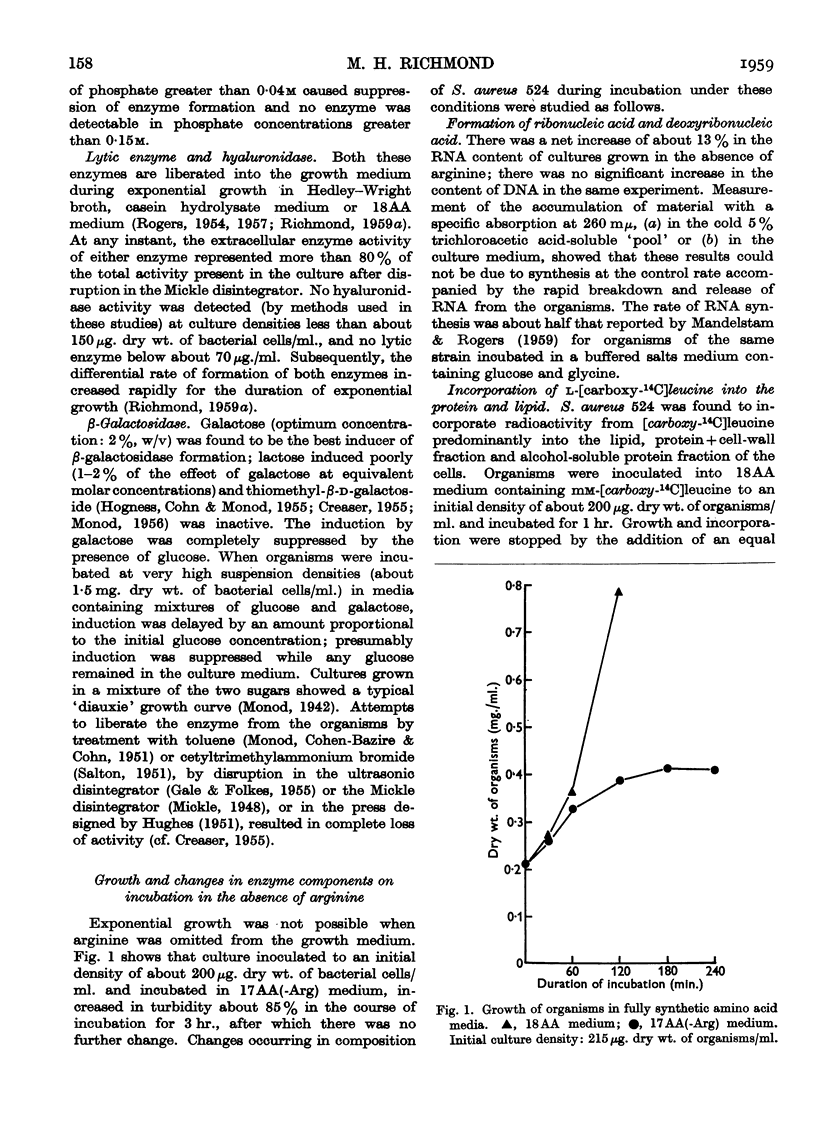
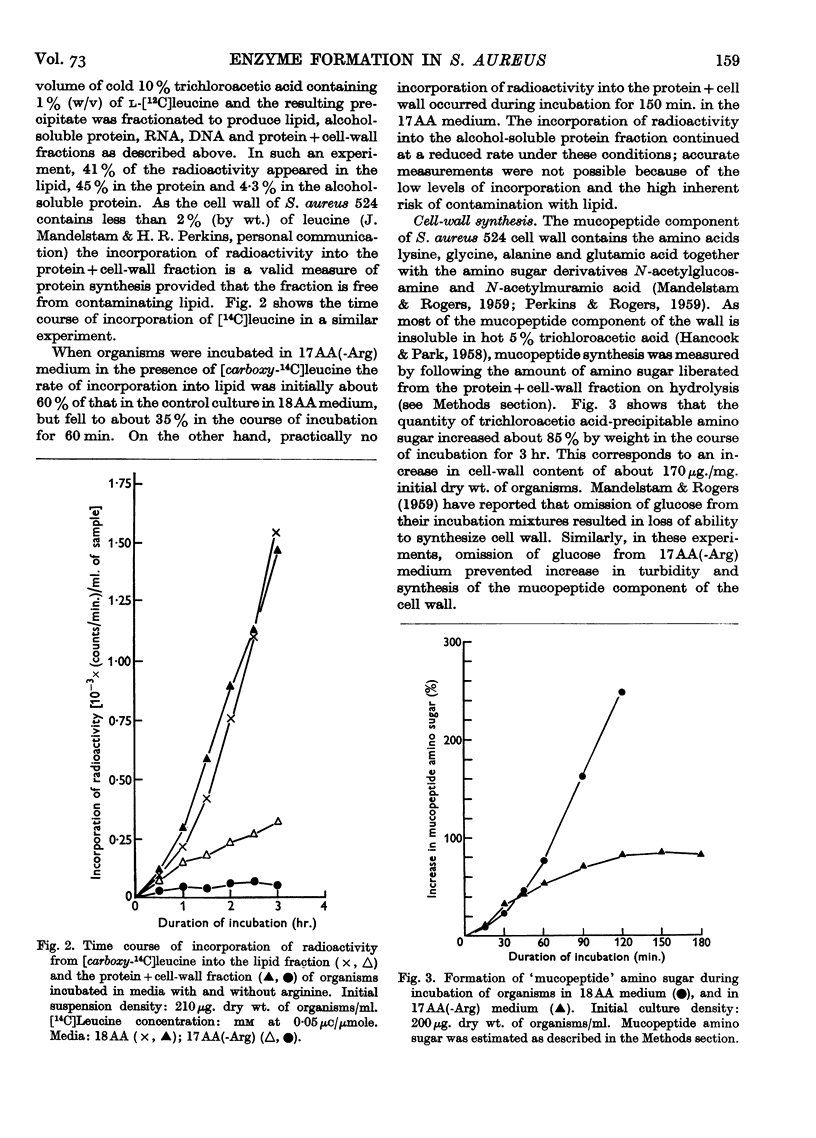
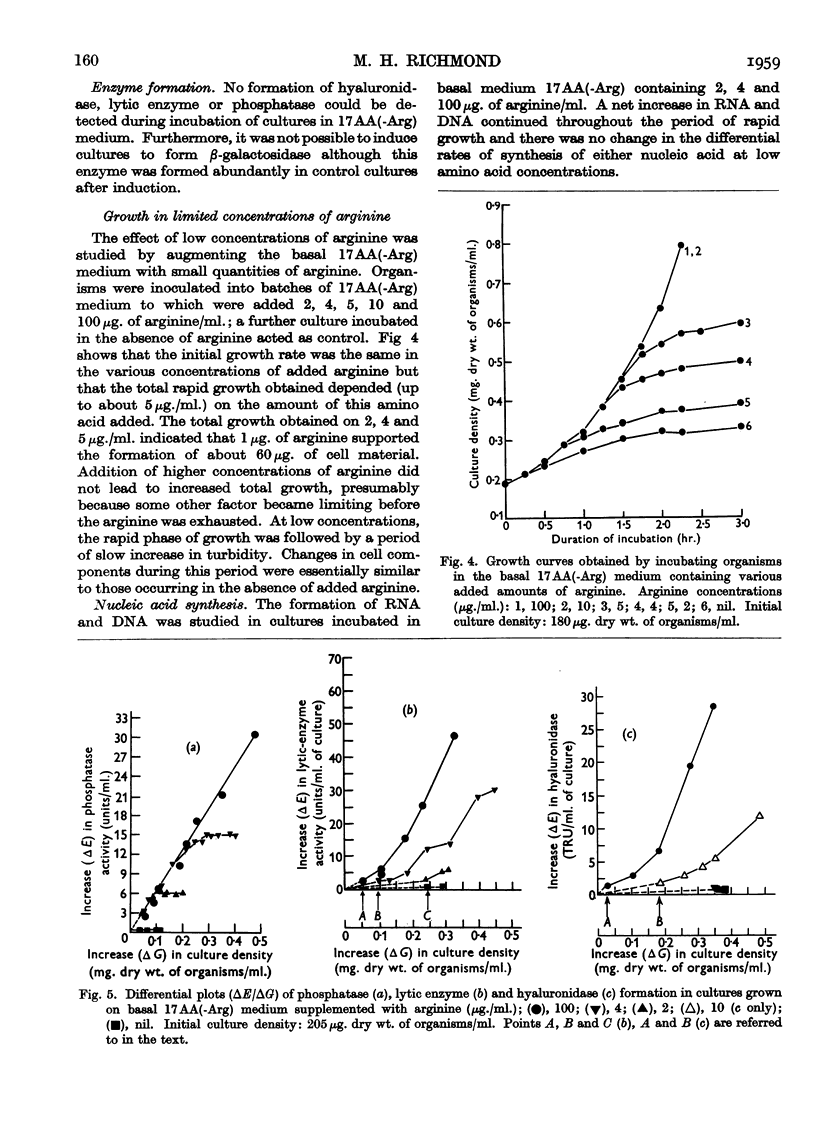
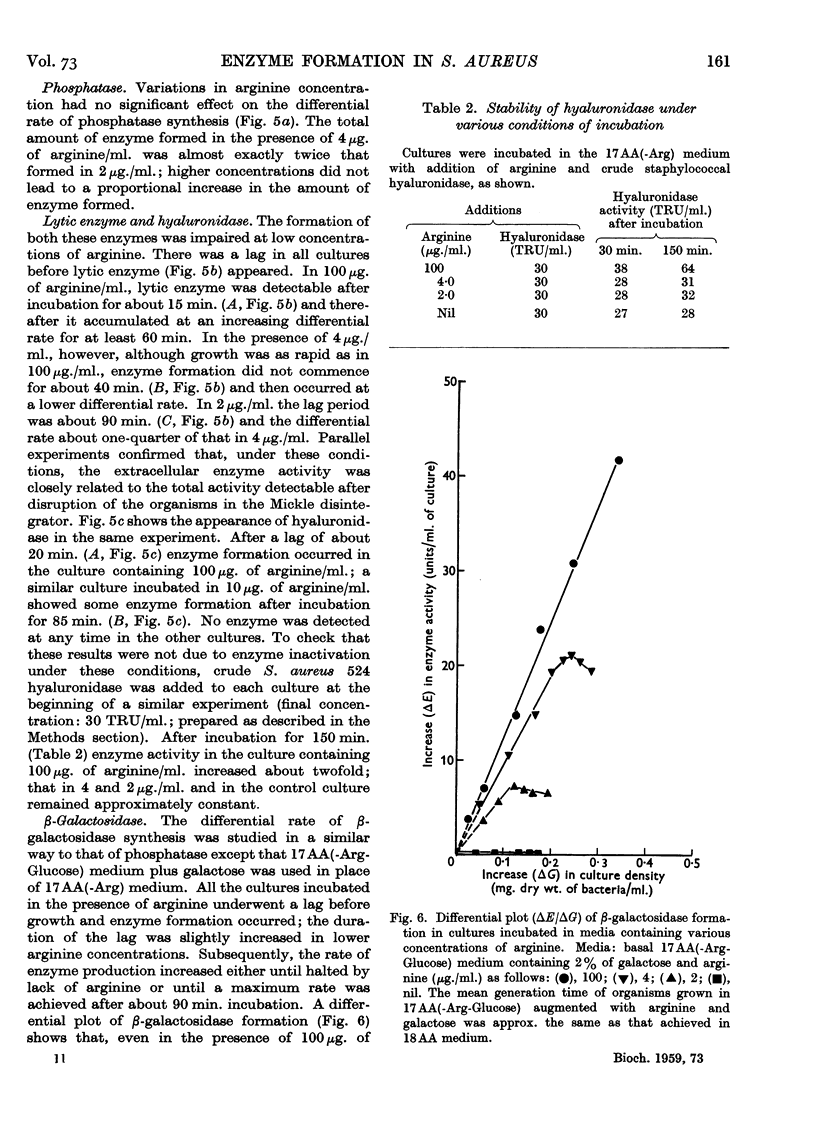
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. J., BADDILEY J., BUCHANAN J. G., CARSS B. Nucleotides and the bacterial cell wall. Nature. 1958 Jun 21;181(4625):1692–1693. doi: 10.1038/1811692a0. [DOI] [PubMed] [Google Scholar]
- BRAWERMAN G., YCAS M. Incorporation of the amino acid analog tryptazan into the protein of Escherichia coli. Arch Biochem Biophys. 1957 May;68(1):112–117. doi: 10.1016/0003-9861(57)90331-4. [DOI] [PubMed] [Google Scholar]
- BURTON K. The relation between the synthesis of deoxyribonucleic acid and the synthesis of protein in the multiplication of bacteriophage T2. Biochem J. 1955 Nov;61(3):473–483. doi: 10.1042/bj0610473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., MUNIER R. Incorporation d'analogues structuraux d'aminoacides dans les protéines bactériennes. Biochim Biophys Acta. 1956 Sep;21(3):592–593. doi: 10.1016/0006-3002(56)90207-4. [DOI] [PubMed] [Google Scholar]
- COHN M. Contributions of studies on the beta-galactosidase of Escherichia coli to our understanding of enzyme synthesis. Bacteriol Rev. 1957 Sep;21(3):140–168. doi: 10.1128/br.21.3.140-168.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COWIE D. B., COHEN G. N. Biosynthesis by Escherichia coli of active altered proteins containing selenium instead of sulfur. Biochim Biophys Acta. 1957 Nov;26(2):252–261. doi: 10.1016/0006-3002(57)90003-3. [DOI] [PubMed] [Google Scholar]
