Abstract
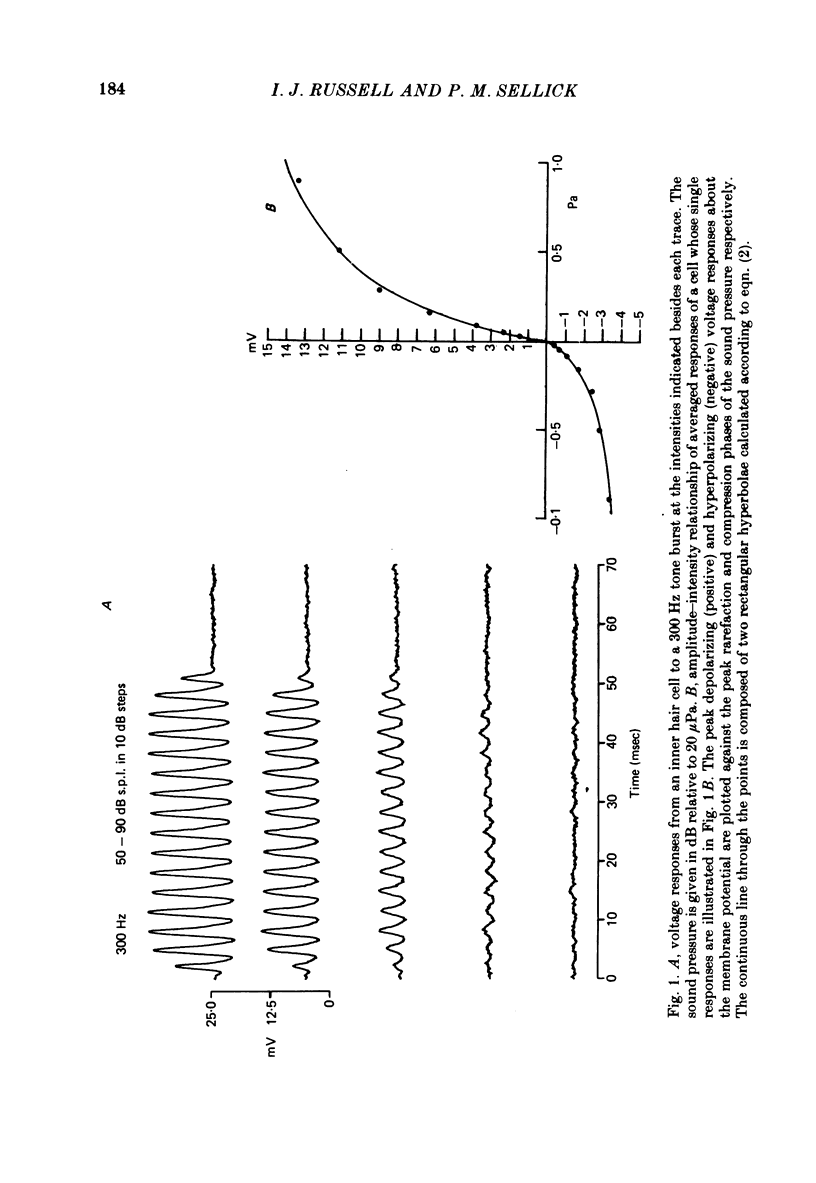
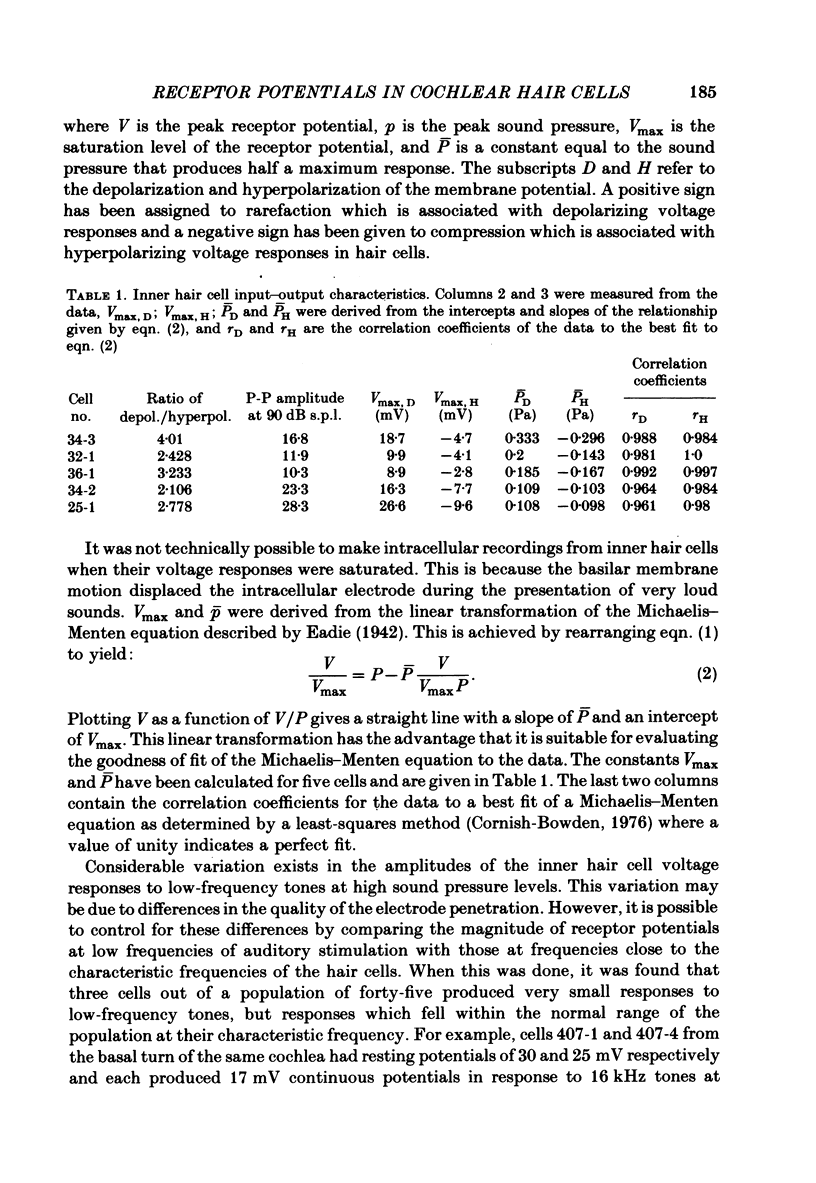
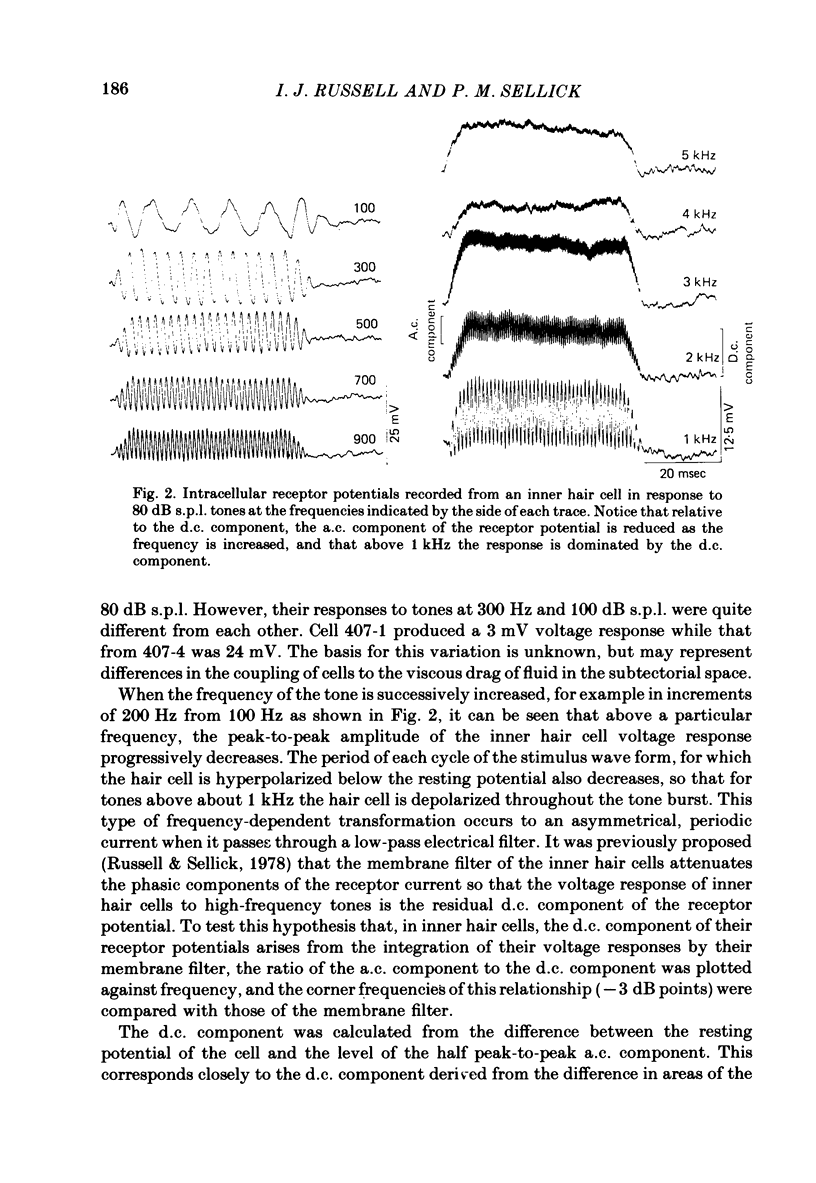
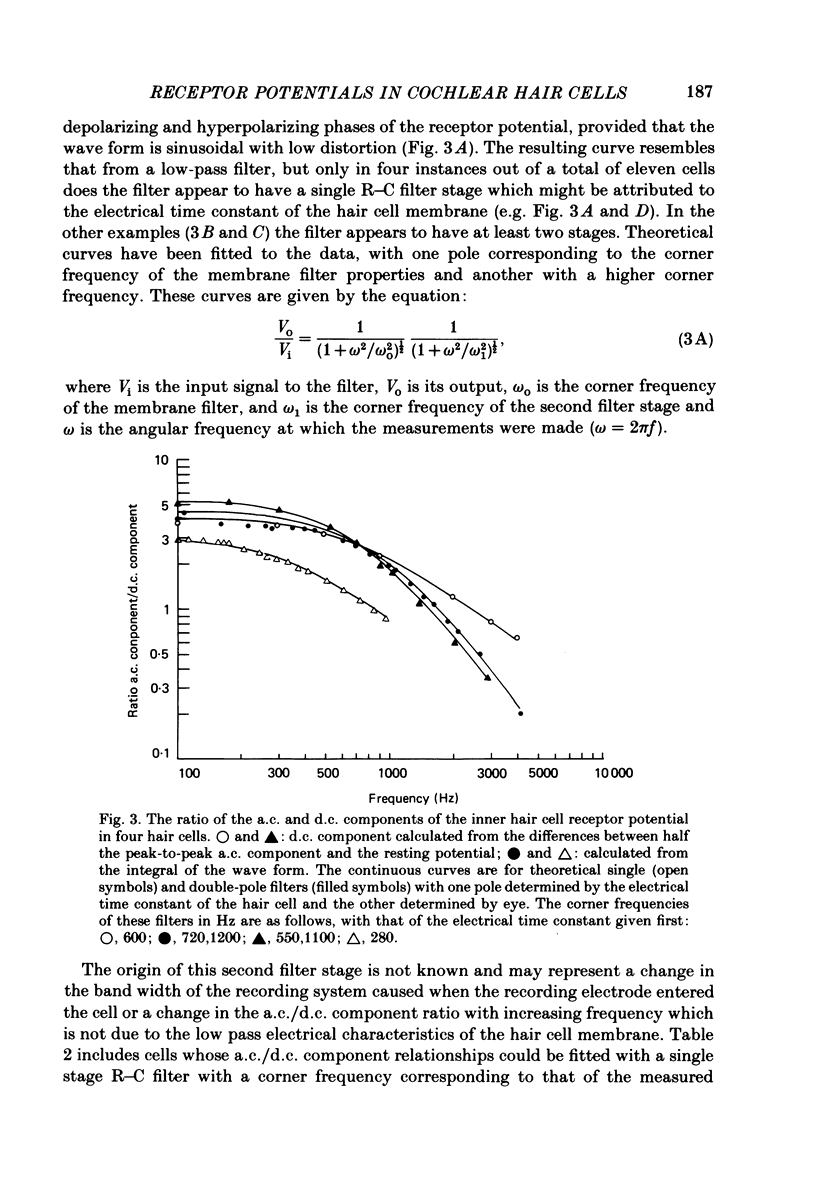
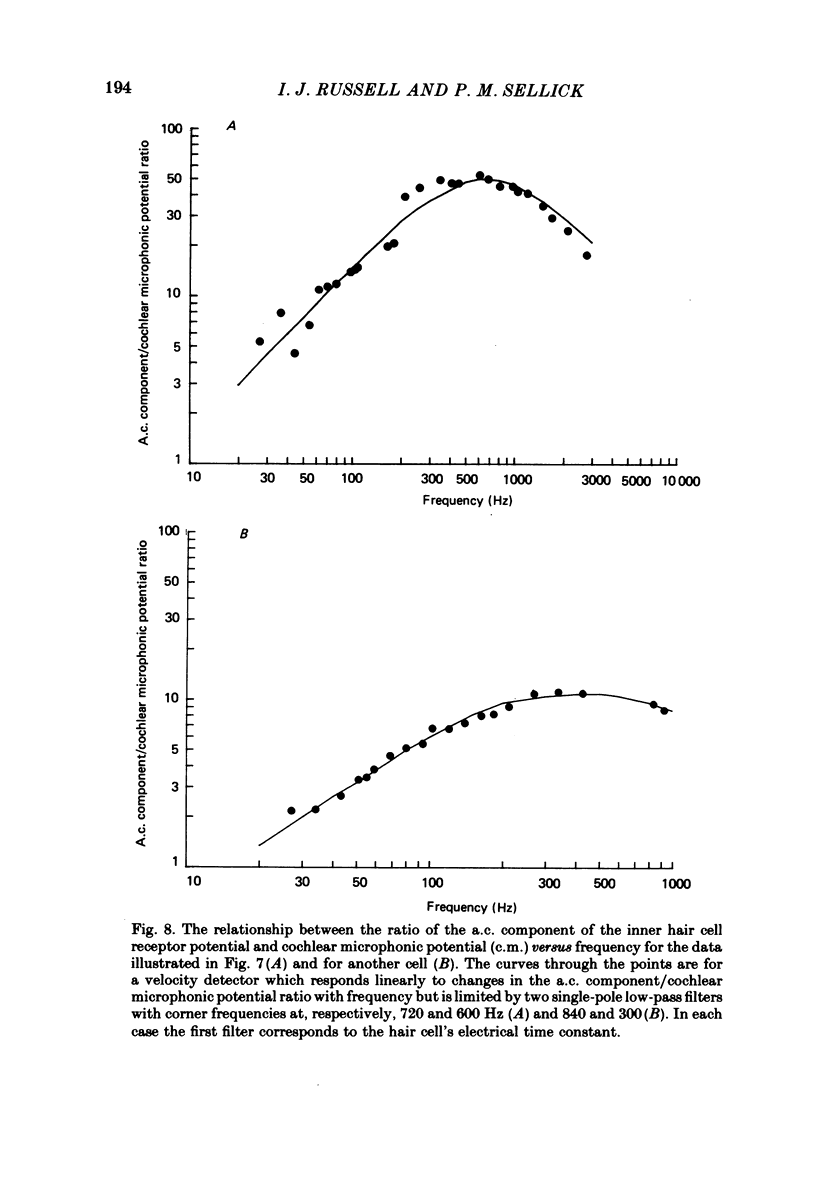
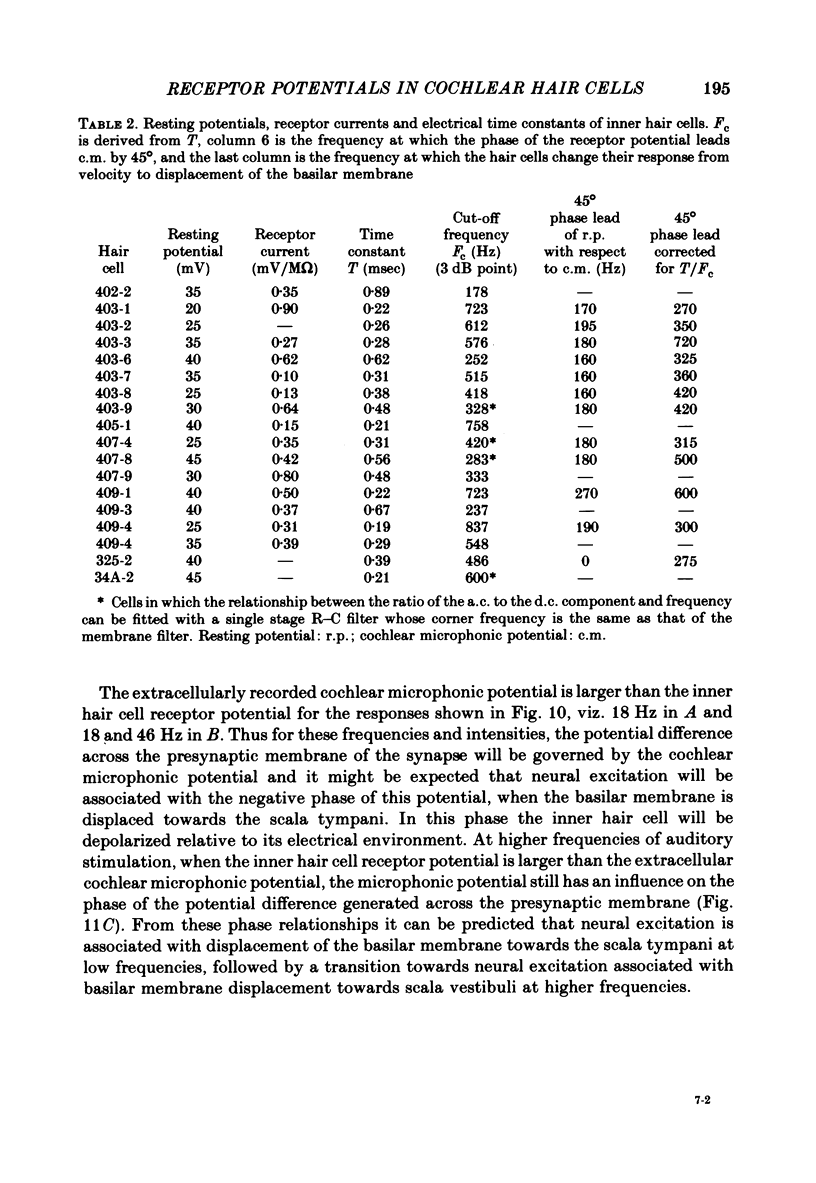
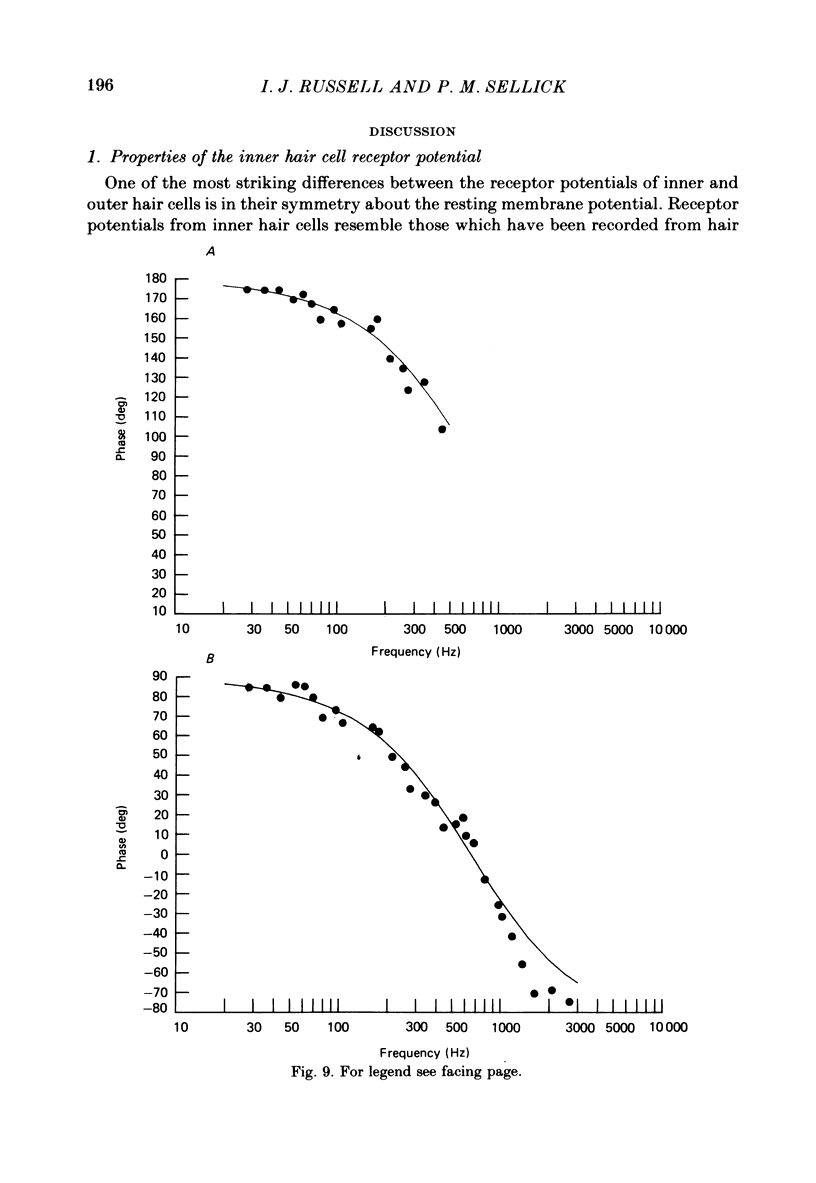
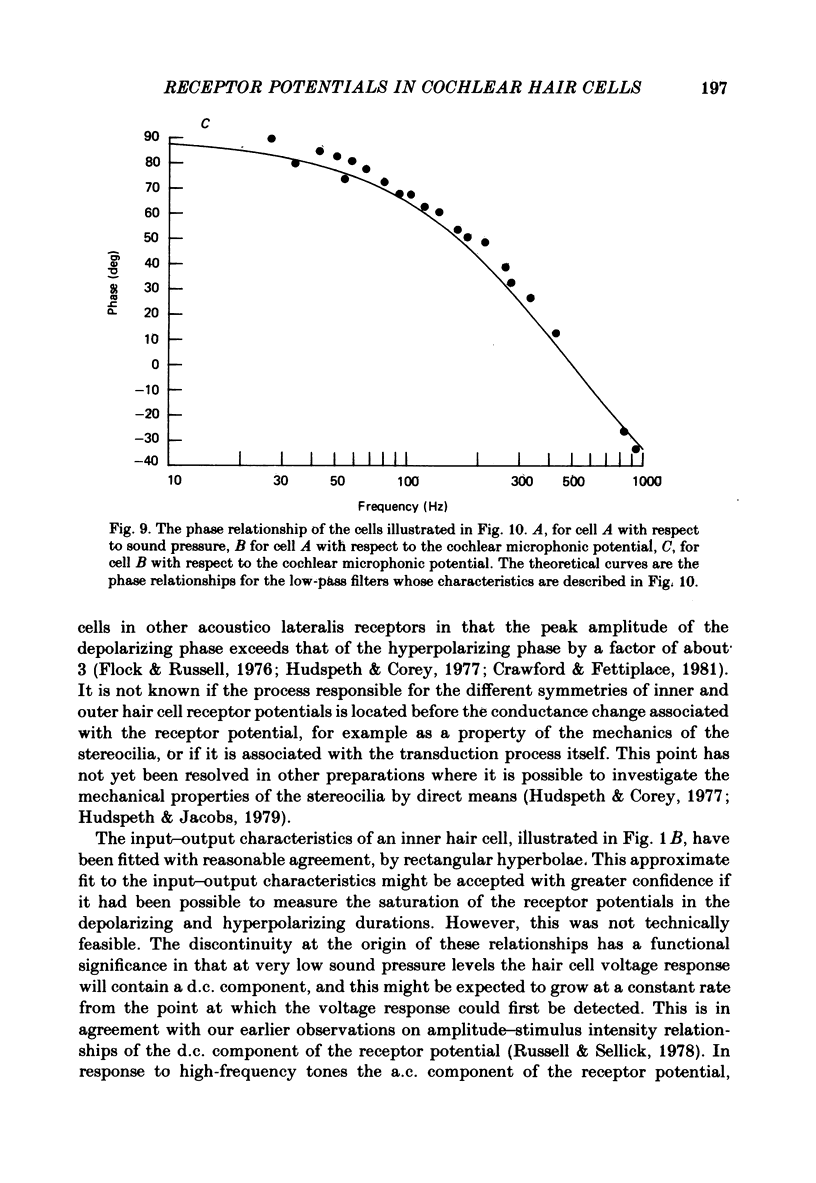
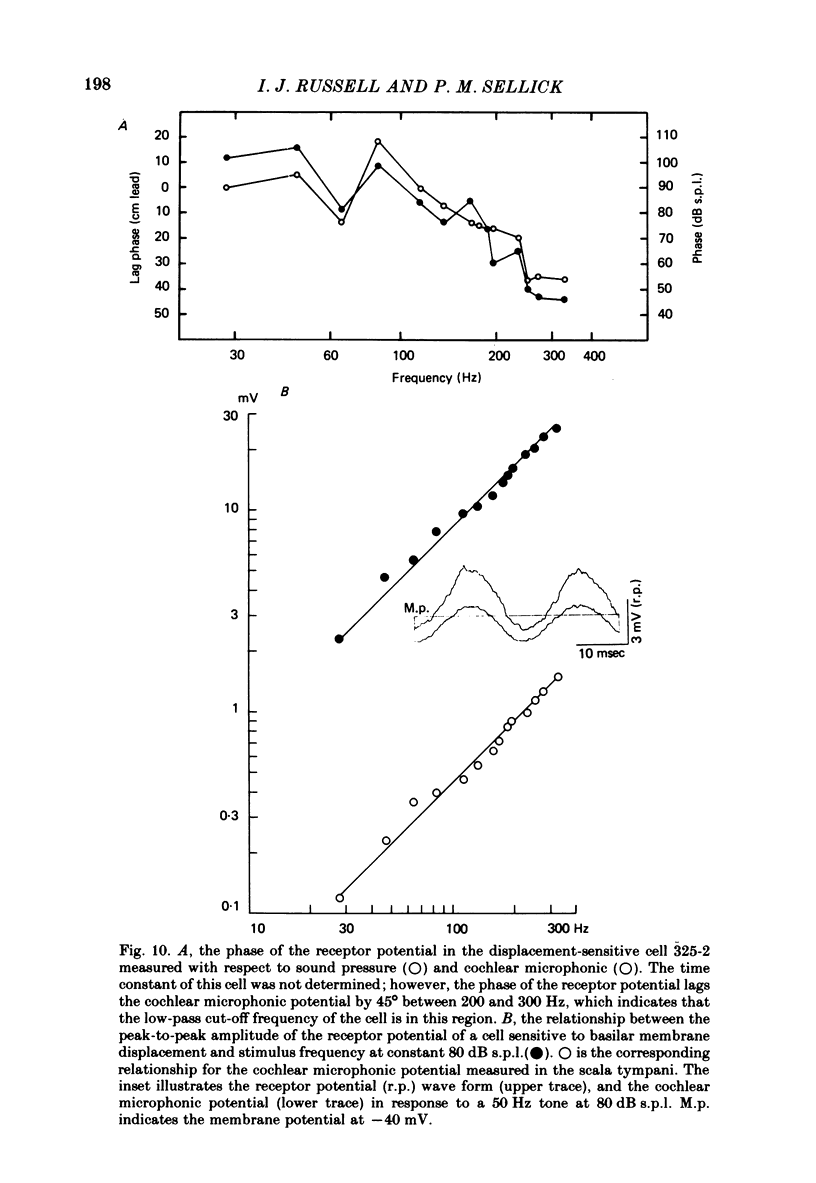
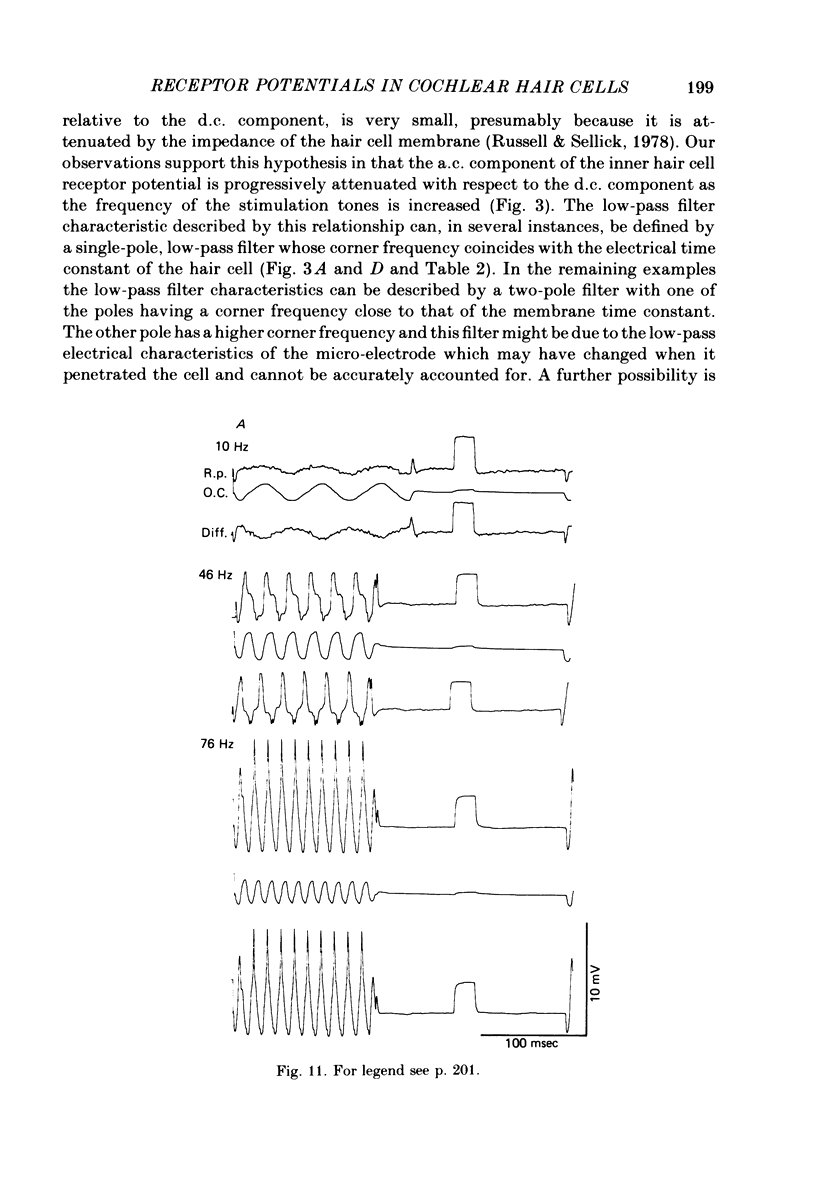
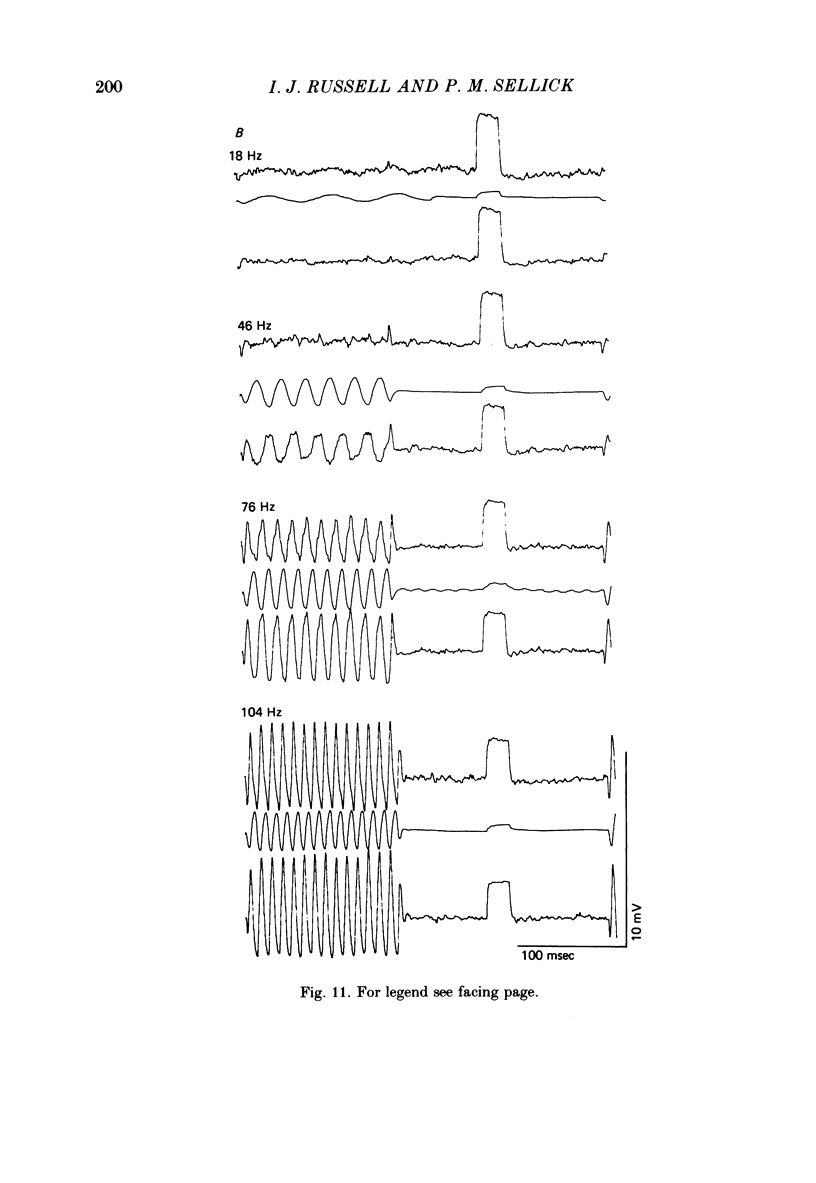
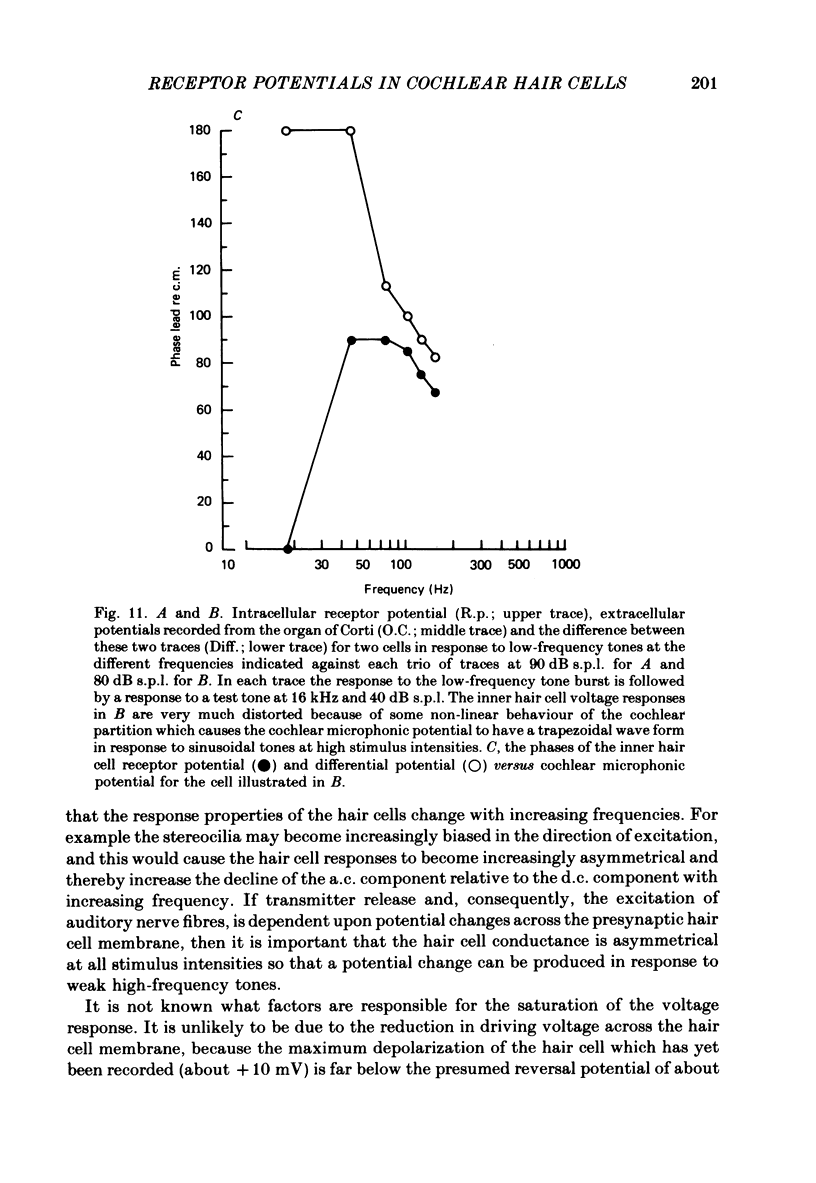
Intracellular receptor potentials were recorded from inner and outer hair cells in response to low-frequency tones, from the basal, high-frequency region of the guinea-pig cochlea. The receptor potentials recorded from inner hair cells are asymmetrical about the resting membrane potential with the depolarizing phase, which corresponds to rarefaction in sound pressure, exceeding the phase of hyperpolarization by a factor of about 3. It was found that the relationship between the peak-to-peak voltage responses and sound pressure level could be described by rectangular hyperbolae. When the frequency of the sound stimulus was progressively increased from 100 Hz