Abstract
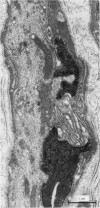
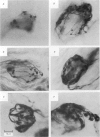
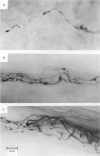
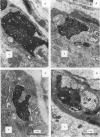
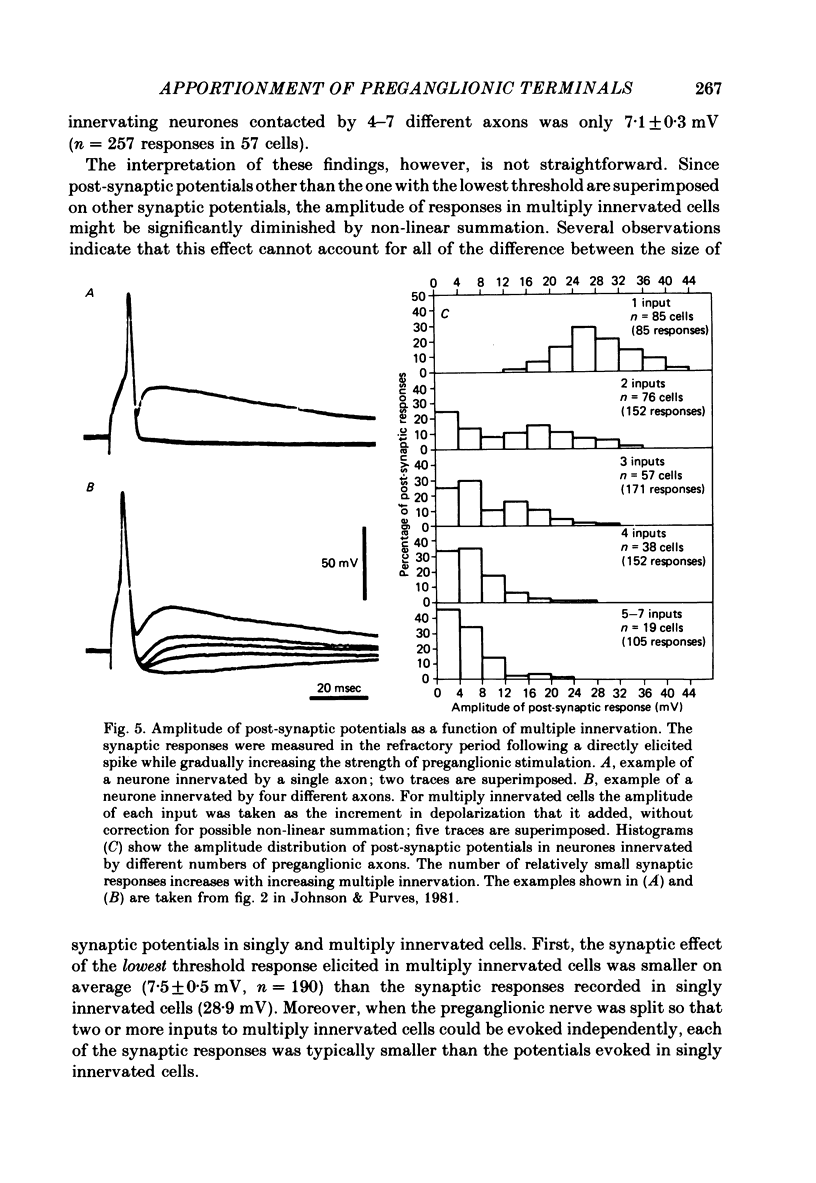
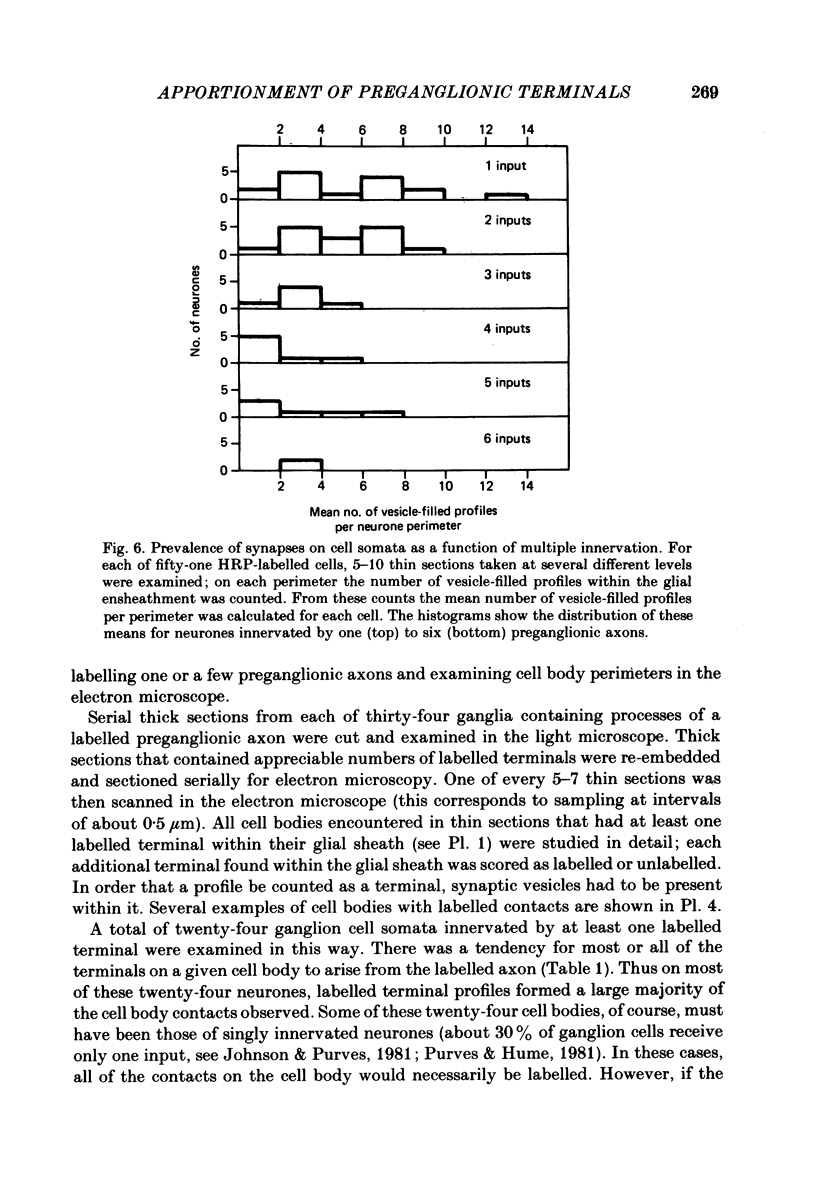
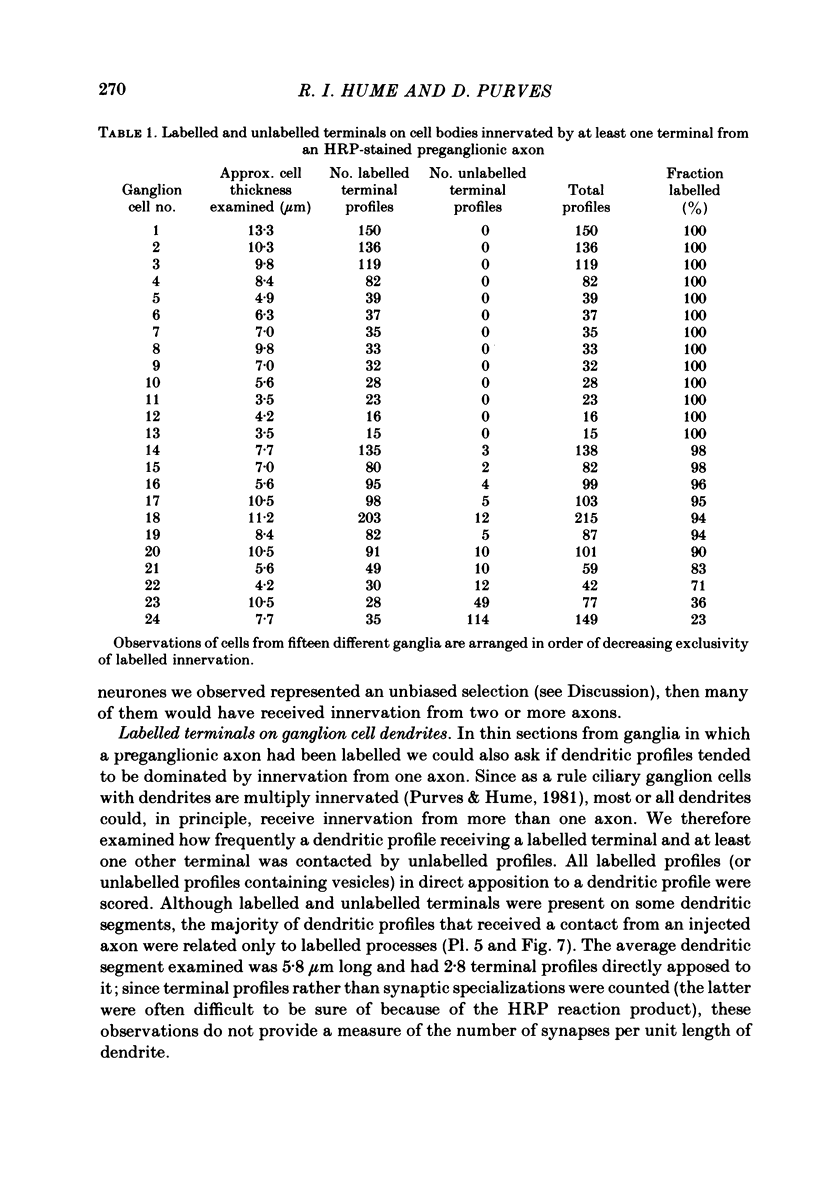
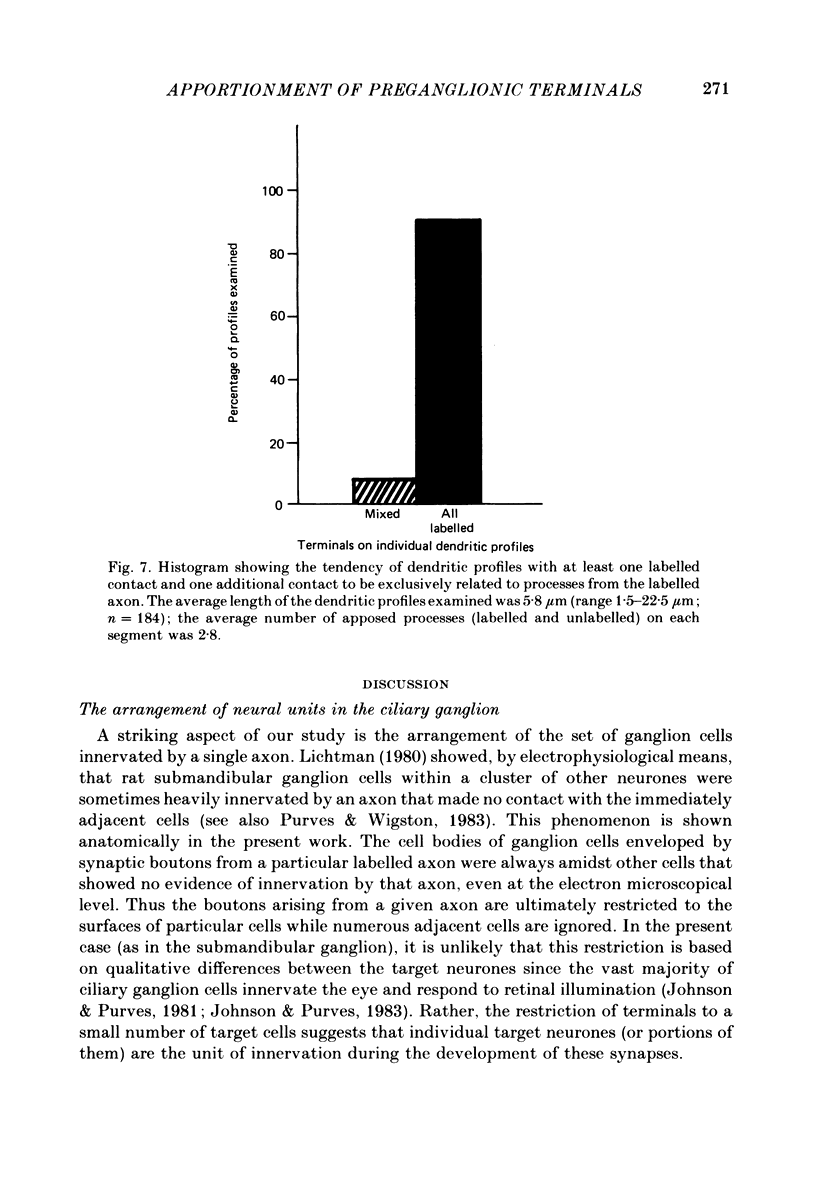
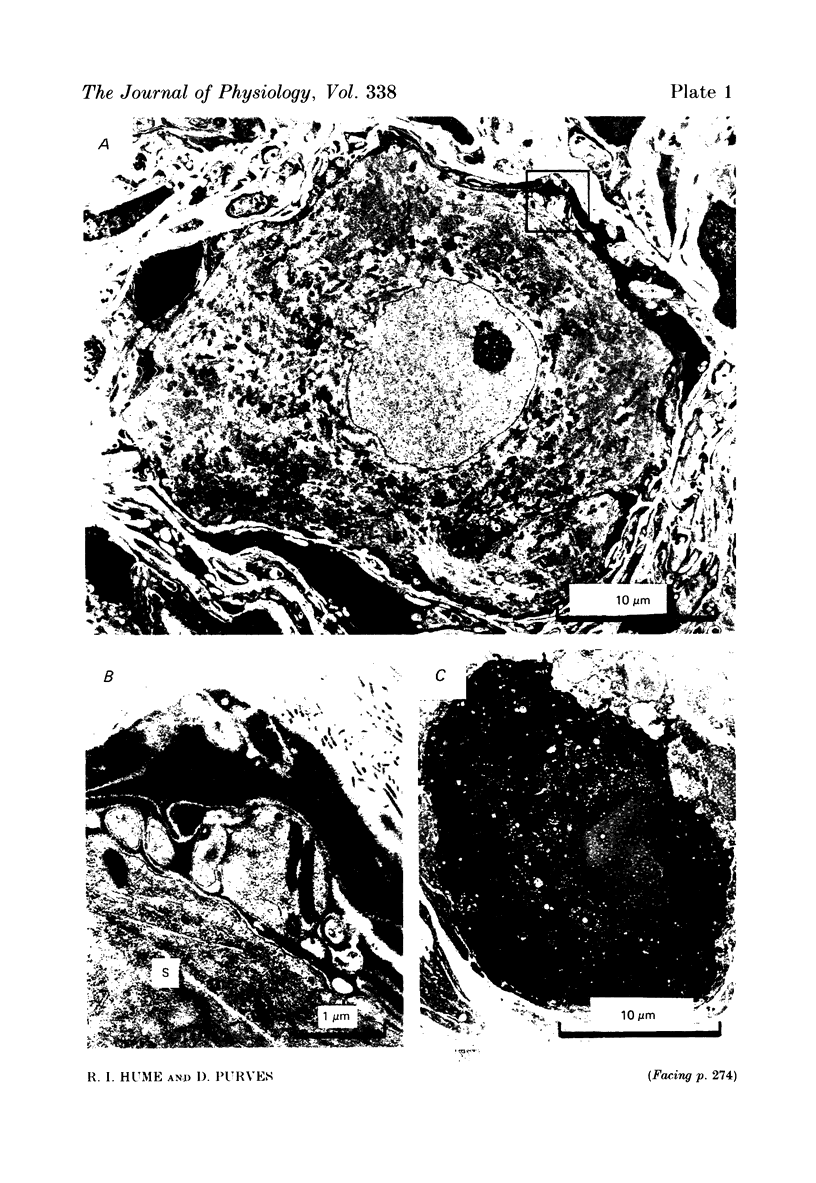
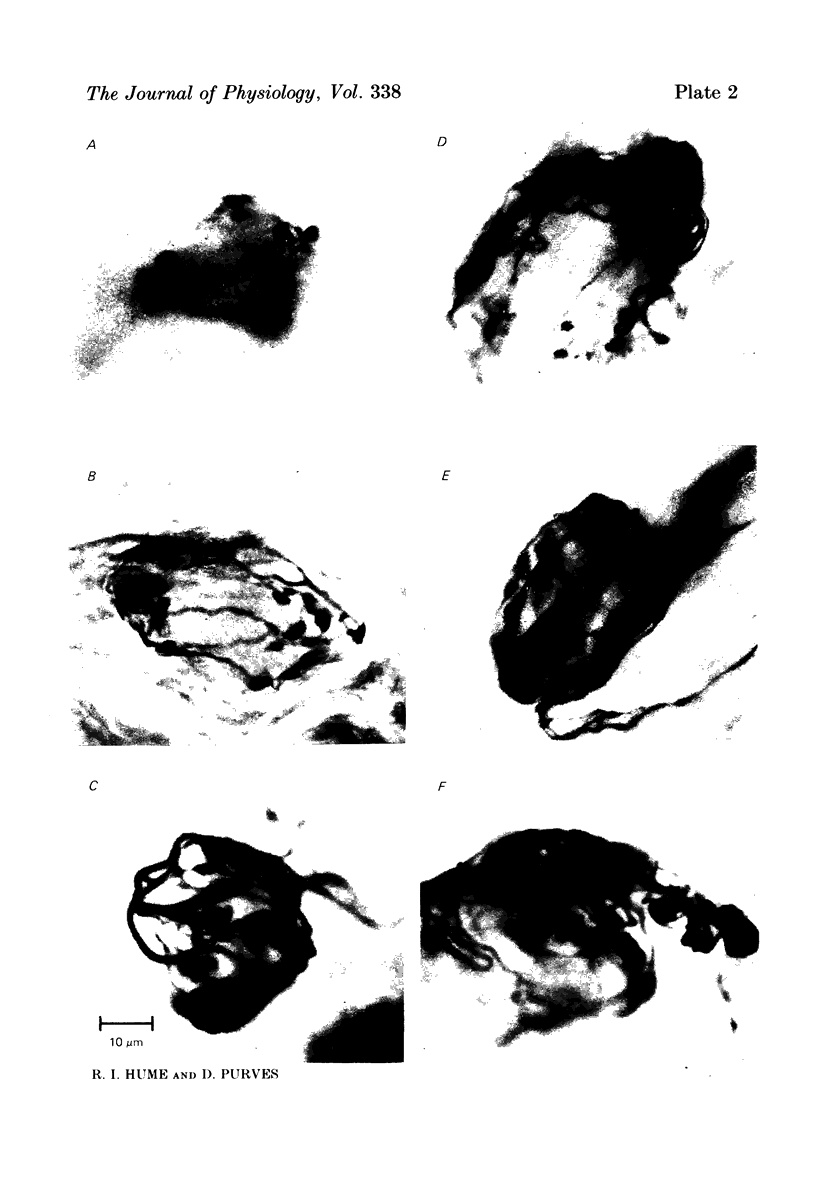
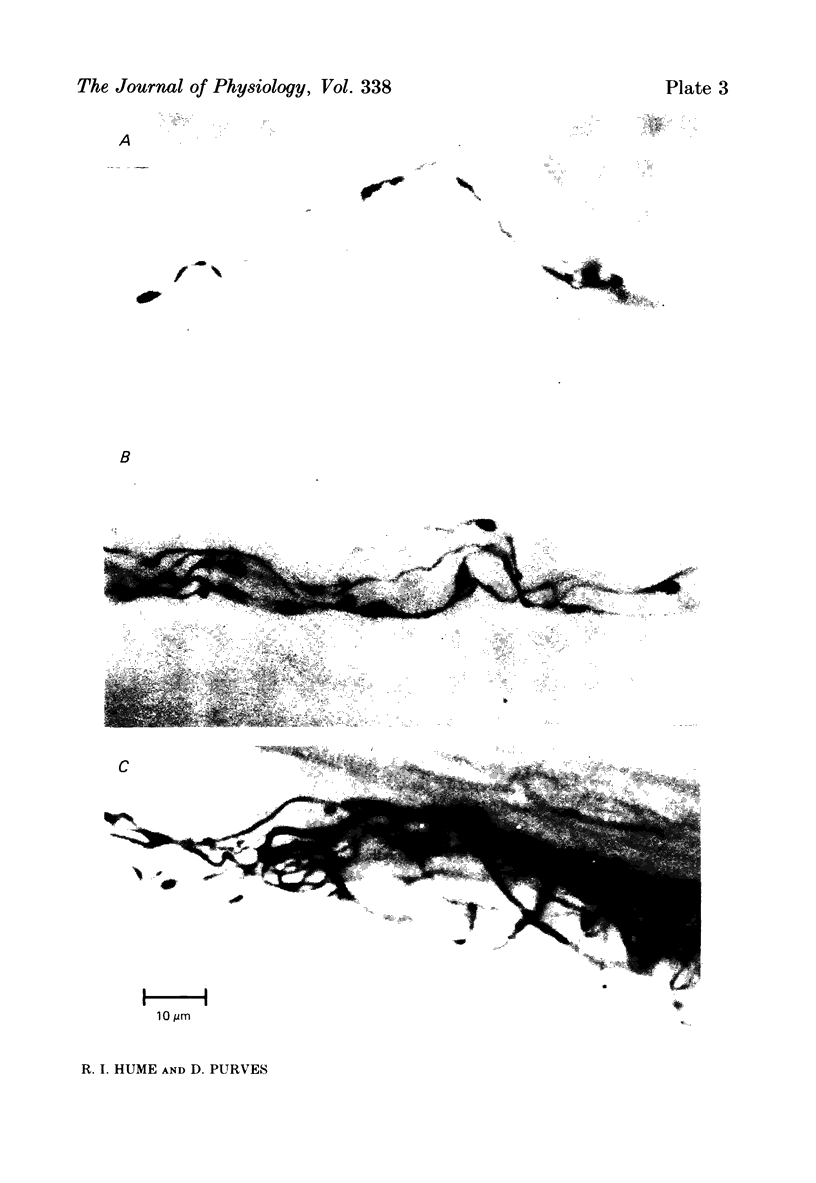
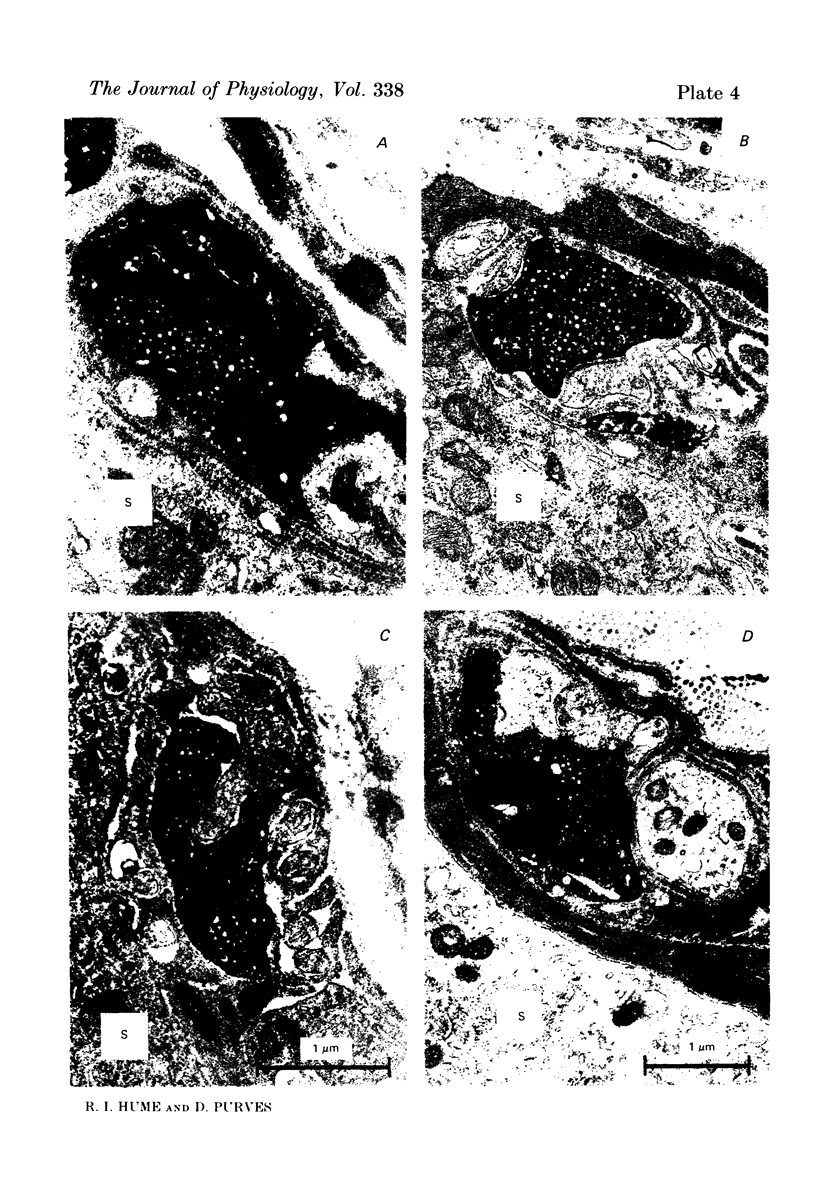
We have studied the apportionment of terminals from single preganglionic axons to target neurones in the ciliary ganglion of adult rabbits. Both electrical recording and intra-axonal injection of horseradish peroxidase (HRP) showed that each preganglionic axon innervates only a small fraction of the ganglion cell population (about 10-20 