomes rather than by potential contaminants.
4. Therapeutic applications of exosomes in biomedicine
As mentioned earlier, exosomes are natural intercellular communicators in normal biological processes but also in pathologies. They transport proteins, lipids, and nucleic acids specific for their parenteral pathogenic cells. From a clinical perspective, most applications use exosomes as biomarkers of diseases (69). The content of the exosome has been shown to be disease-specific, such as in NDs, prion diseases, viral infections, and cancer (69). Furthermore, exosomes play a transformative role in regenerative medicine, offering innovative therapeutic interventions. Their bioactive cargo, including growth factors and signaling molecules, has demonstrated significant potential in modulating immune responses and promoting tissue repair (70). In addition, exosomes serve as carriers for therapeutic cargo loading, holding promise for targeted drug delivery in disease treatment (69, 71, 72). Currently, 150 clinical trials registered on ClinicalTrials.gov are investigating exosome-based therapies for various diseases (73). The majority of applications utilizing exosomes for both therapy and diagnosis focus on their utilization in the fields of cancer and NDs, as depicted in Figure 3 (74). For this reason, this review will focus on the use of exosomes in these diseases.
Figure 3.
Distribution of exosome therapy and diagnostics concerning the target diseases (74). The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
4.1. Exosomes in regenerative medicine
Nowadays, one of the main applications of exosomes is in regenerative medicine, a promising field dedicated to the regeneration and reconstruction of diseased or injured organs and tissues (72). Since the 1970s, mesenchymal stem cells (MSCs) have been under investigation in this field for their multipotent characteristics and their ability to migrate to injury sites. They are used in regenerative medicine due to their robust self-renewal capacity and ability to differentiate into adipogenic, chondrogenic, osteogenic, endothelial, neural, and epithelial cells, as proven in both in vivo and in vitro experiments (70, 73–75). MSCs, like every cell in the human body, release exosomes, which have started to be extensively researched due to their regenerative properties. Currently, at least 31 clinical trials are exploring the use of exosomes derived from MSCs (MSCs-EXOs) as an alternative to basic MSCs therapy (73). MSCs-EXOs have shown comparable or superior therapeutic efficacy compared to MSCs alone (72, 73). They exhibit lower immunogenicity, present an enhanced safety profile by avoiding concerns related to uncontrolled differentiation and tumorigenicity associated with live cells, and offer improved storage conditions, simplifying logistical challenges compared to live cell storage. In addition, there are no ethical issues, and their small size allows for sterilization by filtration (73–77). The comparison between exosomal and stem cell therapy is provided in Table 2.
Table 2.
Exosomes versus stem cells in preparation and therapy.
| Advantages | Disadvantages | |
|---|---|---|
| MSC preparation | Ease to isolate, easy to expand at a large scale, highly proliferative, well established FDA guidelines | Harsh storage and transportation conditions |
| Exosome preparation | Small size, stable upon freezing and thawing | Difficult to isolate and purify, no established regulations and standards |
| Therapeutic application of MSCs | Multilineage differentiation potential, extensive preclinical and clinical studies | Immunogenicity, oncological complications, fusion toxicity, ethical issues |
| Therapeutic application of exosomes | Minimal risk of immune responses and tumor formation, no ethical issues, multiple delivery routes, can be engineered to specifically target and deliver drug cargoes | Rapid clearance from blood after administration |
The greatest attention in regenerative medicine is focused on skin healing, a tissue which plays a crucial multifunctional role as a protective barrier, a temperature regulator, and facilitating tactile and pain sensations (78). However, the use of exosomes for wound treatment could be challenging due to their rapid clearance from the application site, which limited therapeutic effectiveness (79). To address this limitation, current research focuses on the combination of exosomes and biomaterials. This innovative approach extends the retention time of exosomes on the wound surface without compromising their biological activity, enabling the development of advanced exosome-based therapies (80, 81). Hydrogels as biomaterials exhibit a synergistic effect in exosome-induced wound healing and can serve as a versatile platform for the incorporation of therapeutic exosomes, enhancing their efficacy in tissue regeneration. To date, hyaluronic acid, gelatin, chitosan, and polypeptide-based hydrogels have been used for encapsulating exosomes from different cell sources (75, 80, 81). Chitosan is the often-used material for hydrogel preparation. Chitosan hydrogel enriched with MSCs-EXOs, specifically human endometrial stem cell-derived and human placenta-derived exosomes, demonstrated notable wound closure ability by promoting the formation of new epithelial cells, significant retention of MSCs-EXOs at injury sites, promotion of angiogenesis, and acceleration of the recovery of ischemic hind limbs (82). For the best contact of the hydrogel with the wounded skin and effective wound filling, thermosensitive hydrogels are used. Thermosensitive pluronic hydrogel combined with human umbilical cord MSCs-EXOs (HUCMSCs-EXOs) significantly accelerated wound closure, promoted angiogenesis, and improved skin healing of chronic diabetic wounds (83). For effective diabetic wound treatment, polyvinyl alcohol/alginate nanohydrogel with HUCMSCs-EXOs and an injectable antibacterial polypeptide-based hydrogel with adipose derived MSCs-EXOs were used. This type of hydrogels demonstrated the ability to promote proliferation, migration, and angiogenesis of human umbilical vein endothelial cells, expediting diabetic wound closure thus presenting a novel approach for complete skin regeneration (84, 85).
Further applications in regenerative medicine are focused on hard tissue regeneration, essential for bone and cartilage repair. Traditionally, MSCs, scaffolds, and growth factors are used in this field. While scaffolds have been proven beneficial for bone regeneration, the avascular nature of cartilage poses unique challenges (75). Osteoarthritis (OA), a prevalent joint disease extending beyond cartilage, demands innovative regenerative procedures (81). Promisingly, exosome-integrated scaffolds and MSCs-EXO therapy show potential in OA treatment. Articular cavity injection with HUCMSCs-EXOs in PBS demonstrated significant efficacy in preventing severe damage to knee articular cartilage in a rat OA model. These therapies not only promoted chondrocyte proliferation and migration but also exhibited anti-apoptotic effects and reversed cellular injuries. Moreover, HUCMSCs-EXOS played a crucial role in regulating the polarization of M2 macrophages, fostering chondrocyte survival by producing anti-inflammatory cytokines to suppress adverse inflammation (86). To enhance therapeutic efficiency and retention time in vivo, HUCMSCs-EXOs were engineered to specifically target chondrocytes and encapsulated within hyaluronic acid hydrogel, presenting a “two-phase” releasing system. This approach synergistically facilitated OA cartilage repair in a rat model and proved the rejuvenating effects of HUCMSCs-EXOs on aging chondrocytes in OA, offering a promising cell-free OA treatment strategy (87). Therapeutic potential of bone marrow MSCs-EXOs was explored in the context of mitochondrial dysfunction and oxidative stress in OA. 3D printed scaffolds, composed of extracellular matrix, gelatin methacrylate, and exosomes, effectively restored chondrocyte mitochondrial function, enhanced chondrocyte migration, and polarized the synovial macrophage response in vitro. Notably, a 3D printed scaffold significantly facilitated cartilage regeneration in a rabbit model, highlighting its potential as an early treatment strategy for OA (88).
In recent years, MSCs-EXOs have also been used increasingly in ophthalmology. MSCs-EXOs have shown promise in various applications, such as promoting ocular tissue regeneration and addressing vision-related disorders. MSCs-EXOs have explored the therapeutic potential of stem cell exosomes in treating ocular surface diseases, corneal injuries, and retinal degenerative conditions (89). Exosomes derived from bone marrow stem cells have demonstrated the ability to enhance corneal epithelialization and maintain corneal transparency in diabetic mice (90). MSCs-EXOs have been explored for their regenerative effects on corneal injuries (91). In retinal diseases, including age-related macular degeneration and retinitis pigmentosa, MSCs-EXOs have been investigated for their neuroprotective and regenerative properties. These exosomes may influence retinal cell survival, angiogenesis, and anti-inflammatory responses, offering a novel approach for treating degenerative conditions affecting the retina (92). As exosome research in regenerative medicine continues to progress, MSCs-EXOs are beginning to be applied into various fields, such as periodontitis (93, 94). Research on exosomes in regenerative medicine is expected to gain prominence, highlighting the growing need to integrate exosome-based regenerative therapies into clinical practice (95).
4.2. Exosomes in early diagnosis
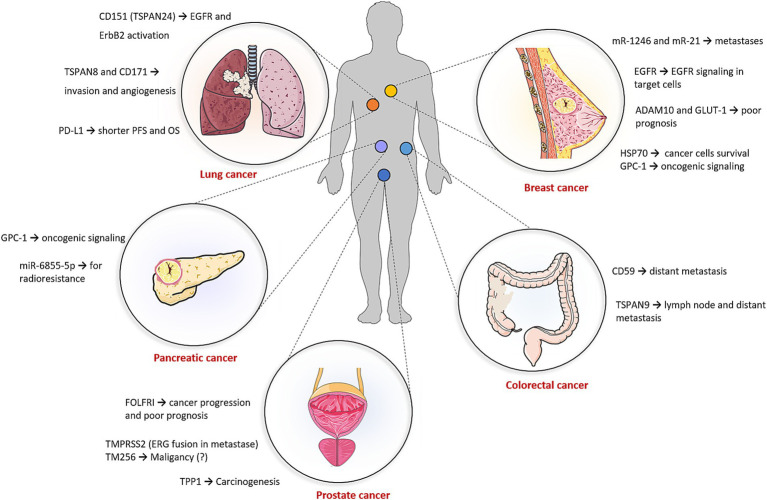
As already mentioned, exosomes are promising biomarkers for the diagnosis of several diseases. Most attention has been paid to cancer (Figure 4; Supplementary Table S1) (95–105) with less focus on NDs.
Figure 4.
Potential of exosomes in cancer diagnostics. The applicability of exosomes in cancer diagnostics is detailed in Supplementary Table S1. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Tumor-derived exosomes (TEXs) have emerged as critical players in cancer progression. Cancer cells release TEXs in large quantities, leading to a rapid increase in the concentration of total exosomes in the serum or plasma of cancer patients, which correlates with poor prognosis (106). TEXs provide immunosuppressive effects by inducing dysfunction in various immune cells, thereby suppressing anti-tumor immune responses (107). Initial interactions between TEXs and immune cells occur through ligand–receptor recognition, followed by either direct fusion with the plasma membrane or receptor-mediated uptake, resulting in the release of TEX cargo into the cytoplasm of immune cells. While T lymphocytes do not efficiently internalize TEXs, they interact with surface molecules to trigger sustained Ca2+ flux and downstream signaling cascades, ultimately altering the transcriptome of recipient cells. In contrast, phagocytic cells, such as dendritic cells and macrophages, rapidly internalize TEXs (108).
Macrophages are a prominent component of the tumor microenvironment (TME) and can make up more than half of the tumor mass in some cases. Moreover, tumor progression is associated mainly with macrophages. Macrophages can be induced to either tumor-suppressive immunological type M1 or tumor-promoting inflammatory M2 macrophages (109, 110). Tumor-associated macrophages predominantly exhibit an M2 phenotype, characterized by the secretion of pro-angiogenic factors and cytokines that promote angiogenesis, tumor growth, and metastasis (111, 112). Many studies have shown evidence on the role of TEXs in macrophage M2 polarization to promote tumor progression. For instance, exposure of macrophages to TEXs decreased the expression of M1 markers, such as IFNγ, while upregulating IL-1β, a marker of inflammation, suggesting that TEXs may help maintain a macrophage phenotype supportive of tumor survival and proliferation (113). In triple-negative breast cancer (TNBC), TEXs have been reported to promote M2 macrophage polarization, facilitating lymph node metastasis. Co-culture of TNBC-TEXs with macrophages led to significant morphological changes and an increased expression of M2 markers, including Fizz1, CD206, and Arg-1. An orthotopic TNBC model further confirmed the role of TEXs in driving M2 polarization, with enhanced tumor growth and axillary lymph node metastasis observed in vivo (114).