to 4 kHz, the 'periodic' (a.c.) component of the receptor potential was attenuated with respect to the 'continuous' (d.c.) component. The characteristics of the inner hair cells could be described by two stages of low-pass filtering, with one of the filters having the same corner frequency as the electrical time constants which varied in different cells between 178 and 840 Hz. Receptor potentials recorded intracellularly from two morphologically identified outer hair cells were symmetrical about the resting membrane potential (about -65-70 mV) and had a maximal amplitude of only 5 mV at frequencies and intensities which yield 20-30 mV voltage responses from inner hair cells. No d.c. component receptor potentials were recorded in response to high-frequency tones. Phase and amplitude measurements were made from receptor potentials from inner hair cells, and from 'cochlear microphonic potentials' which were recorded from the organ of Corti and scala tympani. The phase of depolarization in both potentials was associated with displacement of the basilar membrane towards the scala vestibuli. The phase of the intracellular receptor potentials leads the cochlear microphonic by about 90 degrees and the sound pressure by about 180 degrees at frequencies below 100 Hz. Above this frequency the phase lead progressively declines and at higher frequencies becomes a phase lag. These phase relationships indicate that inner hair cells respond to the velocity of the basilar membrane at frequencies below 200-600 Hz, and to its displacement above this, and that the voltage responses of the inner hair cells are limited by their membrane time constants. It is suggested that outer hair cells respond to basilar membrane displacement throughout their frequency range. It is shown that, with respect to frequency, the different growth rates of the cochlear microphonic potentials and inner hair cell receptor potentials, and the dominance of cochlear microphonic potentials in the organ of Corti, result in an effective electrical interaction between inner hair cells and cochlear microphonic potentials.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boston J. R. A model of lateral line microphonic response to high-level stimuli. J Acoust Soc Am. 1980 Mar;67(3):875–881. doi: 10.1121/1.383967. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R. Non-linearities in the responses of turtle hair cells. J Physiol. 1981 Jun;315:317–338. doi: 10.1113/jphysiol.1981.sp013750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallos P., Billone M. C., Durrant J. D., Wang C., Raynor S. Cochlear inner and outer hair cells: functional differences. Science. 1972 Jul 28;177(4046):356–358. doi: 10.1126/science.177.4046.356. [DOI] [PubMed] [Google Scholar]
- Dallos P., Cheatham M. A. Production of cochlear potentials by inner and outer hair cells. J Acoust Soc Am. 1976 Aug;60(2):510–512. doi: 10.1121/1.381086. [DOI] [PubMed] [Google Scholar]
- Dallos P. Low-frequency auditory characteristics: Species dependence. J Acoust Soc Am. 1970 Aug;48(2):489–499. doi: 10.1121/1.1912163. [DOI] [PubMed] [Google Scholar]
- Dallos P., Santos-Sacchi J., Flock A. Intracellular recordings from cochlear outer hair cells. Science. 1982 Nov 5;218(4572):582–584. doi: 10.1126/science.7123260. [DOI] [PubMed] [Google Scholar]
- Davis H. A model for transducer action in the cochlea. Cold Spring Harb Symp Quant Biol. 1965;30:181–190. doi: 10.1101/sqb.1965.030.01.020. [DOI] [PubMed] [Google Scholar]