- CREASER E. H. The induced (adaptive) biosynthesis of beta-galactosidase in Staphylococcus aureus. J Gen Microbiol. 1955 Apr;12(2):288–297. doi: 10.1099/00221287-12-2-288. [DOI] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE E. F. Assimilation of amino acids by Gram-positive bacteria and some actions of antibiotics thereon. Adv Protein Chem. 1953;8:285–391. doi: 10.1016/s0065-3233(08)60094-7. [DOI] [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. The assimilation of amino acids by bacteria. 18. The incorporation of glutamic acid into the protein fraction of Staphylococcus aureus. Biochem J. 1953 Dec;55(5):721–729. doi: 10.1042/bj0550721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. The assimilation of amino acids by bacteria. 20. The incorporation of labelled amino acids by disrupted staphylococcal cells. Biochem J. 1955 Apr;59(4):661–675. doi: 10.1042/bj0590661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROS F., GROS Françoise Role des acides amines dans la synthèse des acides nucléiques chez Escherichia coli. Exp Cell Res. 1958 Feb;14(1):104–131. doi: 10.1016/0014-4827(58)90218-0. [DOI] [PubMed] [Google Scholar]
- HANCOCK R., PARK J. T. Cell-wall synthesis by Staphylococcus aureus in the presence of chloramphenicol. Nature. 1958 Apr 12;181(4615):1050–1052. doi: 10.1038/1811050a0. [DOI] [PubMed] [Google Scholar]
- HOGNESS D. S., COHN M., MONOD J. Studies on the induced synthesis of beta-galactosidase in Escherichia coli: the kinetics and mechanism of sulfur incorporation. Biochim Biophys Acta. 1955 Jan;16(1):99–116. doi: 10.1016/0006-3002(55)90188-8. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- KANTOROWICZ O. Shaking apparatus for the aeration of bacterial cultures. J Gen Microbiol. 1951 May;5(2):276–278. doi: 10.1099/00221287-5-2-276. [DOI] [PubMed] [Google Scholar]