of the approximately 400 ganglion cells). Examination of ganglia in whole mounts showed that neurones whose cell bodies were enveloped by HRP-labelled boutons from a single axon were often surrounded by other neurones which received no contacts from the labelled fibre. Electron microscopical examination of labelled presynaptic terminals on individual ganglion cells confirmed that the boutons of single axons were sharply confined to particular target cells. This suggests that individual target neurones (or portions of them) are the unit of innervation during the development of these synaptic connexions. Comparison of the amplitudes of synaptic responses in singly and multiply innervated ganglion cells indicated that, on average, an individual axon made a weaker synaptic connexion with a multiply innervated neurone than with neurone that received only one input. Moreover, neurones innervated by several different axons tended to have fewer synapses on their somata than neurones innervated by only one or two preganglionic axons. Individual post-synaptic profiles were often contacted exclusively by labelled terminals when examined in the electron microscope. Since many of these neurones are multiply innervated, this observation suggests some regional separation of the several inputs contacting the same cell. For several reasons, however, this inference must be regarded as tentative. Taken together, these findings provide a possible explanation of the correlation between the dendritic geometry of ganglion cells and the number of different axons that innervate them (Purves & Hume, 1981). The several axons that initially innervate ganglion cells without dendrites evidently compete during early life until only a single input remains. On ganglion cells with dendrites, however, the number of inputs that persists is proportional to dendritic complexity. The present results suggest that the diminished competition between axons innervating neurones with dendrites may result from some degree of terminal segregation on dendritic arborizations.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angaut-Petit D., Mallart A. Dual innervation of end-plate sites and its consequences for neuromuscular transmission in muscles of adult Xenopus laevis. J Physiol. 1979 Apr;289:203–218. doi: 10.1113/jphysiol.1979.sp012733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Haimann C., Mallart A., Ferré J. T., Zilber-Gachelin N. F. Interaction between motor axons from two different nerves reinnervating the pectoral muscle of Xenopus laevis. J Physiol. 