- Evans E. F. Neuroleptanesthesia for the guinea pig. An ideal anesthetic procedure for long-term physiological studies of the cochlea. Arch Otolaryngol. 1979 Apr;105(4):185–186. doi: 10.1001/archotol.1979.00790160019004. [DOI] [PubMed] [Google Scholar]
- Flock A., Russell I. Inhibition by efferent nerve fibres: action on hair cells and afferent synaptic transmission in the lateral line canal organ of the burbot Lota lota. J Physiol. 1976 May;257(1):45–62. doi: 10.1113/jphysiol.1976.sp011355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino T. Relationship of the tectorial membrane on the organ of Corti. A scanning electron microscope study of cats and guinea pigs. Arch Histol Jpn. 1974 Jul;37(1):25–39. [PubMed] [Google Scholar]
- Hudspeth A. J., Corey D. P. Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2407–2411. doi: 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A. J., Jacobs R. Stereocilia mediate transduction in vertebrate hair cells (auditory system/cilium/vestibular system). Proc Natl Acad Sci U S A. 1979 Mar;76(3):1506–1509. doi: 10.1073/pnas.76.3.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone B. M., Sellick P. M. The peripheral auditory apparatus. Q Rev Biophys. 1972 Feb;5(1):1–57. doi: 10.1017/s0033583500000032. [DOI] [PubMed] [Google Scholar]