- KIHARA H., SNELL E. E. The enzymatic cleavage of canavanine to O-ureidohomoserine and ammonia. J Biol Chem. 1957 May;226(1):485–495. [PubMed] [Google Scholar]
- MANDELSTAM J., ROGERS H. J. Chloramphenicol-resistant incorporation of amino-acids into Staphylococci and cell-wall synthesis. Nature. 1958 Apr 5;181(4614):956–957. doi: 10.1038/181956a0. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J., ROGERS H. J. The incorporation of amino acids into the cell-wall mucopeptide of staphylococci and the effect of antibiotics on the process. Biochem J. 1959 Aug;72:654–662. doi: 10.1042/bj0720654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHN M. La biosynthèse induite des enzymes; adaptation enzymatique. Adv Enzymol Relat Subj Biochem. 1952;13:67–119. [PubMed] [Google Scholar]
- MONOD J., PAPPENHEIMER A. M., Jr, COHEN-BAZIRE G. La cinétique de la biosynthèse de la beta-galactosidase chez E. coli considérée comme fonction de la croissance. Biochim Biophys Acta. 1952 Dec;9(6):648–660. doi: 10.1016/0006-3002(52)90227-8. [DOI] [PubMed] [Google Scholar]
- McQUILLEN K. Lysis resulting from metabolic disturbance. J Gen Microbiol. 1958 Apr;18(2):498–512. doi: 10.1099/00221287-18-2-498. [DOI] [PubMed] [Google Scholar]
- McQUILLEN K., ROBERTS R. B. The utilization of acetate for synthesis in Escherichia coli. J Biol Chem. 1954 Mar;207(1):81–95. [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Effects of azatryptophan on bacterial enzymes and bacteriophage. Biochim Biophys Acta. 1958 Feb;27(2):330–344. doi: 10.1016/0006-3002(58)90340-8. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S., SHORE V. G. Incorporation of azatryptophan into proteins of bacteria and bacteriophage. Biochim Biophys Acta. 1956 Aug;21(2):406–407. doi: 10.1016/0006-3002(56)90044-0. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The dependence of nucleic acid synthesis on the presence of amino acids in Escherichia coli. J Bacteriol. 1956 Jun;71(6):677–683. doi: 10.1128/jb.71.6.677-683.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLLOCK M. R., KRAMER M. Intermediates in the biosynthesis of bacterial penicillinase. Biochem J. 1958 Dec;70(4):665–681. doi: 10.1042/bj0700665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHMOND M. H. Formation of a lytic enzyme by a strain of Bacillus subtilis. Biochim Biophys Acta. 1959 May;33(1):78–92. doi: 10.1016/0006-3002(59)90500-1. [DOI] [PubMed] [Google Scholar]
- RICHMOND M. H. Lytic enzymes of Staphylococcus aureus 524. Biochim Biophys Acta. 1959 Feb;31(2):564–565. doi: 10.1016/0006-3002(59)90041-1. [DOI] [PubMed] [Google Scholar]
- RICKENBERG H. V., LESTER G. The preferential synthesis of beta-galactosidase in Escherichia coli. J Gen Microbiol. 1955 Oct;13(2):279–284. doi: 10.1099/00221287-13-2-279. [DOI] [PubMed] [Google Scholar]
- ROGERS H. J. The preferential suppression of hyaluronidase formation in cultures of Staphylococcus aureus. J Gen Microbiol. 1957 Feb;16(1):22–37. doi: 10.1099/00221287-16-1-22. [DOI] [PubMed] [Google Scholar]
- ROGERS H. J. The rate of formation of hyaluronidase, coagulase and total extracellular protein by strains of Staphylococcus aureus. J Gen Microbiol. 1954 Apr;10(2):209–220. doi: 10.1099/00221287-10-2-209. [DOI] [PubMed] [Google Scholar]