1981 Jan;310:257–272. doi: 10.1113/jphysiol.1981.sp013547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimann C., Mallart A., Ferré J. T., Zilber-Gachelin N. F. Patterns of motor innervation in the pectoral muscle of adult Xenopus laevis: evidence for possible synaptic remodelling. J Physiol. 1981 Jan;310:241–256. doi: 10.1113/jphysiol.1981.sp013546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Purves D. Geometry of neonatal neurones and the regulation of synapse elimination. Nature. 1981 Oct 8;293(5832):469–471. doi: 10.1038/293469a0. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Purves D. Post-natal reduction of neural unit size in the rabbit ciliary ganglion. J Physiol. 1981 Sep;318:143–159. doi: 10.1113/jphysiol.1981.sp013855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. On the predominantly single innervation of submandibular ganglion cells in the rat. J Physiol. 1980 May;302:121–130. doi: 10.1113/jphysiol.1980.sp013233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D. The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol. 1980 Apr;301:213–228. doi: 10.1113/jphysiol.1980.sp013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W. The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol. 1977 Dec;273(1):155–177. doi: 10.1113/jphysiol.1977.sp012087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The formation of synapses in mammalian sympathetic ganglia reinnervated with preganglionic or somatic nerves. J Physiol. 1974 Feb;237(1):217–242. doi: 10.1113/jphysiol.1974.sp010479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Hume R. I. The relation of postsynaptic geometry to the number of presynaptic axons that innervate autonomic ganglion cells. J Neurosci. 1981 May;1(5):441–452. doi: 10.1523/JNEUROSCI.01-05-00441.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Formation and maintenance of synaptic connections in autonomic ganglia. Physiol Rev. 1978 Oct;58(4):821–862. doi: 10.1152/physrev.1978.58.4.821. [DOI] [PubMed] [Google Scholar]
- Purves D., Wigston D. J. Neural units in the superior cervical ganglion of the guinea-pig. J Physiol. 1983 Jan;334:169–178. doi: 10.1113/jphysiol.1983.sp014487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen A., Raisman G. Synapse formation in the rat superior cervical ganglion during normal development and after neonatal deafferentation. Brain Res. 1980 Jan 13;181(2):315–323. doi: 10.1016/0006-8993(80)90615-0. [DOI] [PubMed] [Google Scholar]