In cancer metastasis, epithelial–mesenchymal transition (EMT) is the key process during which cancer cells lose their epithelial properties and adopt a more mesenchymal and invasive phenotype. Generally, EMT initiation is characterized by loss of cell–cell adhesions and apicobasal polarity, leading to the formation of cells with increased migratory and invasion capabilities that are able to invade the extracellular matrix (115, 116). At a molecular level, EMT involves the downregulation of epithelial-type proteins, such as E-cadherin, and the acquisition of mesenchymal markers, such as vimentin (117). TEXs from bladder cancer cells have been shown to induce EMT-like changes in urothelial cells, enhancing their invasive potential. This effect was mediated by TEX-induced upregulation of vimentin and downregulation of E-cadherin through the TGF-β1 signaling pathway (118). Similar EMT-inducing effects of TEXs have been observed in glioblastoma, lung carcinoma, and gastric cancer models (119–121). TEXs can also influence EMT through their miRNA cargo. For example, miR-23a within TEXs promotes EMT by inhibiting E-cadherin synthesis in lung carcinoma and melanoma cells, while miR-191 and let-7a, present in TEXs from patients with melanoma, gastric, and colorectal cancers, have also been implicated in EMT regulation (122–126). In addition, TEX-derived miR-105 has been shown to promote vascular invasion by downregulating ZO-1 in endothelial cells. Notably, elevated miR-105 levels in the serum of breast cancer patients have been correlated with metastatic progression and poor prognosis (127). Given their critical role in modulating the TME, promoting EMT, and facilitating immune evasion, profiling TEXs in blood and other body fluids holds significant promise as a non-invasive method for cancer diagnosis and prognosis (128).
The WHO Global Cancer Observatory (GLOBOCAN) 2022 registry provides a list of the most common types of cancers. The three most common types are lung (12.4%), breast (11.5%), and colorectal (9.6%) cancers. Lung cancer is the leading cause of cancer death worldwide (129). Surface-enhanced Raman spectroscopy (SERS) of exosomes, combined with AI deep learning software, allows for accurate diagnosis of early-stage lung cancer. The deep learning model was trained with SERS signals of exosomes derived from normal and lung cancer cell lines and subsequently its ability to detect cancer was verified using exosome samples from patients’ blood. The model identified the lung cancer patients and even detected stage I patients with an accuracy of 90.7% (130). Regarding TEX protein biomarkers, CD151, TSPAN8, and CD171 were overexpressed in lung cancer samples. Of these, CD171 has been associated with EMT, metastases, and poor prognosis (96, 131, 132). Determination of PD-L1 can also provide important diagnostic information. Akbar et al. reported that exoPD-L1 from non-small cell lung cancer (NSCLC) patients and healthy controls showed a significantly higher difference than corresponding serum and tissue PD-L1 (97). For example, exoPD-L1 was found in all the patients (100%), while tissue PD-L1 was observed only in 71% patients. Furthermore, exoPD-L1 can also be used for the prediction of immune inhibitors efficiency. ROC curve analysis of change from baseline in exoPD-L1 levels between responders and non-responders showed 87% sensitivity and 100% specificity (p = 0.0015), indicating strong discriminatory power. An increasing number of studies have demonstrated that non-coding RNAs are closely correlated with the initiation and development of lung cancer as well (133). Detection of exosomal long non-coding RNA as potential lung cancer diagnosis is also performed in a current clinical study (NCT03830619) (134). Breast cancer is the most common type of cancer and the second leading cause of cancer-related deaths in women. In this type of cancer, media from breast cancer cell lines were analyzed to identify specific exosomal proteins such as glucose transporter-1, glypican-1, and the metalloproteinase domain-containing protein 10 (98). Epidermal growth factor receptor (EGFR) is a transmembrane protein that plays a key role in cell signaling pathways involved in cell growth, proliferation, and survival and is often overexpressed or mutated in various cancers. It was reported that triple-negative breast cancer cells (MDA-MB-468) can produce exosomes with encapsulated EGFR (protected from EGFR inhibitor), which can induce EGFR signaling in target cells, thereby promoting cancer progression or resistance to therapy (99). Typical non-coding RNA cancer biomarkers are miR-1246 and miR-21, which were also overexpressed in breast cancer cell lines (100). The plasma level of miR-1246 was measured in breast cancer patients and healthy controls using an Au nanoflare probe. This biomarker-based probe distinguished breast cancer patients from healthy individuals with 100% sensitivity and 92.9% specificity (95). Currently, a clinical trial (NCT02662621) is underway, focusing on an exosomal detection protocol for diagnosing various cancers, including breast cancer. The study indicates that exosomes displaying the stress protein HSP70 on their membrane may serve as cancer-specific exosomes (134). In colorectal cancer, tetraspanin-1 was found to be upregulated in plasma exosomes from patients compared to healthy controls, showing 75.7% sensitivity (135). Pancreatic cancer, one of the deadliest cancer types, involves TEXs that carry the glypican-1 biomarker. This co-receptor for various signaling molecules regulates key processes such as cell growth, motility, and differentiation (102). Currently, two clinical trials are underway to assess the efficacy of diagnostic exosomes in colon and liver cancers (NCT03432806) and in pancreatic cancer (NCT03334708). These studies aim to identify biomarkers circulating in blood and analyze the corresponding tissues (134). While previous studies have focused on TEXs from blood, urinary exosomes play a role in urological tumors (136). In prostate cancer, biomarkers such as TM256 and LAMTOR proteins found in urinary exosomes exhibited very high sensitivity (105). In addition, potential biomarkers such as TPP1, TMPRSS2, and FOLR1 were highly upregulated in urinary exosomes derived from the bladder. Notably, despite being histologically tumor-free at cystectomy, patients’ urinary exosomes displayed a carcinogenic metabolic profile, likely originating from undetected or partially transformed cancer cells (137).
Current diagnosis of NDs relies on clinical assessments, medical history, imaging techniques, and diagnostic tests based on observed symptoms (138, 139). However, predicting these diseases remains challenging due to the typically late-stage diagnosis. Emerging research highlights the potential of exosomes as biomarkers for early detection, offering a promising approach for more effective and timely diagnoses in the future (Figure 5) (140). Early diagnosis of NDs is crucial for enabling timely interventions that can significantly improve patient outcomes (141).
Figure 5.
Potential of exosomes in the diagnosis of neurological diseases. In AD, protein markers such as total tau, phosphorylated T181-tau (p-T181-tau), phosphorylated S396-tau (p-S396-tau), and amyloid-beta 42 (Aβ42) derived from neural-derived blood exosomes are indicative of neurodegeneration and plaque formation. Notably, GAP43, SNAP25, and synaptotagmin 1 can be detected even in asymptomatic stages of AD. Furthermore, exosomal Aβ42, Aβ40, and P-T181-tau have been implicated in schizophrenia. In the context of PD and ALS, exosomal α-synuclein and neurofilament light chain exhibit promising potential as biomarkers for disease monitoring and progression. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
In Alzheimer’s disease (AD), neural-derived blood exosomes have emerged as a valuable source of AD-related overexpressed protein markers, including total tau, P-T181-tau, P-S396-tau, and Aβ42 (33). Exosomal synaptic proteins, such as growth-associated GAP43, neurogranin, SNAP25, and synaptotagmin 1, show promise in predicting AD at asymptomatic stages, potentially detecting the disease 5 to 7 years before cognitive impairment occurs (34). In addition, miRNA markers, including miR-137, miR-181c, miR-9, and miR-29a/b, are downregulated in blood serum and offer additional potential for early AD detection (142, 143). Among other types of biomarkers, lipids such as LDL-C, TG, and HDL-C are associated with AD (144). Furthermore, it was also shown that exosomal protein markers offer comparable diagnostic capacity to cerebrospinal markers, further enhancing the potential of blood-based diagnostics for AD (145). Exosomal levels of Aβ42, Aβ40, and P-T181-tau were also measured in the blood plasma of patients diagnosed with schizophrenia (36). Specifically, these markers were determined in neural and astrocytic exosomes. While Aβ42 levels were higher in astrocytic exosomes than in neural exosomes, other markers were similar between these two groups. It was also found that higher astrocytic P-T181-tau levels were associated with worse executive functioning, and astrocytic Aβ42 levels were more sensitive and specific in differentiating diagnostic groups. Other types of neural cells explored in the context of NDs are oligodendrocytes. These cells are associated with MS. Oligodendrocyte-derived extracellular vesicles showed higher concentrations of myelin basic protein, which could serve as potential biomarkers across diverse MS phenotypes (35). For Parkinson disease (PD), it was found that the most reliable biomarker of blood neural exosomes is α-synuclein (146). In amyotrophic lateral sclerosis (ALS), exosomal biomarkers, such as neurofilament light chain, have been identified in both cerebrospinal fluid and blood. This marker can serve for early diagnosis and monitoring disease progression (147).
4.3. Exosomes in targeted delivery and disease treatment
The two main areas in which exosome applications are greatly investigated and hold great potential are cancer and NDs (74, 76). In cancer treatment, significant attention is paid to nanocarriers which can entrap chemotherapeutic drugs and deliver them to the diseased site, reducing the side effects associated with the systemic administration of conventional anticancer drugs (148, 149). In recent years, exosomes started to be explored as promising nanocarriers (48) that can affect tumor growth, metastasis, and even sensitize cancer cells to conventional therapies (Figure 6).
Figure 6.
Exosomes represent a promising delivery system for the transport of anticancer agents. In murine cancer models, specifically in breast and ovarian carcinoma, exosomal formulations exhibit lower cardiotoxicity and higher efficacy compared to free doxorubicin. In the case of paclitaxel, exosomal formulations provide significantly improved intracellular delivery into 3LL-M27 cells (Lewis carcinoma expressing P-glycoprotein, which is associated with drug resistance) when compared to liposomal formulations and polystyrene nanoparticles. Furthermore, in these murine models, exosomes have been shown to suppress the development of metastases. Regarding natural agents, curcumin-loaded exosomes display promising efficacy against colon and pancreatic carcinomas, exhibiting antimetastatic effects and reduced inflammation. Exosomes are also suitable for the transport of biological agents such as microRNAs. For instance, exosomes loaded with miR-379 and miR-144-5p demonstrate potent effects against breast cancer and pancreatic ductal adenocarcinoma. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
Their ability to target specific cell types and deliver therapeutic cargos makes them a valuable asset in the fight against malignancies (150–153). For example, exosomes loaded with doxorubicin (ExoDOX) were used in a mouse model of breast and ovarian cancers. It was found that ExoDOX are less cardiotoxic than free DOX, enabling the use of higher concentrations of ExoDOX, thus increasing the efficacy of DOX (154). ExoDOX conjugated with gold nanoparticles (ExoDOX-GNPs) were used in vitro with lung cancer cell lines and normal lung fibroblasts. The pH sensitive conjugation bond enables the enhanced rate of drug release under acidic conditions and successful uptake of the ExoDOX-GNPs by the recipient cells. Cell viability assays indicated that ExoDOX-GNPs exhibit preferential cytotoxicity toward cancer cells and have minimal activity on non-cancerous cells (155). Exosomes loaded with paclitaxel (ExoPTX) were developed. The incorporation of paclitaxel into exosomes enhanced drug cytotoxicity more than 50-fold in drug-resistant MDCKMDR1 (Pgp+) cells (156). Furthermore, ExoPTX displayed a potent anticancer effect in a mouse model of murine Lewis Lung Carcinoma pulmonary metastases. In addition to separate therapeutics, other compounds are also used as exosomal cargo. Exosomes loaded with gemcitabine in combination with the survivin protein with mutation T34A induce apoptosis and enhance gemcitabine-killing effects in pancreatic adenocarcinoma cells (157). For cancer treatment, exosomes can also deliver nucleic acids, including miRNAs, siRNAs, and mRNAs (158). For instance, exosomes containing miR-379, a potential tumor suppressor, showed a significant reduction in the rate of tumor formation and growth for the in vitro and in vivo therapies of breast cancer (159). Exosomes loaded with miR-145-5p, which inhibits pancreatic ductal adenocarcinoma (PDAC) cell proliferation, invasion, and increase apoptosis, significantly reduced the growth of xenograft tumors in a PDAC mouse model (160). Natural products are also used in exosomal cancer therapies, including the use of curcumin. This molecule can mitigate cancer initiation and metastasis (161, 162). Exosomes loaded with curcumin induced the apoptosis of pancreatic cancer cells and significantly delayed brain tumor growth with reduced inflammation when delivered to a GL26 brain tumor model via an intranasal route (163, 164). Plant exosomes with curcumin are used also in Phase I clinical trial investigating the ability of plant exosomes to deliver curcumin to normal and malignant colon tissue (NCT01294072). This study is now recruiting patients (165). Grape exosomes are investigated in preliminary active clinical trial to abrogate oral mucositis induced by combined chemotherapy and radiation in head and neck cancer patients (NCT01668849) (165). Vaccination with tumor antigen-loaded dendritic cell-derived exosomes on patients with unresectable NSCLC lung cancer responding to induction chemotherapy was explored in another clinical study (NCT01159288), where the first phase of this study has now been completed. The primary endpoint was progression-free survival at 4 months after chemotherapy cessation, with a target of at least 50% of patients achieving this endpoint. However, this target was not met as only 32% of patients experienced disease stabilization at 4 months (165, 166). A study of mesenchymal stromal cell-derived exosomes with KrasG12D siRNA for metastatic pancreas cancer patients harboring the KrasG12D mutation is now recruiting patients for the NCT03608631 clinical trial (166). G12D is the most common KRAS mutation detected in carcinomas and confers a unique structural conformation that influences downstream signaling and may lead to its potent oncogenic activity (167).