- ROGERS H. J. Variant populations within a hyaluronidase-producing culture of Staphylococcus aureus. J Pathol Bacteriol. 1953 Oct;66(2):545–551. doi: 10.1002/path.1700660226. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. The conditions controlling the production of hyaluronidase by micro-organisms grown in simplified media. Biochem J. 1945;39(5):435–443. doi: 10.1042/bj0390435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. J. The adsorption of cetyltrimethylammonium bromide by bacteria, its action in releasing cellular constituents and its bactericidal effects. J Gen Microbiol. 1951 May;5(2):391–404. doi: 10.1099/00221287-5-2-391. [DOI] [PubMed] [Google Scholar]
- SANDS M. K., ROBERTS R. B. The effects of a tryptophan-histidine deficiency in a mutant of Escherichia coli. J Bacteriol. 1952 Apr;63(4):505–511. doi: 10.1128/jb.63.4.505-511.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZAFIR J. J., BENNETT E. O. The adaptation of the Voges-Proskauer reaction for the quantitative assay of streptomycin. Science. 1953 Jun 26;117(3052):717–718. doi: 10.1126/science.117.3052.717. [DOI] [PubMed] [Google Scholar]
- WALKER J. B. Canavanine and homoarginine as antimetabolites of arginine and lysine in yeast and algae. J Biol Chem. 1955 Jan;212(1):207–215. [PubMed] [Google Scholar]
- YCAS M., BRAWERMAN G. Interrelations between nucleic acid and protein biosynthesis in microorganisms. Arch Biochem Biophys. 1957 May;68(1):118–129. doi: 10.1016/0003-9861(57)90332-6. [DOI] [PubMed] [Google Scholar]