- Jones G. M., Milsum J. H. Frequency-response analysis of central vestibular unit activity resulting from rotational stimulation of the semicircular canals. J Physiol. 1971 Dec;219(1):191–215. doi: 10.1113/jphysiol.1971.sp009657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R. S. Hairs of the cochlear sensory cells and their attachment to the tectorial membrane. Acta Otolaryngol. 1966 Jan-Feb;61(1):55–72. doi: 10.3109/00016486609127043. [DOI] [PubMed] [Google Scholar]
- Lim D. J. Fine morphology of the tectorial membrane. Its relationship to the organ of Corti. Arch Otolaryngol. 1972 Sep;96(3):199–215. doi: 10.1001/archotol.1972.00770090321001. [DOI] [PubMed] [Google Scholar]
- Mountain D. C. Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alter cochlear mechanics. Science. 1980 Oct 3;210(4465):71–72. doi: 10.1126/science.7414321. [DOI] [PubMed] [Google Scholar]
- Peake W. T., Ling A., Jr Basilar-membrane motion in the alligator lizard: its relation to tonotopic organization and frequency selectivity. J Acoust Soc Am. 1980 May;67(5):1736–1745. doi: 10.1121/1.384300. [DOI] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Intracellular studies of hair cells in the mammalian cochlea. J Physiol. 1978 Nov;284:261–290. doi: 10.1113/jphysiol.1978.sp012540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell I. J., Sellick P. M. Tuning properties of cochlear hair cells. Nature. 1977 Jun 30;267(5614):858–860. doi: 10.1038/267858a0. [DOI] [PubMed] [Google Scholar]
- Schmiedt R. A., Zwislocki J. J., Hamernik R. P. Effects of hair cell lesions on responses of cochlear nerve fibers. I. Lesions, tuning curves, two-tone inhibition, and responses to trapezoidal-wave patterns. J Neurophysiol. 1980 May;43(5):1367–1389. doi: 10.1152/jn.1980.43.5.1367. [DOI] [PubMed] [Google Scholar]
- Sellick P. M., Patuzzi R., Johnstone B. M. Measurement of basilar membrane motion in the guinea pig using the Mössbauer technique. J Acoust Soc Am. 1982 Jul;72(1):131–141. doi: 10.1121/1.387996. [DOI] [PubMed] [Google Scholar]
- Sellick P. M., Russell I. J. The responses of inner hair cells to basilar membrane velocity during low frequency auditory stimulation in the guinea pig cochlea. Hear Res. 1980 Jun;2(3-4):439–445. doi: 10.1016/0378-5955(80)90080-5. [DOI] [PubMed] [Google Scholar]
- Sokolich W. G., Hamernik R. P., Zwislocki J. J., Schmiedt R. A. Inferred response polarities of cochlear hair cells. J Acoust Soc Am. 1976 Apr;59(4):963–974. doi: 10.1121/1.380955. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Innervation patterns in the organ of corti of the cat. Acta Otolaryngol. 1969 Feb-Mar;67(2):239–254. doi: 10.3109/00016486909125448. [DOI] [PubMed] [Google Scholar]
- Stewart W. W. Functional connections between cells as revealed by dye-coupling with a highly fluorescent naphthalimide tracer. Cell. 1978 Jul;14(3):741–759. doi: 10.1016/0092-8674(78)90256-8. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Asanuma A., Yanagisawa K. Potentials of outer hair cells and their membrane properties in cationic environments. Hear Res. 1980 Jun;2(3-4):431–438. doi: 10.1016/0378-5955(80)90079-9. [DOI] [PubMed] [Google Scholar]
- Wilson J. P., Johnstone J. R. Basilar membrane and middle-ear vibration in guinea pig measured by capacitive probe. J Acoust Soc Am. 1975 Mar;57(3):705–723. doi: 10.1121/1.380472. [DOI] [PubMed] [Google Scholar]