It is noteworthy that anti-tumor exosomes could be produced by activated T cells themselves (Figure 7). It is well established that tumor cells often express programmed death-ligand 1 (PD-L1) to deactivate T cells through the activation of programmed death-1 (PD-1) signaling pathways (168). Furthermore, research has shown that these tumor cells also release exosomes containing PD-L1, which exhibit similar immunosuppressive functionality. However, Qiu et al. have reported that activated T cells produce exosomes containing PD-1, which serve to neutralize exosomes carrying PD-L1 (169) and induce the degradation of PD-L1 on the surface of TNBC cells. This dynamic interplay highlights the potential of T-cell-derived exosomes in counteracting tumor-mediated immune evasion.
Figure 7.
Anti-tumor effects of T-cell-derived exosomes. Following activation by antigen-presenting cells (1), T cells generate exosomes containing PD-1 (2), which directly attenuate the immunosuppressive effects of cancer cells (4) by facilitating the degradation of PD-L1 (5) present on the surface of tumor cells. In addition, T-cell-derived exosomes (3) inhibit the functionality of immunosuppressive exosomes expressing PD-L1 (5), produced by cancer cells. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
The ability of exosomes to cross the blood–brain barrier (BBB) provides the opportunity for their use in the treatment of NDs (45, 170). Many publications highlight the application of MSCs-EXOs in the treatment of NDs (Figure 8) (171, 172).
Figure 8.
Exosomes in the treatment of neurodegenerative diseases. Due to their ability to cross the BBB, exosomes are being investigated for the treatment of neurodegenerative diseases. In a rat model of PD, bone marrow-derived mesenchymal stem cell exosomes (MSCs-EXOs) have been shown to improve motor function. Similarly, MSCs-EXOs with a high content of sphingosine-1-phosphate have been found to decrease Aβ deposition and enhance cognitive function in a mouse model of AD. In a mouse model of schizophrenia, MSCs-EXOs have been reported to reduce cerebrospinal fluid glutamate levels and alleviate schizophrenia-related behaviors. Exosomes derived from murine microglial cells contain IL-4, an anti-inflammatory cytokine, and lactadherin, a phagocytic “eat me” signal, which reduce neuroinflammation in a mouse model of experimental autoimmune encephalomyelitis (a MS model). Similarly, effects were observed with macrophage-derived exosomes that were modified with derivatives of sialic acid, containing resveratrol. The figure was partly generated using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
For example, the effect of bone marrow-derived MSCs-EXOs was examined in rats with induced Parkinson’s disease (PD). Rats treated with these exosomes showed significant improvements in motor function and histopathological outcomes, demonstrating a greater suppression of PD symptoms compared to L-DOPA treatment, which is a medication commonly used to treat PD (173). In AD treatment, bone marrow MSCS-EXOs with high content of sphingosine-1-phosphate (second messenger downregulated in the AD tissue) were injected into double transgenic AD mice (174). Their application led to reduce Aβ deposition and promote cognitive function recovery. According to the observed results, sphingosine kinase inhibitor (SKI-II) or sphingosine-1-phosphate 1 receptor blocker (VPC23019) repress the therapeutic effects of exosomes. Intracerebral injection of bone marrow MSCS-EXOs in a preclinical mouse model of early stage of AD suggests the possibility of intervening before overt clinical manifestations. The study indicated that bone marrow MSC-EXOs are effective at reducing the Aβ plaque burden and the number of dystrophic neurites in both cortex and hippocampus (175). Adipose-MSCs-EXOs administered intranasally and HUCMSCs-EXOs administered intravenously have also shown promise in AD treatment. These treatments effectively improved neurological damage in entire brain regions, increased newborn neurons, powerfully rescued memory deficits, and reduced Aβ expression in transgenic AD mice (176–179). MSCS-EXOs were also used in a mouse model of schizophrenia, where they improved the core schizophrenia-like behavior and biochemical markers of schizophrenia (e.g., cerebrospinal fluid glutamate level) (180). In addition to MSCs-EXOs, macrophage-derived EXOs loaded with resveratrol, nature antioxidant, or exosomes from murine microglia cell line containing anti-inflammatory cytokine IL-4 were also used in mice with MS (181, 182). The efficacy of this approach can be increased by suitable derivatization of the exosome membrane with compounds such as lactadherin (“eat me” signal for the phagocytes) or sialic acid derivatives (BBB transport). Both exosome agents significantly inhibited inflammatory responses in the CNS and the peripheral system in a mouse model and effectively improved the clinical evolution of MS in vivo (182, 183). In clinical studies, there is just a single trial registered in CLinicalTrials.gov. This study evaluates the safety and the efficacy of adipose MSC-EXOs in AD (NCT04388982). It was found that the intranasal administration of MSCs-EXOs was safe and well tolerated for a semi-weekly treatment frequency. A dose of at least 4 × 108 particles was selected for a randomized phase II and phase III clinical trial in further steps (181).
5. Future direction
Exosomes are increasingly recognized for their pivotal role in the diagnostic realm of cancer and NDs. Nonetheless, a multitude of high-impact clinical trials have unveiled their promising potential in diagnosing a spectrum of additional serious conditions. Hyperuricemia (HUA) is acknowledged as a significant risk factor for chronic heart failure (CHF), a disease frequently linked to elevated morbidity and mortality rates (184, 185). Notably, fluctuations in miRNA expression have been correlated with cardiovascular diseases, including CHF and HUA (186, 187). Analysis of miRNA patterns in serum exosomes revealed that miR-27a-5p was upregulated (p < 0.01), while miR-139-3p was downregulated (p < 0.01) in patient groups (CHF with HUA) (188). When used in combination, these markers exhibited an AUC of 0.899 (95%) with 79.2% sensitivity and 91.7% specificity. Moreover, exosomes are integral to the pathogenesis of osteoarthritis, with those isolated from synovial fluids emerging as promising diagnostic instruments for affected patients (189, 190). However, exosome isolation methods can be invasive. In contrast, urine biomarkers present a non-invasive alternative for osteoarthritis diagnostics (191), positioning urine-derived exosomes as potential diagnostic assets. Cao et al. introduced a nanopolymer modified with an exosome-affinity component (CD63 aptamer and distearoyl phosphoethanolamine) (192). This innovative approach leads to aggregation of exosomes upon binding, facilitating easy centrifugation (4,000 g for 3 min at pH 6). Notably, these precipitated exosomes can be re-dissolved in a basic pH environment. Metabolomic analyses of urine exosomes have identified 30 biomarkers, including catechol (AUC = 0.917, p < 0.001), which effectively differentiate between healthy individuals and those with early osteoarthritis.
In the future, exosomes such as natural nanocarriers should emerge as a novel therapeutic alternative in the fields of oncology, immunology, and regenerative medicine. Future research may focus on refining loading techniques, optimizing targeting strategies, and exploring novel applications in diverse disease contexts (193). For example, in ND treatment to enhance targeted drug delivery efficiency, exosomes can be surface-modified with RVG protein/peptides to specifically bind to the acetylcholine receptor expressed on neuronal cells (194). Comparing RVG-tagged MSC-EXOs to MSCS-EXOs, EXOs tagged with RVG exhibit improved targeting to the cortex and hippocampus after being administered intravenously, plaque deposition and Aβ levels are decreased sharply, and the activation of astrocytes is obviously reduced compared to the observations made in the group of AD mice treated only with MSCs-EXOs. In the group of AD mice injected with RVG-EXOs, there is a significant improvement in learning and memory capabilities with reduced plaque deposition and Aβ levels (195).
Exosomes can also be combined with other biomaterials or inorganic materials for biomedical uses. These hybrid nanoparticles can be further loaded with specific cargo or drug and surface engineered to increase the local concentration of the particles at the diseased site, thereby reducing toxicity and side effects and maximizing therapeutic efficacy (196). The surface engineered exosomal hybrid nanoparticles with specific cargo loading, and modifications conferring desired properties such as pH sensitivity or photosensitivity can be called “ExoBots.” This term reflects the hybrid nature of these structures, which incorporate elements from both biological (exosomes) and technological (robotics) realms to form advanced nanoparticle system. ExoBots combine the advantageous properties of exosomes and other nanoparticles, holding great promise for advancing therapeutic interventions across various biomedical applications. For instance, an engineered core-shell hybrid system was prepared for the in vivo treatment of PD mice. This hybrid system consisted of a curcumin-loaded polymer nanoparticle core and an RVG-modified exosome shell. This hybrid was able to clear α-synuclein aggregates, reduce their cytotoxicity in neurons, and improve the motor behavior of PD mice (197).
Another type of hybrid is composed from exosomes and liposomes. Long circulating and pH sensitive hybrids loaded with DOX were investigated for anti-tumor effect on a mouse model of breast cancer. The results indicated that this hybrid system may be a promising nanocarrier for the treatment of breast cancer, reducing toxicity and inhibiting metastasis mainly in the lungs (198). This hybrid approach was also used to overcome chemotherapy resistance in OC. The hybrid system was developed by fusing cRGD-modified liposomes loaded with miR-497 and triptolide with CD47-expressing tumor-derived exosomes. RGD peptides specifically bind to integrin receptors overexpressed on the surface of many cancer cells. Overexpression of miR-497 may overcome OC chemotherapy resistance, and triptolide was confirmed to possess a superior killing effect on cisplatin-resistant cell lines. The in vitro results indicated that these hybrids were efficiently taken up by tumor cells, thus significantly enhancing tumor cell apoptosis and exerting significant anticancer activity without any negative effects observed in vivo. These hybrids may provide a translational strategy to overcome cisplatin-resistant OC (199). In addition, paclitaxel was used as the cargo of hybrids for in vivo colon cancer treatment. The study revealed that hybrids significantly suppressed tumor growth in a colon tumor-bearing mouse model, reduced the expression of M2 type tumor-associated macrophages, and decreased regulatory T cells (200). Many other types of hybrids were explored, such as thermosensitive hybrids for improved treatment of metastatic peritoneal cancer, pH sensitive macrophage hybrids loaded with DOX for tumor targeted drug delivery, or long circulated pH sensitive hybrids loaded with dasatinib for pancreatic cancer treatment. All these ExoBots show positive therapeutic results and can thus serve as potential therapeutics for cancer treatment (201–203).
Recently, alongside the extensive research on exosomes derived from eukaryotic cells, there has been a growing interest in exploring plant-derived exosomes (P-ELNs; puerarin) as a novel source of exosomes with potential applications in various biotechnological and therapeutic fields. Polyphenolic compounds found in various plant exosomes show great promise in treating serious health disturbances. High-impact studies (204, 205) suggest that their therapeutic effects may be partially attributed to their ability to target ferroptosis, a process linked to numerous pathological states (206, 207).
For instance, exosome-like nanovesicles derived from P. lobata roots have been shown to alleviate alcoholic intoxication, enhance alcohol metabolism, and reduce alcohol levels in the liver and serum of mouse models (204). These effects are associated with the induction of acetaldehyde dehydrogenase activity and a decrease in glutathione peroxidase 4 and glutathione levels, as well as with the suppression of acyl-CoA synthetase long-chain family member 4, likely contributing to the repression of ferroptosis. In addition, Robinia pseudoacacia L. flower-derived P-ELNs, when administered orally, significantly reduce hypoxia-induced ferroptosis and mucosal injury in the gastrointestinal tract of mouse models (205). Their effects are mediated through the modulation of HIF-1α and HIF-2α expression, subsequently influencing ROS production and lipid peroxidation via NOX4 and ALOX5 pathways.
Another promising application of P-ELNs is their potential to mitigate obesity, a well-known risk factor for cancer, metabolic disorders, cardiovascular diseases, and inflammation (208–210). The global prevalence of obesity has been on the rise, making this research particularly relevant. Wang et al. reported that turmeric-derived ELNs exhibit potent anti-obesity effects, achieving weight reductions of 8.68 and 14.56% through intragastric and subcutaneous delivery, respectively (211). This effect is linked to the stimulation of adipocyte apoptosis, the induction of lipolysis, and the inhibition of lipogenesis, highlighting the therapeutic potential of these natural compounds. However, the biological functions of P-ELNs are not fully understood, standard isolation protocols are lacking, and P-ELNs are a promising new frontier in precision medicine. Their plant origin offers advantages in terms of biocompatibility, scalability, and reduced immunogenicity. Moreover, bioactive molecules from plants are associated with disease-preventive effects making P-ELNs an attractive alternative to mammalian EVs in future biomedical innovations (212–214).
6. Conclusion
Although further fundamental research, especially regarding the biogenesis of exosomes and the optimization of their isolation techniques and characterization methods, is necessary, their significant potential has been demonstrated in several biomedical areas, particularly in regenerative medicine, disease diagnosis, and treatment. The continued growth of the exosome market is also evidenced by the recent announcement of four collaborations between pharmaceutical companies, including two potentially worth close to $1 billion each. One of the largest is Lilly’s partnership with Evox Therapeutics of Oxford, UK. In this deal, which could bring in up to $1.2 billion in milestone payments, CNS-targeting exosomes developed by Evox will be loaded with RNA interference and antisense oligonucleotide therapies from Lilly, targeting up to five undisclosed targets. In another major deal, potentially worth over $900 million, Carmine Therapeutics has partnered with Takeda to develop gene therapies for two undisclosed rare diseases targets (215). Exosomes may thus be the future of medicine, used as ExoBots programmed to deliver specific drugs to specific locations within the organism with minimal side effects and high therapeutic efficacies.
Glossary
Glossary
- AD
Alzheimer’s disease
- ALS
amyotrophic lateral sclerosis
- BBB
blood–brain barrier
- CHF
chronic heart failure
- DGUC
density gradient ultracentrifugation
- DLS
dynamic light scattering
- DUC
differential ultracentrifugation
- EEs
early endosomes
- EGFR
epidermal growth factor receptor
- ELISA
enzyme-linked immunosorbent assay
- EMT
epithelial–mesenchymal transition
- EVs
extracellular vehicles
- ExoDOX
exosomes loaded with doxorubicin
- ExoDOX-GNPs
exosomes loaded with doxorubicin conjugated with gold nanoparticles
- Exo-L
large exosomes
- ExoPTX
exosomes loaded with paclitaxel
- Exo-S
small exosomes
- HUA
hyperuricemia
- HUCMSCs-EXOs
mesenchymal stem cell exosomes derived from human umbilical cord
- ILVs
intraluminal vesicles
- ISEV
International Society for Extracellular Vesicles
- LC–MS
liquid chromatography–mass spectrometry tandem technique
- LEs
late endosomes
- MS
multiple sclerosis
- MSCs
mesenchymal stem cells
- MSCs-EXOs
mesenchymal stem cell-derived exosomes
- MVBs
multi-vesicular bodies
- nanoFCM
nanoflow cytometry
- NDs
neurodegenerative diseases
- NGS
next-generation sequencing
- NSCLC
non-small cell lung cancer
- NTA
nanoparticle tracking analysis
- PD
Parkinson disease
- PDAC
pancreatic ductal adenocarcinoma
- PD-1
programmed death-1
- PD-L1
programmed death-ligand 1
- P-ELNs
plant-derived exosomes
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- RPS
resistive pulse sensing
- SEC
size exclusion chromatography
- SERS
surface-enhanced Raman spectroscopy
- TEXs
tumor-derived exosomes
- TFF
tangential flow filtration
- TME
tumor microenvironment
- TNBC
triple-negative breast cancer
- OA
osteoarthritis
- UF
ultrafiltration
Funding Statement
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the project of C2P (NEXARS IP 073-23-04). This study was supported by the projects of Charles University in Prague (SVV260637; SVV260521; UNCE 204064; Cooperatio DIAG). This study was also supported by the Ministry of Education, Youth, and Sports grant no. LM2023053 (EATRIS-CZ), the Technology Agency of the Czech Republic within project TN02000122. The study was also supported by the Ministry of Health grant no. RVO-VFN 64165. We are also grateful for the support from the project National Institute for Cancer Research (Program EXCELES, ID Project No. LX22NPO5102) funded by the European Union—Next Generation. The study was also supported by the National Institute for Neurological Research (Programme EXCELES, ID Project No. LX22NPO5107) funded by the European Union—Next Generation EU. The study was supported from the project MULTIOMICS_CZ (Programme Johannes Amos Comenius, Ministry of Education, Youth and Sports of the Czech Republic,__ ID Project CZ.02.01.01/00/23_020/0008540) co-funded by the European Union. We also acknowledge the Operational Programme Research, Development, and Education within the project Center for Tumor Ecology—Research of the Cancer Microenvironment Supporting Cancer Growth and Spread (reg. no. CZ.02.1.01/0.0/0.0/16_019/0000785).
Author contributions
NO: Writing – original draft. VŠ: Writing – original draft. RH: Formal analysis, Writing – review & editing. MS: Writing – original draft. PD: Writing – original draft. DH: Writing – review & editing. ZK: Writing – review & editing. JH: Writing – original draft. FV: Writing – review & editing. MV: Writing – original draft. PM: Writing – review & editing, Formal analysis. MJ: Supervision, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1539714/full#supplementary-material
References
- 1.Bayraktar HRME, Abd-Ellah KHGMF, Amero P, Chavez-Reyes A, Rodriguez-Aguayo C. Exosomes: from garbage bins to promising therapeutic targets. Int J Mol Sci. (2017) 18:538. doi: 10.3390/ijms18030538, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burtenshaw D, Regan B, Owen K, Collins D, McEneaney D, Megson IL, et al. Exosomal composition, biogenesis and profiling using point-of-care diagnostics-implications for cardiovascular disease. Front Cell Dev Biol. (2022) 10:853451. doi: 10.3389/fcell.2022.853451, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheta M, Taha EA, Lu Y, Eguchi T. Extracellular vesicles: new classification and tumor immunosuppression. Biology. (2023) 12:110. doi: 10.3390/biology12010110, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. (1985) 101:942–8. doi: 10.1083/jcb.101.3.942, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. (1987) 262:9412–20. doi: 10.1016/S0021-9258(18)48095-7, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367:eaau6977. doi: 10.1126/science.aau6977, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, et al. Review on strategies and Technologies for Exosome Isolation and Purification. Front Bioeng Biotechnol. (2021) 9:811971. doi: 10.3389/fbioe.2021.811971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yakubovich EI, Polischouk AG, Evtushenko VI. Principles and problems of exosome isolation from biological fluids. Biochem Suppl Ser A Membr Cell Biol. (2022) 16:115–26. doi: 10.1134/S1990747822030096, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dilsiz N. A comprehensive review on recent advances in exosome isolation and characterization: toward clinical applications. Transl Oncol. (2024) 50:102121. doi: 10.1016/j.tranon.2024.102121, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumari S, Lausted C, Scherler K, Ng AHC, Lu Y, Lee I, et al. Approaches and challenges in characterizing the molecular content of extracellular vesicles for biomarker discovery. Biomol Ther. (2024) 14:1599. doi: 10.3390/biom14121599, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YF, Luh F, Ho YS, Yen Y. Exosomes: a review of biologic function, diagnostic and targeted therapy applications, and clinical trials. J Biomed Sci. (2024) 31:67. doi: 10.1186/s12929-024-01055-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Borg EGF, Liaci AM, Vos HR, Stoorvogel W. A novel three step protocol to isolate extracellular vesicles from plasma or cell culture medium with both high yield and purity. J Extracell Vesicles. (2020) 9:1791450. doi: 10.1080/20013078.2020.1791450, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jafari N, Llevenes P, Denis GV. Exosomes as novel biomarkers in metabolic disease and obesity-related cancers. Nat Rev Endocrinol. (2022) 18:327–8. doi: 10.1038/s41574-022-00666-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh S, Rajendran RL, Mahajan AA, Chowdhury A, Bera A, Guha S, et al. Harnessing exosomes as cancer biomarkers in clinical oncology. Cancer Cell Int. (2024) 24:278. doi: 10.1186/s12935-024-03464-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Zou Y, Song C, Cao K, Cai K, Chen S, et al. Advances in the study of exosomes in cardiovascular diseases. J Adv Res. (2024) 66:133–53. doi: 10.1016/j.jare.2023.12.014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park C, Weerakkody JS, Schneider R, Miao S, Pitt D. CNS cell-derived exosome signatures as blood-based biomarkers of neurodegenerative diseases. Front Neurosci. (2024) 18:1426700. doi: 10.3389/fnins.2024.1426700, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bano A, Vats R, Verma D, Yadav P, Kamboj M, Bhardwaj R. Exploring salivary exosomes as early predictors of oral cancer in susceptible tobacco consumers: noninvasive diagnostic and prognostic applications. J Cancer Res Clin Oncol. (2023) 149:15781–93. doi: 10.1007/s00432-023-05343-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozyk N, Tang KD, Zhang X, Batstone M, Kenny L, Vasani S, et al. Salivary exosomes as biomarkers for early diagnosis of oral squamous cell carcinoma. Oral Oncol Rep. (2023) 6:100017. doi: 10.1016/j.oor.2023.100017 [DOI] [Google Scholar]
- 19.Nijakowski K, Surdacka A. Salivary biomarkers for diagnosis of inflammatory bowel diseases: a systematic review. Int J Mol Sci. (2020) 21:7477. doi: 10.3390/ijms21207477, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun IO, Lerman LO. Urinary extracellular vesicles as biomarkers of kidney disease: from diagnostics to therapeutics. Diagnostics. (2020) 10:311. doi: 10.3390/diagnostics10050311, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee N, Canagasingham A, Bajaj M, Shanmugasundaram R, Hutton A, Bucci J, et al. Urine exosomes as biomarkers in bladder cancer diagnosis and prognosis: from functional roles to clinical significance. Front Oncol. (2022) 12:1019391. doi: 10.3389/fonc.2022.1019391, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO, et al. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. (2009) 100:1603–7. doi: 10.1038/sj.bjc.6605058, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou B, Xu K, Zheng X, Chen T, Wang J, Song Y, et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. (2020) 5:144. doi: 10.1038/s41392-020-00258-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosquera-Heredia MI, Morales LC, Vidal OM, Barcelo E, Silvera-Redondo C, Velez JI, et al. Exosomes: potential disease biomarkers and new therapeutic targets. Biomedicines. (2021) 9:1061. doi: 10.3390/biomedicines9081061, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranyai T, Herczeg K, Onodi Z, Voszka I, Modos K, Marton N, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. (2015) 10:e0145686. doi: 10.1371/journal.pone.0145686, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chhoy P, Brown CW, Amante JJ, Mercurio AM. Protocol for the separation of extracellular vesicles by ultracentrifugation from in vitro cell culture models. STAR Protoc. (2021) 2:100303. doi: 10.1016/j.xpro.2021.100303, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plaschke K, Brenner T, Fiedler MO, Holle T, von der Forst M, Wolf RC, et al. Extracellular vesicles as possible plasma markers and mediators in patients with Sepsis-associated delirium-a pilot study. Int J Mol Sci. (2023) 24:15781. doi: 10.3390/ijms242115781, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares Martins T, Catita J, Martins Rosa I, Silva OAB, Henriques AG. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS One. (2018) 13:e0198820. doi: 10.1371/journal.pone.0198820, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tangwattanachuleeporn M, Muanwien P, Teethaisong Y, Somparn P. Optimizing concentration of Polyethelene glycol for exosome isolation from plasma for downstream application. Medicina (Kaunas). (2022) 58:1600. doi: 10.3390/medicina58111600, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coughlan C, Bruce KD, Burgy O, Boyd TD, Michel CR, Garcia-Perez JE, et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr Protoc Cell Biol. (2020) 88:e110. doi: 10.1002/cpcb.110, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu WZ, Ma ZJ, Kang XW. Current status and outlook of advances in exosome isolation. Anal Bioanal Chem. (2022) 414:7123–41. doi: 10.1007/s00216-022-04253-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdouh M, Hamam D, Gao ZH, Arena V, Arena M, Arena GO. Exosomes isolated from cancer patients' sera transfer malignant traits and confer the same phenotype of primary tumors to oncosuppressor-mutated cells. J Exp Clin Cancer Res. (2017) 36:113. doi: 10.1186/s13046-017-0587-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fiandaca MS, Kapogiannis D, Mapstone M, Boxer A, Eitan E, Schwartz JB, et al. Identification of preclinical Alzheimer's disease by a profile of pathogenic proteins in neurally derived blood exosomes: a case-control study. Alzheimers Dement. (2015) 11:600–7.e1. doi: 10.1016/j.jalz.2014.06.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia L, Zhu M, Kong C, Pang Y, Zhang H, Qiu Q, et al. Blood neuro-exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage. Alzheimers Dement. (2021) 17:49–60. doi: 10.1002/alz.12166, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agliardi C, Guerini FR, Zanzottera M, Bolognesi E, Picciolini S, Caputo D, et al. Myelin basic protein in oligodendrocyte-derived extracellular vesicles as a diagnostic and prognostic biomarker in multiple sclerosis: a pilot study. Int J Mol Sci. (2023) 24:894. doi: 10.3390/ijms24010894, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee EE, Winston-Gray C, Barlow JW, Rissman RA, Jeste DV. Plasma levels of neuron- and astrocyte-derived Exosomal amyloid Beta1-42, amyloid Beta1-40, and phosphorylated tau levels in schizophrenia patients and non-psychiatric comparison subjects: relationships with cognitive functioning and psychopathology. Front Psych. (2020) 11:532624. doi: 10.3389/fpsyt.2020.532624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YT, Shi T, Srivastava S, Kagan J, Liu T, Rodland KD. Proteomic analysis of exosomes for discovery of protein biomarkers for prostate and bladder Cancer. Cancers (Basel). (2020) 12:2335. doi: 10.3390/cancers12092335, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Liu S, Qiao Z, Shang Z, Xia Z, Niu X, et al. Systematic comparison of exosomal proteomes from human saliva and serum for the detection of lung cancer. Anal Chim Acta. (2017) 982:84–95. doi: 10.1016/j.aca.2017.06.005, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Logozzi M, Di Raimo R, Mizzoni D, Fais S. Immunocapture-based ELISA to characterize and quantify exosomes in both cell culture supernatants and body fluids. Methods Enzymol. (2020) 645:155–80. doi: 10.1016/bs.mie.2020.06.011, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elkommos-Zakhary M, Rajesh N, Beljanski V. Exosome RNA sequencing as a tool in the search for cancer biomarkers. Noncoding RNA. (2022) 8:75. doi: 10.3390/ncrna8060075, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang S, Cheng J, Yao Y, Lou C, Wang L, Huang X, et al. Combination of four serum exosomal MiRNAs as novel diagnostic biomarkers for early-stage gastric cancer. Front Genet. (2020) 11:237. doi: 10.3389/fgene.2020.00237, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu Z, Yin H, Zhang H, Zhang H, Liu X, Zeng X, et al. Optimization of a method for the clinical detection of serum exosomal miR-940 as a potential biomarker of breast cancer. Front Oncol. (2022) 12:956167. doi: 10.3389/fonc.2022.956167, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi J, Kantoff PW, Wooster R, Farokhzad OC. Cancer nanomedicine: progress, challenges and opportunities. Nat Rev Cancer. (2017) 17:20–37. doi: 10.1038/nrc.2016.108, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Azzi S, Hebda JK, Gavard J. Vascular permeability and drug delivery in cancers. Front Oncol. (2013) 3:211. doi: 10.3389/fonc.2013.00211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdelsalam M, Ahmed M, Osaid Z, Hamoudi R, Harati R. Insights into exosome transport through the blood-brain barrier and the potential therapeutical applications in brain diseases. Pharmaceuticals (Basel). (2023) 16:571. doi: 10.3390/ph16040571, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu S, Wang Y, Xia X, Zheng JC. Exosome engineering: current progress in cargo loading and targeted delivery. Nanoimpact. (2020) 20:100261. doi: 10.1016/j.impact.2020.100261 [DOI] [Google Scholar]
- 47.Cecchin R, Troyer Z, Witwer K, Morris KV. Extracellular vesicles: the next generation in gene therapy delivery. Mol Ther. (2023) 31:1225–30. doi: 10.1016/j.ymthe.2023.01.021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J. Trends in developing extracellular vesicle-based therapeutics. Brain Tumor Res Treat. (2024) 12:153–61. doi: 10.14791/btrt.2024.0027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z, Wang C, Li T, Liu Z, Li L. Comparison of ultracentrifugation and density gradient separation methods for isolating Tca8113 human tongue cancer cell line-derived exosomes. Oncol Lett. (2014) 8:1701–6. doi: 10.3892/ol.2014.2373, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. (2015) 4:27031. doi: 10.3402/jev.v4.27031, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collino F, Pomatto M, Bruno S, Lindoso RS, Tapparo M, Sicheng W, et al. Exosome and microvesicle-enriched fractions isolated from mesenchymal stem cells by gradient separation showed different molecular signatures and functions on renal tubular epithelial cells. Stem Cell Rev Rep. (2017) 13:226–43. doi: 10.1007/s12015-016-9713-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Busatto S, Vilanilam G, Ticer T, Lin WL, Dickson DW, Shapiro S, et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells. (2018) 7:273. doi: 10.3390/cells7120273, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo H, Zhang J, Yang A, Ouyang W, Long S, Lin X, et al. Large-scale isolation of exosomes derived from NK cells for anti-tumor therapy. Bio Protoc. (2023) 13:e4693. doi: 10.3389/fimmu.2022.1087689, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim JY, Rhim WK, Seo HJ, Lee JY, Park CG, Han DK. Comparative analysis of MSC-derived exosomes depending on cell culture Media for Regenerative Bioactivity. Tissue Eng Regen Med. (2021) 18:355–67. doi: 10.1007/s13770-021-00352-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawai-Harada Y, Nimmagadda V, Harada M. Scalable isolation of surface-engineered extracellular vesicles and separation of free proteins via tangential flow filtration and size exclusion chromatography (TFF-SEC). BMC Methods. (2024) 1:9. doi: 10.1186/s44330-024-00009-0 [DOI] [Google Scholar]
- 56.Ahn SH, Ryu SW, Choi H, You S, Park J, Choi C. Manufacturing therapeutic exosomes: from bench to industry. Mol Cells. (2022) 45:284–90. doi: 10.14348/molcells.2022.2033, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guerreiro EM, Vestad B, Steffensen LA, Aass HCD, Saeed M, Ovstebo R, et al. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS One. (2018) 13:e0204276. doi: 10.1371/journal.pone.0204276, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen VVT, Witwer KW, Verhaar MC, Strunk D, van Balkom BWM. Functional assays to assess the therapeutic potential of extracellular vesicles. J Extracell Vesicles. (2020) 10:e12033. doi: 10.1002/jev2.12033, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. (2018) 7:1535750. doi: 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Welsh JA, Goberdhan DCI, O'Driscoll L, Buzas EI, Blenkiron C, Bussolati B, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. (2024) 13:e12404. doi: 10.1002/jev2.12404, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kowal EJK, Ter-Ovanesyan D, Regev A, Church GM. Extracellular vesicle isolation and analysis by Western blotting. Methods Mol Biol. (2017) 1660:143–52. doi: 10.1007/978-1-4939-7253-1_12, PMID: [DOI] [PubMed] [Google Scholar]
- 62.Takizawa K, Nishimura T, Harita Y. Enzyme-linked immunosorbent assay to detect surface marker proteins of extracellular vesicles purified from human urine. STAR Protoc. (2023) 4:102415. doi: 10.1016/j.xpro.2023.102415, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noble JM, Roberts LM, Vidavsky N, Chiou AE, Fischbach C, Paszek MJ, et al. Direct comparison of optical and electron microscopy methods for structural characterization of extracellular vesicles. J Struct Biol. (2020) 210:107474. doi: 10.1016/j.jsb.2020.107474, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Pol E, Coumans FA, Grootemaat AE, Gardiner C, Sargent IL, Harrison P, et al. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. (2014) 12:1182–92. doi: 10.1111/jth.12602, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Koritzinsky EH, Street JM, Star RA, Yuen PS. Quantification of exosomes. J Cell Physiol. (2017) 232:1587–90. doi: 10.1002/jcp.25387, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rupert DLM, Claudio V, Lasser C, Bally M. Methods for the physical characterization and quantification of extracellular vesicles in biological samples. Biochim Biophys Acta Gen Subj. (1861) 2017:3164–79. doi: 10.1016/j.bbagen.2016.07.028 [DOI] [PubMed] [Google Scholar]
- 67.Fortunato D, Mladenovic D, Criscuoli M, Loria F, Veiman KL, Zocco D, et al. Opportunities and pitfalls of fluorescent labeling methodologies for extracellular vesicle profiling on high-resolution single-particle platforms. Int J Mol Sci. (2021) 22:10510. doi: 10.3390/ijms221910510, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dehghani M, Montange RK, Olszowy MW, Pollard D. An emerging fluorescence-based technique for quantification and protein profiling of extracellular vesicles. SLAS Technol. (2021) 26:189–99. doi: 10.1177/2472630320970458, PMID: [DOI] [PubMed] [Google Scholar]
- 69.Chung IM, Rajakumar G, Venkidasamy B, Subramanian U, Thiruvengadam M. Exosomes: current use and future applications. Clin Chim Acta. (2020) 500:226–32. doi: 10.1016/j.cca.2019.10.022, PMID: [DOI] [PubMed] [Google Scholar]
- 70.Mager E.L.A. S I., Breakefield X.O., and Wood M.J., Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov 12 (2013) 347–357. doi: 10.1038/nrd3978 [DOI] [PubMed] [Google Scholar]
- 71.Aheget H, Tristan-Manzano M, Mazini L, Cortijo-Gutierrez M, Galindo-Moreno P, Herrera C, et al. Exosome: a new player in translational nanomedicine. J Clin Med. (2020) 9:2380. doi: 10.3390/jcm9082380, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tienda-Vazquez MA, Hanel JM, Marquez-Arteaga EM, Salgado-Alvarez AP, Scheckhuber CQ, Alanis-Gomez JR, et al. Exosomes: a promising strategy for repair, regeneration and treatment of skin disorders. Cells. (2023) 12:1625. doi: 10.3390/cells12121625, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang K, Cheng K. Stem cell-derived exosome versus stem cell therapy. Nat Rev Bioeng. (2023) 1:608–9. doi: 10.1038/s44222-023-00064-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X. Exosome therapy and diagnostics: The path to becoming clinical giants. Washington, USA: CAS; (2022). [Google Scholar]
- 75.Nooshabadi VT, Khanmohamadi M, Valipour E, Mahdipour S, Salati A, Malekshahi ZV, et al. Impact of exosome-loaded chitosan hydrogel in wound repair and layered dermal reconstitution in mice animal model. J Biomed Mater Res A. (2020) 108:2138–49. doi: 10.1002/jbm.a.36959, PMID: [DOI] [PubMed] [Google Scholar]
- 76.Tan F, Li X, Wang Z, Li J, Shahzad K, Zheng J. Clinical applications of stem cell-derived exosomes. Signal Transduct Target Ther. (2024) 9:17. doi: 10.1038/s41392-023-01704-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prasai A, Jay JW, Jupiter D, Wolf SE, El Ayadi A. Role of exosomes in dermal wound healing: a systematic review. J Invest Dermatol. (2022) 142:662–678.e8. doi: 10.1016/j.jid.2021.07.167, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shi L, Song D, Meng C, Cheng Y, Wang B, Yang Z. Opportunities and challenges of engineered exosomes for diabetic wound healing. Giant. (2024) 18:100251. doi: 10.1016/j.giant.2024.100251 [DOI] [Google Scholar]
- 79.Fan MH, Pi JK, Zou CY, Jiang YL, Li QJ, Zhang XZ, et al. Hydrogel-exosome system in tissue engineering: a promising therapeutic strategy. Bioact Mater. (2024) 38:1–30. doi: 10.1016/j.bioactmat.2024.04.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xie Y, Guan Q, Guo J, Chen Y, Yin Y, Han X. Hydrogels for exosome delivery in biomedical applications. Gels. (2022) 8:328. doi: 10.3390/gels8060328, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khayambashi P, Iyer J, Pillai S, Upadhyay A, Zhang Y, Tran SD. Hydrogel encapsulation of mesenchymal stem cells and their derived exosomes for tissue engineering. Int J Mol Sci. (2021) 22:684. doi: 10.3390/ijms22020684, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang K, Zhao X, Chen X, Wei Y, Du W, Wang Y, et al. Enhanced therapeutic effects of mesenchymal stem cell-derived exosomes with an injectable hydrogel for Hindlimb ischemia treatment. ACS Appl Mater Interfaces. (2018) 10:30081–91. doi: 10.1021/acsami.8b08449, PMID: [DOI] [PubMed] [Google Scholar]
- 83.Yang J, Chen Z, Pan D, Li H, Shen J. Umbilical cord-derived mesenchymal stem cell-derived exosomes combined Pluronic F127 hydrogel promote chronic diabetic wound healing and complete skin regeneration. Int J Nanomedicine. (2020) 15:5911–26. doi: 10.2147/IJN.S249129, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y, Zhang P, Gao X, Chang L, Chen Z, Mei X. Preparation of exosomes encapsulated nanohydrogel for accelerating wound healing of diabetic rats by promoting angiogenesis. Mater Sci Eng C. (2021) 120:111671. doi: 10.1016/j.msec.2020.111671, PMID: [DOI] [PubMed] [Google Scholar]
- 85.Wang C, Wang M, Xu T, Zhang X, Lin C, Gao W, et al. Engineering bioactive self-healing antibacterial exosomes hydrogel for promoting chronic diabetic wound healing and complete skin regeneration. Theranostics. (2019) 9:65–76. doi: 10.7150/thno.29766, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li P, Lv S, Jiang W, Si L, Liao B, Zhao G, et al. Exosomes derived from umbilical cord mesenchymal stem cells protect cartilage and regulate the polarization of macrophages in osteoarthritis. Ann Transl Med. (2022) 10:976. doi: 10.21037/atm-22-3912, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cao H, Chen M, Cui X, Liu Y, Liu Y, Deng S, et al. Cell-free osteoarthritis treatment with sustained-release of chondrocyte-targeting exosomes from umbilical cord-derived mesenchymal stem cells to rejuvenate aging chondrocytes. ACS Nano. (2023) 17:13358–76. doi: 10.1021/acsnano.3c01612, PMID: [DOI] [PubMed] [Google Scholar]
- 88.Chen P, Zheng L, Wang Y, Tao M, Xie Z, Xia C, et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. (2019) 9:2439–59. doi: 10.7150/thno.31017, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu KY, Ahmad H, Lin G, Carbonneau M, Tran SD. Mesenchymal stem cell-derived exosomes in ophthalmology: a comprehensive review. Pharmaceutics. (2023) 15:1167. doi: 10.3390/pharmaceutics15041167, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Di G, Du X, Qi X, Zhao X, Duan H, Li S, et al. Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG-6-dependent stem cell activation and macrophage switch. Invest Ophthalmol Vis Sci. (2017) 58:4344–54. doi: 10.1167/iovs.17-21506, PMID: [DOI] [PubMed] [Google Scholar]
- 91.Samaeekia R, Rabiee B, Putra I, Shen X, Park YJ, Hematti P, et al. Effect of human corneal mesenchymal stromal cell-derived exosomes on corneal epithelial wound healing. Invest Ophthalmol Vis Sci. (2018) 59:5194–200. doi: 10.1167/iovs.18-24803, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mead B, Tomarev S. Bone marrow-derived mesenchymal stem cells-derived exosomes promote survival of retinal ganglion cells through miRNA-dependent mechanisms. Stem Cells Transl Med. (2017) 6:1273–85. doi: 10.1002/sctm.16-0428, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang T, Zhou Y, Zhang W, Xue Y, Xiao Z, Zhou Y, et al. Exosomes and exosome composite scaffolds in periodontal tissue engineering. Front Bioeng Biotechnol. (2023) 11:1287714. doi: 10.3389/fbioe.2023.1287714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin H, Chen H, Zhao X, Ding T, Wang Y, Chen Z, et al. Advances of exosomes in periodontitis treatment. J Transl Med. (2022) 20:279. doi: 10.1186/s12967-022-03487-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhai LY, Li MX, Pan WL, Chen Y, Li MM, Pang JX, et al. In situ detection of plasma Exosomal MicroRNA-1246 for breast Cancer diagnostics by a au Nanoflare probe. ACS Appl Mater Interfaces. (2018) 10:39478–86. doi: 10.1021/acsami.8b12725, PMID: [DOI] [PubMed] [Google Scholar]
- 96.Sandfeld-Paulsen B, Jakobsen KR, Baek R, Folkersen BH, Rasmussen TR, Meldgaard P, et al. Exosomal proteins as diagnostic biomarkers in lung Cancer. J Thorac Oncol. (2016) 11:1701–10. doi: 10.1016/j.jtho.2016.05.034, PMID: [DOI] [PubMed] [Google Scholar]
- 97.Akbar S, Raza A, Mohsin R, Kanbour A, Qadri S, Parray A, et al. Circulating exosomal immuno-oncological checkpoints and cytokines are potential biomarkers to monitor tumor response to anti-PD-1/PD-L1 therapy in non-small cell lung cancer patients. Front Immunol. (2022) 13:1097117. doi: 10.3389/fimmu.2022.1097117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Risha Y, Minic Z, Ghobadloo SM, Berezovski MV. The proteomic analysis of breast cell line exosomes reveals disease patterns and potential biomarkers. Sci Rep. (2020) 10:13572. doi: 10.1038/s41598-020-70393-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hung Y, Wang Y-L, Lin Y-Z, Chiang S-F, Wu W-R, Wang S-C. The exosomal compartment protects epidermal growth factor receptor from small molecule inhibitors. Biochem Biophys Res Commun. (2019) 510:42–7. doi: 10.1016/j.bbrc.2018.12.187, PMID: [DOI] [PubMed] [Google Scholar]
- 100.Hannafon BN, Trigoso YD, Calloway CL, Zhao YD, Lum DH, Welm AL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. (2016) 18:90. doi: 10.1186/s13058-016-0753-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gobbo J, Marcion G, Cordonnier M, Dias AMM, Pernet N, Hammann A, et al. Restoring anticancer immune response by targeting tumor-derived exosomes with a HSP70 peptide Aptamer. JNCI J Natl Cancer Inst. (2015) 108:djv330. doi: 10.1093/jnci/djv330, PMID: [DOI] [PubMed] [Google Scholar]
- 102.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. (2015) 523:177–82. doi: 10.1038/nature14581, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ueda H, Takahashi H, Kobayashi S, Kubo M, Sasaki K, Iwagami Y, et al. miR-6855-5p enhances Radioresistance and promotes migration of pancreatic Cancer by inducing epithelial-mesenchymal transition via suppressing FOXA1: potential of plasma Exosomal miR-6855-5p as an indicator of radiosensitivity in patients with pancreatic cancer. Ann Surg Oncol. (2024) 32:720–35. doi: 10.1245/s10434-024-16115-w [DOI] [PubMed] [Google Scholar]
- 104.Dash S, Wu CC, Wu CC, Chiang SF, Lu YT, Yeh CY, et al. Extracellular vesicle membrane protein profiling and targeted mass spectrometry unveil CD59 and Tetraspanin 9 as novel plasma biomarkers for detection of colorectal cancer. Cancers. (2022) 15:177. doi: 10.3390/cancers15010177, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Overbye A, Skotland T, Koehler CJ, Thiede B, Seierstad T, Berge V, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. (2015) 6:30357–76. doi: 10.18632/oncotarget.4851, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. (2013) 13:421. doi: 10.1186/1471-2407-13-421, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Whiteside TL. Exosomes and tumor-mediated immune suppression. J Clin Invest. (2016) 126:1216–23. doi: 10.1172/JCI81136, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hao Q, Wu Y, Wu Y, Wang P, Vadgama JV. Tumor-derived exosomes in tumor-induced immune suppression. Int J Mol Sci. (2022) 23:1416. doi: 10.3390/ijms23031461, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, et al. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. (2006) 66:11432–40. doi: 10.1158/0008-5472.CAN-06-1867, PMID: [DOI] [PubMed] [Google Scholar]
- 110.Mantovani A, Marchesi F, Porta C, Sica A, Allavena P. Inflammation and cancer: breast cancer as a prototype. Breast. (2007) 16:27–33. doi: 10.1016/j.breast.2007.07.013, PMID: [DOI] [PubMed] [Google Scholar]
- 111.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel). (2014) 6:1670–90. doi: 10.3390/cancers6031670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. (2006) 42:717–27. doi: 10.1016/j.ejca.2006.01.003, PMID: [DOI] [PubMed] [Google Scholar]
- 113.Ham S, Lima LG, Chai EPZ, Muller A, Lobb RJ, Krumeich S, et al. Breast Cancer-derived exosomes Alter macrophage polarization via gp130/STAT3 signaling. Front Immunol. (2018) 9:871. doi: 10.3389/fimmu.2018.00871, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Piao YJ, Kim HS, Hwang EH, Woo J, Zhang M, Moon WK. Breast cancer cell-derived exosomes and macrophage polarization are associated with lymph node metastasis. Oncotarget. (2018) 9:7398–410. doi: 10.18632/oncotarget.23238, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annu Rev Cell Dev Biol. (2011) 27:347–76. doi: 10.1146/annurev-cellbio-092910-154036, PMID: [DOI] [PubMed] [Google Scholar]
- 116.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. (2009) 139:871–90. doi: 10.1016/j.cell.2009.11.007, PMID: [DOI] [PubMed] [Google Scholar]
- 117.Shen Y, TanTai J. Exosomes secreted by metastatic cancer cells promotes epithelial mesenchymal transition in small cell lung carcinoma: the key role of Src/TGF-beta1 axis. Gene. (2024) 892:147873. doi: 10.1016/j.gene.2023.147873, PMID: [DOI] [PubMed] [Google Scholar]
- 118.Franzen CA, Blackwell RH, Todorovic V, Greco KA, Foreman KE, Flanigan RC, et al. Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis. (2015) 4:e163. doi: 10.1038/oncsis.2015.21, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci USA. (2013) 110:17380–5. doi: 10.1073/pnas.1304266110, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liu D, Li C, Trojanowicz B, Li X, Shi D, Zhan C, et al. CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric Cancer. (2016) 19:754–66. doi: 10.1007/s10120-015-0523-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rahman MA, Barger JF, Lovat F, Gao M, Otterson GA, Nana-Sinkam P. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget. (2016) 7:54852–66. doi: 10.18632/oncotarget.10243, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cao M, Seike M, Soeno C, Mizutani H, Kitamura K, Minegishi Y, et al. MiR-23a regulates TGF-beta-induced epithelial-mesenchymal transition by targeting E-cadherin in lung cancer cells. Int J Oncol. (2012) 41:869–75. doi: 10.3892/ijo.2012.1535, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim J, Kim TY, Lee MS, Mun JY, Ihm C, Kim SA. Exosome cargo reflects TGF-beta1-mediated epithelial-to-mesenchymal transition (EMT) status in A549 human lung adenocarcinoma cells. Biochem Biophys Res Commun. (2016) 478:643–8. doi: 10.1016/j.bbrc.2016.07.124, PMID: [DOI] [PubMed] [Google Scholar]
- 124.Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, et al. Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One. (2010) 5:e13247. doi: 10.1371/journal.pone.0013247, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tanaka S, Hosokawa M, Ueda K, Iwakawa S. Effects of Decitabine on invasion and Exosomal expression of miR-200c and miR-141 in Oxaliplatin-resistant colorectal Cancer cells. Biol Pharm Bull. (2015) 38:1272–9. doi: 10.1248/bpb.b15-00129, PMID: [DOI] [PubMed] [Google Scholar]
- 126.Xiao D, Barry S, Kmetz D, Egger M, Pan J, Rai SN, et al. Melanoma cell-derived exosomes promote epithelial-mesenchymal transition in primary melanocytes through paracrine/autocrine signaling in the tumor microenvironment. Cancer Lett. (2016) 376:318–27. doi: 10.1016/j.canlet.2016.03.050, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. (2014) 25:501–15. doi: 10.1016/j.ccr.2014.03.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kumar S, Dhar R, Kumar L, Shivji GG, Jayaraj R, Devi A. Theranostic signature of tumor-derived exosomes in cancer. Med Oncol. (2023) 40:321. doi: 10.1007/s12032-023-02176-6, PMID: [DOI] [PubMed] [Google Scholar]
- 129.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2022) 74:229–63. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 130.Shin H, Oh S, Hong S, Kang M, Kang D, Ji YG, et al. Early-stage lung Cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano. (2020) 14:5435–44. doi: 10.1021/acsnano.9b09119, PMID: [DOI] [PubMed] [Google Scholar]
- 131.Hai J, Zhu CQ, Bandarchi B, Wang YH, Navab R, Shepherd FA, et al. L1 cell adhesion molecule promotes tumorigenicity and metastatic potential in non-small cell lung cancer. Clin Cancer Res. (2012) 18:1914–24. doi: 10.1158/1078-0432.CCR-11-2893, PMID: [DOI] [PubMed] [Google Scholar]
- 132.Tischler V, Pfeifer M, Hausladen S, Schirmer U, Bonde AK, Kristiansen G, et al. L1CAM protein expression is associated with poor prognosis in non-small cell lung cancer. Mol Cancer. (2011) 10:127. doi: 10.1186/1476-4598-10-127, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Y, Ding W, Wang J, Ao X, Xue J. Non-coding RNAs in lung cancer: molecular mechanisms and clinical applications. Front Oncol. (2023) 13:1256537. doi: 10.3389/fonc.2023.1256537, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Makler A, Asghar W. Exosomal biomarkers for cancer diagnosis and patient monitoring. Expert Rev Mol Diagn. (2020) 20:387–400. doi: 10.1080/14737159.2020.1731308, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee CH, Im EJ, Moon PG, Baek MC. Discovery of a diagnostic biomarker for colon cancer through proteomic profiling of small extracellular vesicles. BMC Cancer. (2018) 18:1058. doi: 10.1186/s12885-018-4952-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xu Y, Lou J, Yu M, Jiang Y, Xu H, Huang Y, et al. Urinary exosomes diagnosis of urological tumors: a systematic review and meta-analysis. Front Oncol. (2021) 11:734587. doi: 10.3389/fonc.2021.734587, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hiltbrunner S, Mints M, Eldh M, Rosenblatt R, Holmstrom B, Alamdari F, et al. Urinary exosomes from bladder cancer patients show a residual cancer phenotype despite complete pathological downstaging. Sci Rep. (2020) 10:5960. doi: 10.1038/s41598-020-62753-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shusharina N, Yukhnenko D, Botman S, Sapunov V, Savinov V, Kamyshov G, et al. Modern methods of diagnostics and treatment of neurodegenerative diseases and depression. Diagnostics. (2023) 13:573. doi: 10.3390/diagnostics13030573, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Konickova D, Mensikova K, Tuckova L, Henykova E, Strnad M, Friedecky D, et al. Biomarkers of neurodegenerative diseases: biology, taxonomy, clinical relevance, and current research status. Biomedicines. (2022) 10:1760. doi: 10.3390/biomedicines10071760, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao P, Li X, Du X, Liu S, Xu Y. Diagnostic and therapeutic potential of exosomes in neurodegenerative diseases. Front Aging Neurosci. (2021) 13:790863. doi: 10.3389/fnagi.2021.790863, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rastogi S, Sharma V, Bharti PS, Rani K, Modi GP, Nikolajeff F, et al. The evolving landscape of exosomes in neurodegenerative diseases: exosomes characteristics and a promising role in early diagnosis. Int J Mol Sci. (2021) 22:440. doi: 10.3390/ijms22010440, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Geekiyanage H, Jicha GA, Nelson PT, Chan C. Blood serum miRNA: non-invasive biomarkers for Alzheimer's disease. Exp Neurol. (2012) 235:491–6. doi: 10.1016/j.expneurol.2011.11.026, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manna I, De Benedittis S, Quattrone A, Maisano D, Iaccino E, Quattrone A. Exosomal miRNAs as potential diagnostic biomarkers in Alzheimer's disease. Pharmaceuticals. (2020) 13:243. doi: 10.3390/ph13090243, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Agarwal M, Khan S. Plasma lipids as biomarkers for Alzheimer's disease: a systematic review. Cureus. (2020) 12:e12008. doi: 10.7759/cureus.12008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang J, et al. Concordance between the assessment of Abeta42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. (2019) 15:1071–80. doi: 10.1016/j.jalz.2019.05.002, PMID: [DOI] [PubMed] [Google Scholar]
- 146.Nila IS, Sumsuzzman DM, Khan ZA, Jung JH, Kazema AS, Kim SJ, et al. Identification of exosomal biomarkers and its optimal isolation and detection method for the diagnosis of Parkinson's disease: a systematic review and meta-analysis. Ageing Res Rev. (2022) 82:101764. doi: 10.1016/j.arr.2022.101764, PMID: [DOI] [PubMed] [Google Scholar]
- 147.Barbo M, Ravnik-Glavac M. Extracellular vesicles as potential biomarkers in amyotrophic lateral sclerosis. Genes. (2023) 14:325. doi: 10.3390/genes14020325, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zafar MN, Abuwatfa WH, Husseini GA. Acoustically-activated liposomal Nanocarriers to mitigate the side effects of conventional chemotherapy with a focus on emulsion-liposomes. Pharmaceutics. (2023) 15:421. doi: 10.3390/pharmaceutics15020421, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Srivastava A, Amreddy N, Razaq M, Towner R, Zhao YD, Ahmed RA, et al. Exosomes as Theranostics for lung Cancer. Adv Cancer Res. (2018) 139:1–33. doi: 10.1016/bs.acr.2018.04.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. (2022) 15:83. doi: 10.1186/s13045-022-01305-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Maqsood Q, Sumrin A, Saleem Y, Wajid A, Mahnoor M. Exosomes in Cancer: diagnostic and therapeutic applications. Clin Med Insights Oncol. (2024) 18:11795549231215966. doi: 10.1177/11795549231215966, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cao Y, Xu P, Shen Y, Wu W, Chen M, Wang F, et al. Exosomes and cancer immunotherapy: a review of recent cancer research. Front Oncol. (2022) 12:1118101. doi: 10.3389/fonc.2022.1118101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Araldi RP, Delvalle DA, da Costa VR, Alievi AL, Teixeira MR, Dias Pinto JR, et al. Exosomes as a Nano-Carrier for chemotherapeutics: a new era of oncology. Cells. (2023) 12:2411. doi: 10.3390/cells12172144, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hadla M, Palazzolo S, Corona G, Caligiuri I, Canzonieri V, Toffoli G, et al. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine. (2016) 11:2431–41. doi: 10.2217/nnm-2016-0154, PMID: [DOI] [PubMed] [Google Scholar]
- 155.Srivastava A, Amreddy N, Babu A, Panneerselvam J, Mehta M, Muralidharan R, et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci Rep. (2016) 6:38541. doi: 10.1038/srep38541, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim MS, Haney MJ, Zhao Y, Mahajan V, Deygen I, Klyachko NL, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. (2016) 12:655–64. doi: 10.1016/j.nano.2015.10.012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Aspe JR, Diaz Osterman CJ, Jutzy JM, Deshields S, Whang S, Wall NR. Enhancement of gemcitabine sensitivity in pancreatic adenocarcinoma by novel exosome-mediated delivery of the Survivin-T34A mutant. J Extracell Vesicles. (2014) 3:1–9. doi: 10.3402/jev.v3.23244, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Geis-Asteggiante L, Belew AT, Clements VK, Edwards NJ, Ostrand-Rosenberg S, El-Sayed NM, et al. Differential content of proteins, mRNAs, and miRNAs suggests that MDSC and their exosomes may mediate distinct immune suppressive functions. J Proteome Res. (2018) 17:486–98. doi: 10.1021/acs.jproteome.7b00646, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O'Brien KP, Khan S, Gilligan KE, Zafar H, Lalor P, Glynn C, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. (2018) 37:2137–49. doi: 10.1038/s41388-017-0116-9, PMID: [DOI] [PubMed] [Google Scholar]
- 160.Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y, et al. Exosomes derived from human umbilical cord mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit pancreatic ductal adenocarcinoma progression. Cancer Lett. (2019) 442:351–61. doi: 10.1016/j.canlet.2018.10.039, PMID: [DOI] [PubMed] [Google Scholar]
- 161.Song H, Liu B, Dong B, Xu J, Zhou H, Na S, et al. Exosome-based delivery of natural products in Cancer therapy. Front Cell Dev Biol. (2021) 9:650426. doi: 10.3389/fcell.2021.650426, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mansouri K, Rasoulpoor S, Daneshkhah A, Abolfathi S, Salari N, Mohammadi M, et al. Clinical effects of curcumin in enhancing cancer therapy: a systematic review. BMC Cancer. (2020) 20:791. doi: 10.1186/s12885-020-07256-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Osterman CJ, Lynch JC, Leaf P, Gonda A, Ferguson Bennit HR, Griffiths D, et al. Curcumin Modulates Pancreatic Adenocarcinoma Cell-Derived Exosomal Function. PLoS One. (2015) 10:e0132845. doi: 10.1371/journal.pone.0132845, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol Ther. (2011) 19:1769–79. doi: 10.1038/mt.2011.164, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shahraki K, Boroumand PG, Lotfi H, Radnia F, Shahriari H, Sargazi S, et al. An update in the applications of exosomes in cancer theranostics: from research to clinical trials. J Cancer Res Clin Oncol. (2023) 149:8087–116. doi: 10.1007/s00432-023-04701-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Onco Targets Ther. (2016) 5:e1071008. doi: 10.1080/2162402X.2015.1071008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zeissig MN, Ashwood LM, Kondrashova O, Sutherland KD. Next batter up! Targeting cancers with KRAS-G12D mutations. Trends Cancer. (2023) 9:955–67. doi: 10.1016/j.trecan.2023.07.010, PMID: [DOI] [PubMed] [Google Scholar]
- 168.Han Y, Liu D, Li L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res. (2020) 10:727–42. PMID: [PMC free article] [PubMed] [Google Scholar]
- 169.Qiu Y, Yang Y, Yang R, Liu C, Hsu J-M, Jiang Z, et al. Activated T cell-derived exosomal PD-1 attenuates PD-L1-induced immune dysfunction in triple-negative breast cancer. Oncogene. (2021) 40:4992–5001. doi: 10.1038/s41388-021-01896-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of extracellular vesicles across the blood-brain barrier: brain pharmacokinetics and effects of inflammation. Int J Mol Sci. (2020) 21:4407. doi: 10.3390/ijms21124407, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Fayazi N, Sheykhhasan M, Soleimani Asl S, Najafi R. Stem cell-derived exosomes: a new strategy of neurodegenerative disease treatment. Mol Neurobiol. (2021) 58:3494–514. doi: 10.1007/s12035-021-02324-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Luarte A, Batiz LF, Wyneken U, Lafourcade C. Potential therapies by stem cell-derived exosomes in CNS diseases: focusing on the neurogenic niche. Stem Cells Int. (2016) 2016:5736059. doi: 10.1155/2016/5736059, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mohamed AS, Abdel-Fattah DS, Abdel-Aleem GA, El-Sheikh TF, Elbatch MM. Biochemical study of the effect of mesenchymal stem cells-derived exosome versus L-Dopa in experimentally induced Parkinson's disease in rats. Mol Cell Biochem. (2023) 478:2795–811. doi: 10.1007/s11010-023-04700-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang X, Yang G. Bone marrow mesenchymal stem cells-derived exosomes reduce Abeta deposition and improve cognitive function recovery in mice with Alzheimer's disease by activating sphingosine kinase/sphingosine-1-phosphate signaling pathway. Cell Biol Int. (2021) 45:775–84. doi: 10.1002/cbin.11522, PMID: [DOI] [PubMed] [Google Scholar]
- 175.Elia CA, Tamborini M, Rasile M, Desiato G, Marchetti S, Swuec P, et al. Intracerebral injection of extracellular vesicles from mesenchymal stem cells exerts reduced Abeta plaque burden in early stages of a preclinical model of Alzheimer's disease. Cells. (2019) 8:1059. doi: 10.3390/cells8091059, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ma X, Huang M, Zheng M, Dai C, Song Q, Zhang Q, et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer's disease. J Control Release. (2020) 327:688–702. doi: 10.1016/j.jconrel.2020.09.019, PMID: [DOI] [PubMed] [Google Scholar]
- 177.Chen YA, Lu CH, Ke CC, Chiu SJ, Jeng FS, Chang CW, et al. Mesenchymal stem cell-derived exosomes ameliorate Alzheimer's disease pathology and improve cognitive deficits. Biomedicines. (2021) 9:594. doi: 10.3390/biomedicines9060594, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhai L, Shen H, Sheng Y, Guan Q. ADMSC Exo-MicroRNA-22 improve neurological function and neuroinflammation in mice with Alzheimer's disease. J Cell Mol Med. (2021) 25:7513–23. doi: 10.1111/jcmm.16787, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cone AS, Yuan X, Sun L, Duke LC, Vreones MP, Carrier AN, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer's disease-like phenotypes in a preclinical mouse model. Theranostics. (2021) 11:8129–42. doi: 10.7150/thno.62069, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tsivion-Visbord H, Perets N, Sofer T, Bikovski L, Goldshmit Y, Ruban A, et al. Mesenchymal stem cells derived extracellular vesicles improve behavioral and biochemical deficits in a phencyclidine model of schizophrenia. Transl Psychiatry. (2020) 10:305. doi: 10.1038/s41398-020-00988-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Xie X, Song Q, Dai C, Cui S, Tang R, Li S, et al. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer's disease: a phase I/II clinical trial. Gen Psychiatr. (2023) 36:e101143. doi: 10.1136/gpsych-2023-101143, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zheng X, Sun K, Liu Y, Yin X, Zhu H, Yu F, et al. Resveratrol-loaded macrophage exosomes alleviate multiple sclerosis through targeting microglia. J Control Release. (2023) 353:675–84. doi: 10.1016/j.jconrel.2022.12.026, PMID: [DOI] [PubMed] [Google Scholar]
- 183.Casella G, Colombo F, Finardi A, Descamps H, Ill-Raga G, Spinelli A, et al. Extracellular vesicles containing IL-4 modulate neuroinflammation in a mouse model of multiple sclerosis. Mol Ther. (2018) 26:2107–18. doi: 10.1016/j.ymthe.2018.06.024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Borghi C, Agabiti-Rosei E, Johnson RJ, Kielstein JT, Lurbe E, Mancia G, et al. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. (2020) 80:1–11. doi: 10.1016/j.ejim.2020.07.006, PMID: [DOI] [PubMed] [Google Scholar]
- 185.Tedeschi A, Agostoni P, Pezzuto B, Corra U, Scrutinio D, La Gioia R, et al. Role of comorbidities in heart failure prognosis part 2: chronic kidney disease, elevated serum uric acid. Eur J Prev Cardiol. (2020) 27:35–45. doi: 10.1177/2047487320957793, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang L, Liu J, Xu B, Liu YL, Liu Z. Reduced exosome miR-425 and miR-744 in the plasma represents the progression of fibrosis and heart failure. Kaohsiung J Med Sci. (2018) 34:626–33. doi: 10.1016/j.kjms.2018.05.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bohatá J, Horváthová V, Pavlíková M, Stibůrková B. Circulating microRNA alternations in primary hyperuricemia and gout. Arthritis Res Ther. (2021) 23:186. doi: 10.1186/s13075-021-02569-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen Z, Shi J, Huang X, Yang Y, Cheng Y, Qu Y, et al. Exosomal miRNAs in patients with chronic heart failure and hyperuricemia and the underlying mechanisms. Gene. (2025) 933:148920. doi: 10.1016/j.gene.2024.148920, PMID: [DOI] [PubMed] [Google Scholar]
- 189.Zhao Y, Xu J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int Orthop. (2018) 42:2865–72. doi: 10.1007/s00264-018-4093-6, PMID: [DOI] [PubMed] [Google Scholar]
- 190.Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. (2017) 7:2029. doi: 10.1038/s41598-017-01905-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, Katz JN, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA biomarkers consortium. Ann Rheum Dis. (2017) 76:186–95. doi: 10.1136/annrheumdis-2016-209252, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Cao Y, Liao S, Deng C, Qin H, Li Y. A pH-responsive phase-transition bi-affinity nanopolymer-assisted exosome metabolomics for early screening of osteoarthritis. Talanta. (2025) 283:127144. doi: 10.1016/j.talanta.2024.127144, PMID: [DOI] [PubMed] [Google Scholar]
- 193.Zou Z, Li H, Xu G, Hu Y, Zhang W, Tian K. Current knowledge and future perspectives of exosomes as Nanocarriers in diagnosis and treatment of diseases. Int J Nanomedicine. (2023) 18:4751–78. doi: 10.2147/IJN.S417422, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang Q, Cheng S, Qin F, Fu A, Fu C. Application progress of RVG peptides to facilitate the delivery of therapeutic agents into the central nervous system. RSC Adv. (2021) 11:8505–15. doi: 10.1039/D1RA00550B, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cui GH, Guo HD, Li H, Zhai Y, Gong ZB, Wu J, et al. RVG-modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease. Immun Ageing. (2019) 16:10. doi: 10.1186/s12979-019-0150-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. (2021) 11:3183–95. doi: 10.7150/thno.52570, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu L, Li Y, Peng H, Liu R, Ji W, Shi Z, et al. Targeted exosome coating gene-chem nanocomplex as "nanoscavenger" for clearing α-synuclein and immune activation of Parkinson's disease. Sci Adv. (2020) 6:eaba3967. doi: 10.1126/sciadv.aba3967, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gomes ER, Souza FR, Cassali GD, Sabino AP, Barros ALB, Oliveira MC. Investigation of the antitumor activity and toxicity of tumor-derived exosomes fused with Long-circulating and pH-sensitive liposomes containing doxorubicin. Pharmaceutics. (2022) 14:2256. doi: 10.3390/pharmaceutics14112256, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li L, He D, Guo Q, Zhang Z, Ru D, Wang L, et al. Exosome-liposome hybrid nanoparticle codelivery of TP and miR497 conspicuously overcomes chemoresistant ovarian cancer. J Nanobiotechnol. (2022) 20:50. doi: 10.1186/s12951-022-01264-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wang X, Li D, Li G, Chen J, Yang Y, Bian L, et al. Enhanced therapeutic potential of hybrid exosomes loaded with paclitaxel for Cancer therapy. Int J Mol Sci. (2024) 25:3645. doi: 10.3390/ijms25073645, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lv Q, Cheng L, Lu Y, Zhang X, Wang Y, Deng J, et al. Thermosensitive exosome-liposome hybrid nanoparticle-mediated chemoimmunotherapy for improved treatment of metastatic peritoneal cancer. Adv Sci. (2020) 7:2000515. doi: 10.1002/advs.202000515, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. (2019) 94:482–94. doi: 10.1016/j.actbio.2019.05.054, PMID: [DOI] [PubMed] [Google Scholar]
- 203.Zhou X, Zhuang Y, Liu X, Gu Y, Wang J, Shi Y, et al. Study on tumour cell-derived hybrid exosomes as dasatinib nanocarriers for pancreatic cancer therapy. Artif Cells Nanomed Biotechnol. (2023) 51:532–46. doi: 10.1080/21691401.2023.2264358, PMID: [DOI] [PubMed] [Google Scholar]
- 204.Zhang W, Song Q, Bi X, Cui W, Fang C, Gao J, et al. Preparation of Pueraria lobata root-derived exosome-like Nanovesicles and evaluation of their effects on mitigating alcoholic intoxication and promoting alcohol metabolism in mice. Int J Nanomedicine. (2024) 19:4907–21. doi: 10.2147/IJN.S462602, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang D, Zhang H, Liao X, Li J, Zeng J, Wang Y, et al. Oral administration of Robinia pseudoacacia L. flower exosome-like nanoparticles attenuates gastric and small intestinal mucosal ferroptosis caused by hypoxia through inhibiting HIF-1α- and HIF-2α-mediated lipid peroxidation. J Nanobiotechnol. (2024) 22:479. doi: 10.1186/s12951-024-02663-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Brogyanyi T, Kejík Z, Veselá K, Dytrych P, Hoskovec D, Masařik M, et al. Iron chelators as mitophagy agents: potential and limitations. Biomed Pharmacother. (2024) 179:117407. doi: 10.1016/j.biopha.2024.117407, PMID: [DOI] [PubMed] [Google Scholar]
- 207.Li J, Cao F, Yin H, Huang Z, Lin Z, Mao N, et al. Ferroptosis: past, present and future. Cell Death Dis. (2020) 11:88. doi: 10.1038/s41419-020-2298-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ahima RS, Lazar MA. The health risk of obesity—better metrics imperative. Science. (2013) 341:856–8. doi: 10.1126/science.1241244, PMID: [DOI] [PubMed] [Google Scholar]
- 209.Matarese G. The link between obesity and autoimmunity. Science. (2023) 379:1298–300. doi: 10.1126/science.ade0113, PMID: [DOI] [PubMed] [Google Scholar]
- 210.Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. (2019) 15:288–98. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 211.Wang J, Zhang T, Gu R, Ke Y, Zhang S, Su X, et al. Development and evaluation of reconstructed Nanovesicles from turmeric for multifaceted obesity intervention. ACS Nano. (2024) 18:23117–35. doi: 10.1021/acsnano.4c05309, PMID: [DOI] [PubMed] [Google Scholar]
- 212.Zheng M, Chavda VP, Vaghela DA, Bezbaruah R, Gogoi NR, Patel K, et al. Plant-derived exosomes in therapeutic nanomedicine, paving the path toward precision medicine. Phytomedicine. (2024) 135:156087. doi: 10.1016/j.phymed.2024.156087, PMID: [DOI] [PubMed] [Google Scholar]
- 213.Hazas MCLDL, Tome-Carneiro J, Del Pozo-Acebo L, Del Saz-Lara A, Chapado LA, Balaguer L, et al. Therapeutic potential of plant-derived extracellular vesicles as nanocarriers for exogenous miRNAs. Pharmacol Res. (2023) 198:106999. doi: 10.1016/j.phrs.2023.106999 [DOI] [PubMed] [Google Scholar]
- 214.Gao C, Zhou Y, Chen Z, Li H, Xiao Y, Hao W, et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics. (2022) 12:5596–614. doi: 10.7150/thno.73650, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zipkin M. Big pharma buys into exosomes for drug delivery. Nat Biotechnol. (2020) 38:1226–8. doi: 10.1038/s41587-020-0725-7, PMID: [DOI] [PubMed] [Google Scholar]
- 216.Greening DW, Xu R, Ji H, Tauro BJ, Simpson RJ. A protocol for exosome isolation and characterization: evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol Biol. (2015) 1295:179–209. doi: 10.1007/978-1-4939-2550-6_15, PMID: [DOI] [PubMed] [Google Scholar]
- 217.Onodi Z, Pelyhe C, Terezia Nagy C, Brenner GB, Almasi L, Kittel A, et al. Isolation of high-purity extracellular vesicles by the combination of Iodixanol density gradient ultracentrifugation and bind-elute chromatography from blood plasma. Front Physiol. (2018) 9:1479. doi: 10.3389/fphys.2018.01479, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Shu S, Allen CL, Benjamin-Davalos S, Koroleva M, MacFarland D, Minderman H, et al. A rapid exosome isolation using ultrafiltration and size exclusion chromatography (REIUS) method for exosome isolation from melanoma cell lines. Methods Mol Biol. (2021) 2265:289–304. doi: 10.1007/978-1-0716-1205-7_22, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Cheruvanky A, Zhou H, Pisitkun T, Kopp JB, Knepper MA, Yuen PS, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol. (2007) 292:F1657–61. doi: 10.1152/ajprenal.00434.2006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nordin JZ, Lee Y, Vader P, Mager I, Johansson HJ, Heusermann W, et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. (2015) 11:879–83. doi: 10.1016/j.nano.2015.01.003, PMID: [DOI] [PubMed] [Google Scholar]
- 221.Ansari FJ, Tafti HA, Amanzadeh A, Rabbani S, Shokrgozar MA, Heidari R, et al. Comparison of the efficiency of ultrafiltration, precipitation, and ultracentrifugation methods for exosome isolation. Biochem Biophys Rep. (2024) 38:101668. doi: 10.1016/j.bbrep.2024.101668, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Contreras H, Alarcón-Zapata P, Nova-Lamperti E, Ormazabal V, Varas-Godoy M, Salomon C, et al. Comparative study of size exclusion chromatography for isolation of small extracellular vesicle from cell-conditioned media, plasma, urine, and saliva. Front Nanotechnol. (2023) 5:1146772. doi: 10.3389/fnano.2023.1146772 [DOI] [Google Scholar]
- 223.Navajas R, Corrales FJ, Paradela A. Serum exosome isolation by size-exclusion chromatography for the discovery and validation of preeclampsia-associated biomarkers. Methods Mol Biol. (2019) 1959:39–50. doi: 10.1007/978-1-4939-9164-8_3 [DOI] [PubMed] [Google Scholar]
- 224.Jiao R, Sun S, Gao X, Cui R, Cao G, Wei H, et al. A polyethylene glycol-based method for enrichment of extracellular vesicles from culture supernatant of human ovarian Cancer cell line A2780 and body fluids of high-grade serous carcinoma patients. Cancer Manag Res. (2020) 12:6291–301. doi: 10.2147/CMAR.S228288, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rider MA, Hurwitz SN, Meckes DG, Jr. ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep. (2016) 6:23978. doi: 10.1038/srep23978, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sharma P, Ludwig S, Muller L, Hong CS, Kirkwood JM, Ferrone S, et al. Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles. (2018) 7:1435138. doi: 10.1080/20013078.2018.1435138, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.